Abstract
Purpose
Despite new developments in cancer therapy, chemotherapy and radiotherapy remain the cornerstone of breast cancer treatment. Therefore, finding ways to reduce the toxicity and increase sensitivity is particularly important. Tumor necrosis factor alpha (TNF-α) exerts multiple functions in cell proliferation, differentiation and apoptosis. In the present study, we investigated whether TNF-α could enhance the effect of chemotherapy and radiotherapy against breast cancer cells.
Methods
Cell growth was determined by MTT assay in vitro, and by using nude mouse tumor xenograft model in vivo. Cell cycle and apoptosis/necrosis were evaluated by flow cytometry. DNA damage was visualized by phospho-Histone H2A.X staining. mRNA expression was assessed by using real-time PCR. Protein expression was tested by Western blot assay.
Results
TNF-α strengthened the cytotoxicity of docetaxel, 5-FU and cisplatin against breast cancer cells both in vitro and in vivo. TNF-α activated NF-κB pathway and dependently up-regulated expressions of CyclinD1, CyclinD2, CyclinE, CDK2, CDK4 and CDK6, the key regulators participating in G1→S phase transition. As a result, TNF-α drove cells out of quiescent G0/G1 phase, entering vulnerable proliferating phases. Treatment of TNF-α brought more DNA damage after Cs137-irradiation and strengthened G2/M and S phase cell cycle arrest induced by docetaxel and cisplatin respectively. Moreover, the up-regulation of RIP3 (a necroptosis marker) by 5-FU, and the activation of RIP3 by TNF-α, synergistically triggered necroptosis (programmed necrosis). Knockdown of RIP3 attenuated the synergetic effect of TNF-α and 5-FU.
Conclusion
TNF-α presented radiotherapy- and chemotherapy-sensitizing effects against breast cancer cells.
Similar content being viewed by others
Background
Breast cancer is by far the most frequent cancer among women worldwide and accounts for 30% of all new cancer cases in females with a greater incidence in women over the age of 60 years [1,2,3,4,5]. Although a variety of treatments including surgical resection, adjuvant chemotherapy, hormonal therapy, and radiotherapy have been shown to improve survival and reduce the risk of tumor reoccurrence, breast cancer remains as one of the leading causes of death in women [1,2,3,4,5]. Chemotherapy, as a conventional method of cancer treatment, is generally performed by using cytotoxic agents. Radiotherapy plays a vital role in local treatment of lymph node metastasis in breast cancer. However, chemotherapy and radiotherapy have effects on both normal and tumor cells, which means patient have to suffer side effects that come along, and the toxicity is always dose dependent [1, 5]. Therefore, finding ways to reduce the dose of chemotherapy and radiotherapy, without affecting their therapeutic efficiency, is particularly important to tumor therapy.
Tumor necrosis factor alpha (TNF-α) is a classical member of a ligand family of cytokines that include TNF, lymphotoxin-α (LTα), Fas ligand (FasL), CD40 ligand (CD40L), and TNF-related apoptosis-inducing ligand (TRAIL) [6]. TNF-α is a pleiotropic pro-inflammatory cytokine inducing a broad range of cellular responses, ranging from inflammatory cytokine production, cell survival, cell proliferation, cell differentiation and cell death. TNF-α can trigger different forms of PCD (programmed cell death) that are morphologically distinguished as apoptosis and necroptosis [7]. Lots of studies have confirmed that TNF-α can recruit a variety of signaling molecules to induce apoptosis and necroptosis to exert its cytotoxicity through binding to TNF-α receptors, including TNF receptor-associated death domain (TRADD), TNF receptor-associated factor 2 (TRAF2), and receptor-interacting protein kinase 1 and 3 (RIP1 and RIP 3) [8]. Therefore, TNF-α has been used as an antitumor agent previously because of its broad spectrums of cytotoxic effects against a variety of cancer cells, including colorectal cancer, melanoma and sarcoma [9,10,11]. However, the clinical application of TNF-α has been limited, largely due to its induction of pro-inflammatory and anti-apoptotic gene transcription mainly via activating the NF-κB signal pathway, which leads to systemic toxicity. Recently, studies have shown that combination with low-dose TNF-α could enhance therapeutic effects of chemotherapeutic drugs [12, 13].
