Abstract
Background
Family with sequence similarity 83 member H antisense RNA 1 (FAM83H-AS1) is a novel long non-coding RNA. Increasing studies have reported that FAM83H-AS1 is abnormally expressed in a variety of tumors and is associated with poor outcome. However, the clinical prognostic significance of lncRNA FAM83H-AS1 in tumors is not completely known.
Methods
In this meta-analysis, literature was collected up until February 5, 2020 through multifarious retrieval strategies by searching through electronic databases of PubMed, Cochrane Library, EMBASE, Medline, Web of Science, CNKI, Weipu, and Wanfang. A total of 14 studies that met the inclusion criteria with relevant clinical data and prognostic information were included in the meta-analysis.
Results
The combined results revealed that high expression of FAM83H-AS1 was associated with poor overall survival (OS) (HR = 1.63, 95% CI 1.24–2.14, P = 0.0004) in a variety of cancers. Additionally, upregulated FAM83H-AS1 expression was significantly correlated with tumor TNM stage (III/IV vs. I/II, OR = 2.40, 95% CI 1.36–4.23, P = 0.003) and lymph node metastasis (positive vs. negative, OR = 1.70, 95% CI 1.14–2.52, P = 0.008) in patients with cancer.
Conclusions
Our results of this meta-analysis indicated that elevated FAM83H-AS1 expression could predict poor prognosis in patients with cancer and suggested that FAM83H-AS1 might serve as a novel biomarker for cancer.
Similar content being viewed by others
Introduction
Long non-coding RNA (lncRNA) is a type of non-coding regulatory RNA that is more than 200 nucleotides in length without protein-coding ability [1]. LncRNAs have been a focus of intense investigation in life science research in the past decade. Increasing studies have shown that lncRNAs play an important role in various activities such as epigenetic regulation, cell cycle regulation, cell differentiation regulation and dosage compensation [2]. Dysregulated lncRNA expression is closely related to multiple human diseases, including a variety of cancers, suggesting a potential function of these lncRNAs as biomarkers for diagnosis, prognosis, cancer stage, and response to therapy [3,4,5].
Several studies have indicated that abnormal expression of the lncRNA family with sequence similarity 83 member H antisense RNA 1(FAM83H-AS1) is associated with poor prognosis in patients with cancer. Ma et al. revealed that increased FAM83H-AS1 expression was associated with shorter patient overall survival (OS) in patients with hepatocellular carcinoma [6]. In gastric cancer, upregulated FAM83H-AS1 was a risk factor related to OS and disease-free survival, and FAM83H-AS1 might function as an oncogene [7]. Another study showed that patients with ovarian cancer were more easily accompanied by high expression of FAM83H-AS1, which was correlated with tumor pathological grade, tumor lymph node metastasis (TNM) stage and distant metastasis [8]. Overexpressed FAM83H-AS1 exhibited oncogenic functions in glioma by suppressing expression of CDKN1A, which encoded a key factor that regulated the G1 phase of the cell cycle, leading to increased cell proliferation [9]. Furthermore, high expression of FAM83H-AS1 was related to advanced clinical stage in bladder cancer and FAM83H-AS1 was more likely to lead to invasion of muscle layer, suggesting FAM83H-AS1 could be an independent poor prognostic factor for OS in bladder cancer patients [10]. However, no systematic meta-analysis has so far clarified the prognostic value of FAM83H-AS1 in these cancers.
Therefore, we performed a meta-analysis to explore the clinical prognostic value of the lncRNA FAM83H-AS1 in patients with cancers.
Methods
Literature search
A comprehensive and systematic literature search was performed up until February 5, 2020 through multifarious retrieval strategies. Two authors (Qin Yang and Jie Wang) were responsible for completing the search in electronic databases including PubMed, Cochrane Library, EMBASE, Medline, Web of Science, CNKI, Weipu, and Wanfang. The following keywords were used for searching, including: “(((((family with sequence similarity 83 member H-antisense RNA 1) OR family with sequence similarity 83 member H-AS1)) OR ((((FAM83H-AS1) OR long non-coding RNA FAM83H-AS1) OR FAM83H antisense RNA 1) OR onco-lncRNA-3))) AND ((((((((cancer) OR tumor) OR carcinoma) OR neoplasm)) AND ((((prognosis) OR survival) OR diagnosis) OR clinicopathological)))).”
Inclusion and exclusion criteria
The criteria for inclusion of literature in the study were as follows: (1) studies with a definite diagnosis or histopathological diagnosis of cancer patients; (2) studies examining prognostic characteristics of lncRNA FAM83H-AS1 in patients with malignant tumor; and (3) studies with sufficient information to calculate the combined hazard risk (HR) and 95% confidence interval (CI).
The criteria for exclusion were as follows: (1) studies without prognostic outcomes; (2) duplicate publications; and (3) non-human studies, letters, case reports, letters, review articles and other studies without survival data.
Data extraction and quality assessment
Three authors (Hao Hua, Pingyong Zhong, Tinggang Mou) independently completed data extraction and reached a consensus. After a set of literature was established according to the above criteria, the following information was successively extracted: author, country, year of publication, tumor type, tumor size, follow-up time, detection method and cut-off value. The number of patients in each group was divided according to the presence or absence of lymph node metastasis, distant metastasis, tumor size, TNM stage, and the number of patients with high or low FAM83H-AS1 expression in each group.
If only Kaplan–Meier curves were available, we extracted the data from the graph survival chart using Engauge Digitizer V4.1 to estimate the survival time and HR. If a study reported data from multivariate or univariate analyses of OS, the former one was applied directly. The Newcastle–Ottawa Quality Assessment Scale (NOS) was performed to evaluate the quality of each eligible study with a score ranging from 0 to 9 points [11].
Statistical analysis
Review Manager (RevMan) 5.3 software was used to combine HR or odds ratio for this meta-analysis. Stata 14 software was used to estimate the publication bias of this meta-analysis. The heterogeneity of results was estimated by Q test and I2 statistics. The fixed-effects model was selected for data analysis when I2< 50%. The random-effects model was used for data analysis when the heterogeneity was obvious (I2> 50%) [12]. If the result indicated HR > 1, lncRNA overexpression was significantly correlated with survival difference. HR < 1 indicated that lncRNA overexpression predicted long survival.
Results
Characteristics and basic information in eligible literature
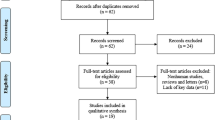
By searching the electronic databases from PubMed, Cochrane Library, EMBASE, Medline, Web of Science, CNKI, Weipu, and Wanfang, we preliminarily retrieved 62 relevant studies. We carefully reviewed the title, abstract, and full content and evaluated whether the study contained available clinical data. A total of 14 studies published between 2016 and 2020 were eligible to be included in this meta-analysis (Fig. 1). The 14 studies contained 2818 patients from three countries including China, United States and Iran. We mainly analyzed nine different types of tumors, including pancreatic ductal adenocarcinoma [13], gastric cancer [7, 14, 15], ovarian cancer [8, 16], glioma [9], colorectal carcinoma [17, 18], bladder cancer [10], hepatocellular carcinoma [6], breast cancer [19], and lung cancer [20]. The expression of FAM83H-AS1 was detected by quantitative real-time fluorescent PCR in all studies. To distinguish the expression levels of FAM83H-AS1, the cut-off value was used. All studies selected median values and used OS to estimate patient survival. Ten studies contained detailed clinical prognosis information to analyze clinical outcome. Detailed information was shown in Table 1.
FAM83H-AS1 expression significantly correlated with OS
13 of 14 studies including 2650 patients assessed the HR and 95% CI of OS. Statistical results of all studies are shown in a forest plot in Fig. 2. The random effects model was selected to estimate the pooled HR because no significant heterogeneous among the studies was found (I2= 68%, P < 0.001). The merged results indicated that high expression of FAM83H-AS1 was significantly associated with poor prognosis (pooled HR = 1.63, 95% CI 1.24–2.14, P = 0.0004). Patients with high expression of FAM83H-AS1 had a worse OS than those with low expression of FAM83H-AS1.