In our present study, we investigate whether TNF-α could enhance the cytotoxicity of chemotherapeutics and radiotherapy against breast cancer cells. The mechanisms involved in the synergistic anticancer effect were also investigated.
Methods
Cell line and cultures
Human breast cancer cell lines MDA-MB-231 and MCF-7 were purchased from American Type Culture Collection (ATCC, VA, USA). Cells were maintained in RPMI-1640 medium (Gibco, NY, USA), supplemented with 10% fetal calf serum (Gibco), 100 units/ml penicillin, and 100 mg/ml streptomycin. The cultures were incubated at 37 °C in a humidified atmosphere with 5% CO2. Cells were passaged every 2–3 days to obtain exponential growth.
Reagents
TNF-α was purchased from Sigma (St Louis, Missouri, USA). Docetaxel was purchased from Jiang Su Heng Rui Medicine CO., LTD. (Liangyungang, Jiangsu, China). 5-Flurouracil (5-FU) was purchased from Shanghai Xudong Haipu Pharmaceutical CO., LTD. (Shanghai, China). Cisplatin was purchased from Nanjing Pharmaceutical Factory CO., LTD. (Nanjing, Jiangsu, China). Bay11-7082 was purchased from Sigma (St. Louis, MO, USA).
MTT assay
Cellular growth was evaluated by MTT (methyl thiazolyl tetrazolium) assay [14]. Cells were seeded into 24-well tissue culture plates at 5 × 104 cells per well. After treatment, MTT (Sigma) was added to each well to a final concentration of 0.5 mg/ml, followed by incubation at 37 °C for 4 h. The medium was then removed, and 800 μl of dimethyl sulfoxide (DMSO) was added per well. The absorbance in each well was measured at 490 nm using a microplate ELISA reader (Bio-Rad Laboratories, CA, USA). The relative cell viability was calculated as follows: relative cell viability = (mean experimental absorbance/mean control absorbance) ×100%. The concentration that caused 50% growth inhibition (IC50) was calculated by the modified Kärbers method [14] according to the formula: IC50 = lg−1 [Xk − i (∑p − 0.5)], in which Xk represents the logarithm of the highest drug concentration; i is that of ratio of adjacent concentration; and ΣP is the sum of the percentage of growth inhibition at various concentrations.
Nude mouse tumor xenograft model and treatment
Four-week-old female BALB/c athymic nude mice were purchased from Shanghai SLAC Laboratory Animal Co. Ltd (Shanghai, China) and received humane care according to the Soochow University Institutional Animal Care and Treatment Committee. MDA-MB-231 cells were injected into the left flanks of the mice in a total volume of 100 μl (0.5 × 107 cells). TNF-α (20 ng each injection, intratumorally) [15], cisplatin (2 mg/kg per body weight, intraperitoneally) [16], and docetaxel (2 mg/kg per body weight, intravenously) [17] were administered every three days. 5-FU was administered intraperitoneally (10 mg/kg per body weight) everyday. Treatments were conducted for 20 days.
Cell cycle analysis
Cell cycle analysis using propidium iodide (PI, Sigma) was performed as previously described [14]. Prior to treatment, cells were synchronized in the cell cycle by serum starvation for 24 h. After treatment, the cells were fixed in 80% ice cold ethanol, and incubated with 0.5% Triton X-100 solution containing 1 mg/ml RNase A at 37 °C for 30 min. PI was added to a final concentration of 50 μg/ml followed by 30 min incubation in the dark. Cellular DNA content was analyzed by a fluorescence-activated cell sorter (FACS; Becton–Dickinson, NJ, USA). Data were processed by ModFit LT software (Verity Software House, ME, USA).