To analyze the pooled HR among different types of cancer, two subgroups (digestive system tumors and non-digestive system tumors) were established. High FAM83H-AS1 expression was related to poor OS in one of the subgroups (digestive system tumors: pooled HR = 1.65 95% CI 1.30–2.10, I2= 44%, P < 0.0001; non-digestive system tumors: pooled HR = 1.47 95% CI 0.98–2.20, I2= 77%, P = 0.06) (Fig. 2). These results indicate that high expression of FAM83H-AS1 might be a significant prognostic factor of OS and more suitable for application in patients with digestive tumors compared with those with non-digestive tumors.
Association between FAM83H-AS1 and clinicopathologic characteristics
Only ten of the eligible studies that contained detailed clinicopathologic characteristics evaluated the correlation between FAM83H-AS1 expression and TNM stage. The results indicated that elevated expression of FAM83H-AS1 was associated with advanced TNM stage (III/IV vs. I/II, OR = 2.40, 95% CI 1.36–4.23, P = 0.003) (Fig. 3a). Moreover, six studies examined characteristics of lymph node metastasis. The results demonstrated that elevated FAM83H-AS1 expression was significantly associated with lymph node metastasis (positive vs. negative, OR = 1.70, 95% CI 1.14–2.52, P = 0.008) (Fig. 3b). The relationships among elevated expression of FAM83H-AS1 and age, gender, tumor size, differentiation and distant metastasis were also investigated. However, no significant correlation was found between FAM83H-AS1 expression and these characteristics (Fig. 4a–e). Detailed information was shown in Table 2.
Publication bias and sensitivity analysis
Begg’s test was performed to evaluate the publication bias of the meta-analysis. The results indicated that there was no significant publication bias in this meta-analysis for OS (P = 0.093) (Fig. 5a). Additionally, sensitivity analysis revealed no significant change in the pooled HR by eliminating any single study, indicating that the results were stable (Fig. 5b).
Discussion
LncRNAs, which are encoded by an unstudied region of the human genome, may contain cancer-missing drivers and have attracted attention in recent years as potentially important regulators of carcinogenesis and cancer development. Increasing evidence has indicated that lncRNAs play an important role in various human diseases, especially in malignant tumors [21,22,23,24]. Antisense long non-coding RNAs, such as TTN-AS1, IDH1-AS1 and AFAP-AS1, have been reported in tumors and closely related to the prognosis, development, invasion and metastasis of tumors [25,26,27].
Accumulated evidences exerted that FAM83H-AS1 acted critical roles in various biological and pathological processes. For example, FAM83H-AS1 could ameliorate SpA-mediated inhibition on the osteogenic differentiation of human bone mesenchymal stem cells during osteomyelitis and promote nucleus pulposus cell growth in intervertebral disc degeneration [28, 29]. However, many studies recently showed that FAM83H-AS1 was overexpressed in some tumors and exhibited an oncogenic role in cell proliferation and metastasis [6, 10, 16]. Dou’s study showed that elevated FAM83H-AS1 expression was correlated with radioresistance and poor OS and was used to effectively predict lymph node metastasis in ovarian cancer [16]. Previous studies confirmed that the expression of HuR was positively correlated with the expression of FAM83H-AS1. Furthermore, HuR promoted cell proliferation and metastasis by interacting with lncRNAs in multiple cancers [30, 31]. The underline mechanism was that FAM83H-AS1 could interact with HuR by increasing the stability of the HuR mRNA. Yang’s study revealed that FAM83H-AS1 acted as a prognostic lncRNA in colon cancer and its expression level was significantly elevated in colon cancer tissues. Additionally, FAM83H-AS1 negatively regulated SMAD1/5/9 [32]. One study suggested that FAM83H-AS1 might inhibit TGF-β signaling pathway by downregulating SMAD1/5/9, preventing the anti-tumor effect of TGF-β signaling [32]. Furthermore, numerous studies showed that FAM83H-AS1 played an important role in gastric cancer progression [7]. Overexpressed FAM83H-AS1 was an independent prognostic predictor of OS in gastric cancer; FAM83H-AS1 expression was also significantly related to lymph node metastasis and showed an important value in the differentiation between cancerous and non-cancerous tissues. Increased FAM83H-AS1 expression has recently been reported to predict poor prognosis and promote malignant phenotypes of bladder cancer [10] and pancreatic ductal adenocarcinoma [13]. A research group found that FAM83H-AS1 was a crucial lncRNA expressed at preliminary stage of breast cancer by RNA sequencing in early-stage tumors, suggesting detection of FAM83H-AS1 expression levels in plasma could be a potential diagnostic and prognostic biomarker for early-stage of breast cancer [33]. However, in contrast to other reports, Baratieh’s study showed that upregulated expression of FAM83H-AS1 in kidney renal papillary cell carcinoma led to longer survival rates [14]. Chemotherapy is one of the most effective methods to treat tumors and is the main treatment for some tumors that have the tendency of metastasis and those that have already metastasized. Whereas, some patients tend to develop resistance to chemotherapy drugs. Interestingly, the latest research showed that silence of FAM83H-AS1 sensitized gastric cancer cells to cisplatin and 5-fluorouracil, which were the first-line treatment scheme for gastric cancer [15]. This suggested that designing drugs to reduce the high expression of FAM83H-AS1 might eliminate chemotherapy resistance in some patients with gastric cancer.
The current study presented the first meta-analysis to comprehensively evaluate the relationship between FAM83H-AS1 expression and prognosis and clinicopathological characteristics of tumors. A total of 14 eligible studies containing 2818 patients were enrolled in this meta-analysis. The pooled results revealed that increased FAM83H-AS1 expression was significantly associated with poor prognosis. Furthermore, the high expression of FAM83H-AS1 might be an important prognostic factor for OS in patients with digestive tumors. The subsequent pooled results also demonstrated that high expression of FAM83H-AS1 was associated with lymph node metastasis and high TNM grade of tumors.
Whereas, this study had some limitations. First, few studies on FAM83H-AS1 were available and thus the number of eligible studies that could be included was limited. Furthermore, most of the studies were from China, which might lead to deviation and might represent the clinical characteristics of Chinese patients with tumors. In addition, because of the relatively small sample size, we were unable to aggregate results based on a single type of tumor. Finally, some studies did not directly give the results and we used an indirect method to obtain HRs and 95% CI. Hence, the clinical significance of high FAM83H-AS1 expression might be overestimated. However, the statistical results could be improved with an increased number of follow-up studies.
In summary, FAM83H-AS1 plays a crucial function as an effective predictive biomarker for tumor prognosis. More relevant studies and in-depth data analysis are needed to further confirm the overall diagnostic value of FAM83H-AS1 in cancers.
Conclusions
Our study first systematically reviewed and estimated the relationship between abnormal FAM83H-AS1 expression and survival and clinical outcomes in patients with tumors. The present results suggested that high expression level of FAM83H-AS1 was associated with poor OS and lncRNA FAM83H-AS1 might be used as a prognostic marker for patients with cancer. Considering the limitations of this study, it is necessary to conduct more large-scale and high-quality studies on various ethnic populations to obtain more value of FAM83H-AS1 in tumors.
Availability of data and materials
All data are included in this article.
Abbreviations
- LncRNA:
-
Long non-coding RNA
- FAM83H-AS1:
-
Family with sequence similarity 83 member H antisense RNA 1
- NA:
-
Not available
- DFS:
-
Disease-free survival
- OS:
-
Overall survival
- PDAC:
-
Pancreatic ductal adenocarcinoma
- GC:
-
Gastric cancer
- KIRP:
-
Kidney renal papillary cell carcinoma
- OC:
-
Ovarian cancer
- CRC:
-
Colorectal carcinoma
- BLCA:
-
Bladder cancer
- HCC:
-
Hepatocellular carcinoma
- BRCA:
-
Breast cancer
- LC:
-
Lung cancer
- LNM:
-
Lymph node metastasis
- DM:
-
Distant metastasis
References
Dey BK, Mueller AC, Dutta A. Long non-coding RNAs as emerging regulators of differentiation, development, and disease. Transcription. 2014;5:e944014.
Marchese FP, Raimondi I, Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017;18:206.
Chen L, Dzakah EE, Shan G. Targetable long non-coding RNAs in cancer treatments. Cancer Lett. 2018;418:119–24.