Real-time PCR
Total RNA was extracted using Trizol reagent (Invitrogen, CA, USA) according to the manufacturer’s protocol. After spectrophotometric quantification, 1 μg total RNA in a final volume of 20 μl was used for reverse transcription with PrimeScript RT Reagent Kit (TaKaRa, Otsu, Shiga, Japan) according to the manufacturer’s protocol. Aliquots of cDNA corresponding to equal amounts of RNA were used for quantification of mRNA by real-time PCR using the LightCycler 96 Real-time Quantitative PCR Detection System (Roche, Indianapolis, IN, USA). The reaction system (25 μl) contained the corresponding cDNA, forward and reverse primers, and SYBR Green PCR master mix (Roche). All data were analyzed using β-actin gene expression as an internal standard. The specific primers were as follows: (1) Cyclin D1, forward, 5′-GCATCTACACCGACAACTCCAT-3′, reverse, 5′-GTTTGTTCTCCTCCGCCTCT-3′, product, 153 bp; (2) Cyclin D2, forward, 5′-CTGAATACTCCTCCCCTCTTCTCTT-3′, reverse, 5′-TCAGCAGGAAGGGGTGTCTCT-3′, product, 142 bp; (3) Cyclin D3, forward, 5′-CAGCCAGACCAGCACTCCTAC-3′, reverse, 5′-GAAGGGGGAACAGACGCC-3′, product, 162 bp; (4) Cyclin E, forward, 5′-AAGGTTTCAGGGTATCAGTGGTG-3′, reverse, 5′-TGCTCGGGCTTTGTCCAG-3′, product, 183 bp; (5) CDK2, forward, 5′-AATAGGCTGGGAGACTGAAGACT-3′, reverse, 5′-ATCCTTGGCAACTGAACAACTAA-3′, product, 228 bp; (6) CDK4, forward, 5′-TGGAGTGTTGGCTGTATCTTTGCT-3′, reverse, 5′-GCAGCCCAATCAGGTCAAAG-3′, product, 106 bp; (7) CDK6, forward, 5′-CCCTGTCTCACCCATACTTCC-3′, reverse, 5′-GCCTCCAGATAGCAATCCTCC-3′, product, 204 bp; (8) β-actin, forward, 5′-TCATGAAGTGTGACGTGGACAT-3′, reverse, 5′-CTCAGGAGGAGCAATGATCTTG-3′, product, 158 bp.
Immunofluorescence
Cells were 4% formaldehyde fixed (10 min) at 0.5 h post 40 Gy Cs137-irradiation and then incubated with 1% BSA and 0.3% triton for 1 h to permeabilise the cells and block non-specific protein–protein interactions. The cells were then incubated with the Phospho-Histone H2A.X (Ser139) (20E3) Rabbit antibody (Cell Signaling Technology, Beverly, MA, USA) (1:400) overnight at 4 °C. The secendary antibody was Alexa Fluor® 488 Conjugated anti-rabbit (Cell Signaling Technology, Beverly, MA, USA) IgG (H+L) used at a 1:2000 dilution for 1 h. DAPI was used to stain the cell nuclei (blue). Fluorescence signals were taken with Leica fluorescence microscope DM4000 (Leica Wetzlar GmbH, Germany).
Luciferase reporter gene assay
The reporter plasmid, pNF-κB-luc, containing the κB-enhancer consensus sequences [(TGGGGACTTTCCGC) ×5] and NF-κB-dependent firefly luciferase gene [18] was purchased from Stratagene (La Jolla, CA, USA). The internal control plasmid, pRL-SV40, which contains the renilla luciferase gene, was obtained from Promega (Madison, WI, USA). Cells were transiently cotransfected with the reporter plasmid (500 ng/well) and the pRL-SV40 plasmid (100 ng/well) for 8 h using Lipofectamine 3000 according to the protocol of the manufacturer. The medium was then renewed and treatments were started. After treatment, the cell lysates were subjected to the dual luciferase reporter assay (Promega) according to the recommendations of the manufacturer and luciferase activities were measured with the GloMax-20/20 luminometer (Promega). The results were expressed as relative luciferase activity, which is the ratio of firefly luciferase activity to renilla luciferase activity.