Vitiello M, Tuccoli A, Poliseno L. Long non-coding RNAs in cancer: implications for personalized therapy. Cell Oncol. 2015;38:17–28.
Arun G, Diermeier SD, Spector DL. Therapeutic targeting of long non-coding RNAs in cancer. Trends Mol Med. 2018;24:257–77.
Ma YK, Shen TH, Yang XY. Upregulation of LncRNA FAM83H-AS1 in hepatocellular carcinoma promotes cell proliferation, migration and invasion by Wnt/beta-catenin pathway. Eur Rev Med Pharmacol Sci. 2019;23:7855–62.
Da J, Liu P, Wang R, Bu L. Upregulation of the long non-coding RNA FAM83H-AS1 in gastric cancer and its clinical significance. Pathol Res Pract. 2019;215:152616.
Gong YB, Zou YF. Clinical significance of lncRNA FAM83H-AS1 in ovarian cancer. Eur Rev Med Pharmacol Sci. 2019;23:4656–62.
Bi YY, Shen G, Quan Y, Jiang W, Xu F. Long noncoding RNA FAM83H-AS1 exerts an oncogenic role in glioma through epigenetically silencing CDKN1A (p21). J Cell Physiol. 2018;233:8896–907.
Shan H, Yang Y, Zhu X, Han X, Zhang P, Zhang X. FAM83H-AS1 is associated with clinical progression and modulates cell proliferation, migration, and invasion in bladder cancer. J Cell Biochem. 2019;120:4687–93.
Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45.
Zhong Y, Wu X, Li Q, Ge X, Wang F, Wu P, et al. Long noncoding RNAs as potential biomarkers and therapeutic targets in gallbladder cancer: a systematic review and meta-analysis. Cancer Cell Int. 2019;19:169.
Arnes L, Liu Z, Wang J, Maurer C, Sagalovskiy I, Sanchez-Martin M, et al. Comprehensive characterisation of compartment-specific long non-coding RNAs associated with pancreatic ductal adenocarcinoma. Gut. 2019;68:499–511.
Baratieh Z, Khalaj Z, Honardoost MA, Emadi-Baygi M, Khanahmad H, Salehi M, et al. Aberrant expression of PlncRNA-1 and TUG1: potential biomarkers for gastric cancer diagnosis and clinically monitoring cancer progression. Biomark Med. 2017;11:1077–90.
Wang B, Guan G, Zhao D. Silence of FAM83H-AS1 promotes chemosensitivity of gastric cancer through Wnt/beta-catenin signaling pathway. Biomed Pharmacother. 2020;125:109961.
Dou Q, Xu Y, Zhu Y, Hu Y, Yan Y, Yan H. LncRNA FAM83H-AS1 contributes to the radioresistance, proliferation, and metastasis in ovarian cancer through stabilizing HuR protein. Eur J Pharmacol. 2019;852:134–41.
Yang L, Xu L, Wang Q, Wang M, An G. Dysregulation of long non-coding RNA profiles in human colorectal cancer and its association with overall survival. Oncol Lett. 2016;12:4068–74.
Lu S, Dong W, Zhao P, Liu Z. lncRNA FAM83H-AS1 is associated with the prognosis of colorectal carcinoma and promotes cell proliferation by targeting the Notch signaling pathway. Oncol Lett. 2018;15:1861–8.
Yang F, Lv SX, Lv L, Liu YH, Dong SY, Yao ZH, et al. Identification of lncRNA FAM83H-AS1 as a novel prognostic marker in luminal subtype breast cancer. Onco Targets Ther. 2016;9:7039–45.
Zhang J, Feng S, Su W, Bai S, Xiao L, Wang L, et al. Overexpression of FAM83H-AS1 indicates poor patient survival and knockdown impairs cell proliferation and invasion via MET/EGFR signaling in lung cancer. Sci Rep. 2017;7:42819.
Ma Y, Xu XL, Huang HG, Li YF, Li ZG. LncRNA TDRG1 promotes the aggressiveness of gastric carcinoma through regulating miR-873-5p/HDGF axis. Biomed Pharmacother. 2019;121:109425.