Apoptosis assays
Apoptosis was evaluated using the Annexin V-FITC/PI Apoptosis Detection kit (Biouniquer Technology, Nanjing, China) according to the manufacturer’s instructions [14]. The cells were resuspended in binding buffer, and Annexin V-FITC and propidium iodide (PI) were added to the buffer and incubated at room temperature for 15 min in the dark, followed by flow cytometry using a Beckman Coulter FC500 dual-laser five-color flow cytometer (Beckman Coulter, Fullerton, CA, USA).
Western blot analysis
Total protein was extracted using a lysis buffer containing 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), 1 mM EDTA, protease inhibitors (10 mg/ml leupeptin, 10 mg/ml aprotinin, 10 mg/ml pepstatin A, 1 mM 4-[2-aminoethyl] benzenesulfonyl fluoride), and phosphatase inhibitors (1 mM NaF, 1 mM Na3VO4). The protein extracts were separated by 10% SDS–polyacrylamide gel electrophoresis (PAGE) and were transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, St Charles, MO, USA). After 1-h blocking in 5% non-fat milk, the membranes were incubated overnight with primary antibodies, rabbit anti-RIP3 (Cell Signaling Technology) or mouse anti-β-actin (Santa Cruz Biotechnologies, CA, USA), at 4 °C. Protein expression was determined using horseradish peroxidase—conjugated antibodies followed by enhanced chemiluminescence) (ECL) detection (Millipore). Protein band analysis was performed using Quantity One 4.6.2 software (Bio-Rad Laboratories, CA, USA). β-actin was used as internal control.
Transfection of small interfering RNA
Target specific small interfering RNAs (siRNAs) were synthesized by Genepharma (Shanghai, China). The specific sequences were as follows: (1) Control-siRNA, 5′-UUCUCCGAACGUGUCACGUdTdT-3′; (2) RIP3-siRNA-1, 5′-UAACUUGACGCACGACAUCAGGCUGUU-3′; (3) RIP3-siRNA-2, 5′-GCAGUUGUAUAUGUUAACGAGCGGUCG-3′ [19]. The transfections were performed with siRNA-Mate Transfection Reagent (Genepharma) according to the manufacturer’s protocol.
Statistical analysis
Each experiment was performed a minimum of three times. Results were expressed as the mean value ± standard deviation (SD). Statistical analysis was performed by unpaired Student’s t test. A P value <0.05 was considered significant.
Results
The chemotherapy sensitization effect of TNF-α against breast cancer cells and xenografts
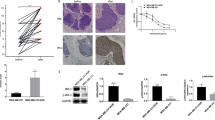
The growth inhibition effects of chemotherapeutics, docetaxel, 5-FU and cisplatin, against breast cancer cells were firstly evaluated by using MTT assays. As shown in Fig. 1a–c, docetaxel, 5-FU and cisplatin repressed growth of MDA-MB-231 and MCF-7 cells through dose- and time-dependent manner. The IC50 values, 48 and 72 h post treatment with each chemotherapeutic, were presented in Table 1.
TNF-α sensitized cytotoxicity of docetaxel (DOC), 5-flurouracil (5-FU) and cisplatin (DDP) against MDA-MB-231 and MCF-7 breast cancer cells. a–c Dose- and time-dependent cytotoxicity of docetaxel (DOC) (a), 5-flurouracil (5-FU) (b) and cisplatin (DDP) (c). **P < 0.01 indicated significant differences from the respective control groups. d–f Enhanced cytotoxicity of docetaxel (DOC) (d), 5-flurouracil (5-FU) (e) and cisplatin (DDP) (f) with combined treatment of TNF-α (5 ng/ml). *P < 0.05 and **P < 0.01 indicated significant differences from the respective control groups; # P < 0.05 and ## P < 0.01 indicated significant differences from the TNF-α (5 ng/ml) treated groups; && P < 0.01 indicated significant differences between folds induction. g, h TNF-α (20 ng each injection, intratumorally, every 3 days) strengthened growth inhibition effect of docetaxel (DOC, 2 mg/kg, intravenously, every 3 days), 5-flurouracil (5-FU, 10 mg/kg, intraperitoneally, everyday) and cisplatin (DDP, 2 mg/kg, intraperitoneally, every 3 days) against breast cancer xenografts in vivo. g Image of stripped xenografts. h Tumor weight of each group. **P < 0.01 indicated significant differences from control group; ## P < 0.01 indicated significant differences from TNF-α treated group; && P < 0.01 indicated significant differences between folds induction
According to IC50 values of chemotherapeutics, chemotherapy sensitization effect of TNF-α was evaluated in vitro. Co-treatment with TNF-α strengthened the growth inhibition induced by docetaxel, 5-FU and cisplatin remarkably (Fig. 1d–f). The chemotherapy sensitization effect was then assessed in vivo. As shown in Fig. 1g, h, TNF-α strengthened the suppression on the formation of xenografts by chemotherapeutics, confirming that TNF-α preserved synergetic effect on breast cancer growth with chemotherapeutics.