Yi T, Wang T, Shi Y, Peng X, Tang S, Zhong L, et al. Long noncoding RNA 91H over-expression contributes to the growth and metastasis of HCC by epigenetically positively regulating IGF2 expression. Liver Int. 2020;40:456–67.
Wu W, Zhao Y, Gao E, et al. LncRNA DLEU2 accelerates the tumorigenesis and invasion of non-small cell lung cancer by sponging miR-30a-5p. J Cell Mol Med. 2020;24:441–50.
Jiang L, Zhao XH, Mao YL, Wang JF, Zheng HJ, You QS. Long non-coding RNA RP11-468E25 curtails colorectal cancer cell proliferation and stimulates apoptosis via the JAK/STAT signaling pathway by targeting STAT5 and STAT6. J Exp Clin Cancer Res. 2019;38:465.
Fu D, Lu C, Qu X, Li P, Chen K, Shan L, et al. LncRNA TTN-AS1 regulates osteosarcoma cell apoptosis and drug resistance via the miR-134-5p/MBTD1 axis. Aging. 2019;11:8374–85.
Shi D, Wu F, Mu S, Hu B, Zhong B, Gao F, et al. LncRNA AFAP1-AS1 promotes tumorigenesis and epithelial-mesenchymal transition of osteosarcoma through RhoC/ROCK1/p38MAPK/Twist1 signaling pathway. J Exp Clin Cancer Res. 2019;38:375.
Zhang N, Li Z, Bai F, Zhang S. PAX5-induced upregulation of IDH1-AS1 promotes tumor growth in prostate cancer by regulating ATG5-mediated autophagy. Cell Death Dis. 2019;10:734.
Wu H, Cao F, Zhou W, Wang G, Liu G, Xia T, et al. Long non-coding RNA FAM83H-AS1 modulates the SpA-inhibited osteogenicdifferentiation in human bone mesenchymal stem cells. Mol Cell Biol. 2020;40:e00362-19.
Wei R, Chen Y, Zhao Z, Gu Q, Wu J. LncRNA FAM83H-AS1 induces nucleus pulposus cell growth via targeting the Notch signaling pathway. J Cell Physiol. 2019;234:22163–71.
Shu C, Yan D, Mo Y, Gu J, Shah N, He J. Long noncoding RNA lncARSR promotes epithelial ovarian cancer cell proliferation and invasion by association with HuR and miR-200 family. Am J Cancer Res. 2018;8:981–92.
He X, Zheng Y, Zhang Y, Gan Y, Zhou Y, Liang H, et al. Long non-coding RNA AK058003, as a precursor of miR-15a, interacts with HuR to inhibit the expression of gamma-synuclein in hepatocellular carcinoma cells. Oncotarget. 2017;8:9451–65.
Yang L, Cui J, Wang Y, Tan J. FAM83H-AS1 is upregulated and predicts poor prognosis in colon cancer. Biomed Pharmacother. 2019;118:109342.
Deva MRA, Patel K, Korivi JS, Meenakumari B, Sundersingh S, Sridevi V, et al. Identification of lncRNAs associated with early-stage breast cancer and their prognostic implications. Mol Oncol. 2019;13:1342–55.
Acknowledgements
We would like to thank Dr. Wang for his guidance on this article and for his editing and proofreading of this English manuscript.
Funding
This meta-analysis was supported by the Key Discipline Construction Fund of The First People’s Hospital of Neijiang.
Author information
Authors and Affiliations
Contributions
Conceptualization: QY, JW. Data curation: QY, HH. Formal analysis: QY, JW, TM, HH, PL. Funding acquisition: XF. Investigation: QY, JW, TM, HH, PL. Project administration: XF. Software: QY, JW. Supervision: XF. Writing—original draft: QY. Writing—review and editing: QY. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Human Research Ethics Committees of the First People’s Hospital of Neijiang, Neijiang, Sichuan.
Consent for publication
All authors agree to publish.
Competing interests
All authors have no conflict of interest in this meta-analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, Q., Wang, J., Zhong, P. et al. The clinical prognostic value of lncRNA FAM83H-AS1 in cancer patients: a meta-analysis. Cancer Cell Int 20, 72 (2020). https://doi.org/10.1186/s12935-020-1148-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-020-1148-8