The radiotherapy sensitization effect of TNF-α against breast cancer cells
To verify the radiotherapy sensitization effect of TNF-α, we measured the dynamic formation of phosphorylated H2A.X (γ-H2A.X), a marker of DNA double-strand breaks (DSBs) [20,21,22], in breast cancer cells after a dose of 40 Gy Cs137-irradiation. As shown in Fig. 2, the number of γ-H2A.X foci increased 30 min post irradiation. TNF-α treatment up-regulated γ-H2A.X foci formation to higher levels, suggesting the co-treatment of TNF-α brought more DNA damages.
TNF-α upregulated cyclin D1, Cyclin D2, Cyclin E, CDK4 and CDK6 through activating NF-κB pathway, and accelerated G1→S cell cycle transition
Cancer cells in G0/G1 cell cycle have been proved to be quiescent and resistant to chemotherapy and radiotherapy. Since TNF-α sensitized chemotherapy and radiotherapy, we then performed cell cycle analysis to explore the effect of TNF-α on cell cycle distribution. As shown in Fig. 3a and b, treatment with TNF-α induced remarkable decreased G0/G1 phase population and increased S plus G2/M phase proportion. These results indicated that TNF-α could drive cells out of quiescent G0/G1 phase, entering chemotherapy- and radiotherapy-sensitive proliferating phases.
TNF-α promoted G1→S cell cycle transition through NF-κB pathway dependent manner. a, b Effects of TNF-α (5 ng/ml) on cell cycle distribution in MDA-MB-231 (a) and MCF-7 (b) cells 24 h post treatment. c Relative luciferase activity of NF-κB after treatment with TNF-α (5 ng/ml) for 24 h. **P < 0.01 indicates significant differences from the respective control groups. d–j Expressions of Cyclin D1 (d), CDK4 (e), CDK6 (f), Cyclin D2 (g), Cyclin D3 (h), Cyclin E (i), and CDK2 (j) after treatment of TNF-α (5 ng/ml) and/or NF-κB inhibitor, Bay11-7082 (Bay, 20 μM). **P < 0.01 indicates significant differences from the respective control groups; ## P < 0.01 indicated significant differences from the TNF-α (5 ng/ml) treated groups; && P < 0.01 indicated significant differences between folds induction
Cyclin D1, Cyclin D2, Cyclin D3, Cyclin E, CDK2, CDK4 and CDK6 are the key regulators participating in G1→S phase transition, and NF-κB is the main pathway at the downstream of TNF-α stimulation. Therefore, we then investigate whether TNF-α could regulate expressions of G1→S phase transition regulators through NF-κB pathway dependent manner. Activation of NF-κB pathway was firstly confirmed by luciferase report gene assays (Fig. 3c). Treatment of TNF-α up-regulated Cyclin D1 and Cyclin D2 in MDA-MB-231 cells, and increased expressions of Cyclin E, CDK4 and CDK6 in MCF-7 cells (Fig. 3d–j). Pre-treatment of Bay11-7082, an inhibitor of NF-κB pathway, attenuated the up-regulation of Cyclin D1, Cyclin D2, Cyclin E, CDK4 and CDK6 (Fig. 3d–j), suggesting TNF-α up-regulated key regulators participating in G1→S cell cycle transition through NF-κB pathway dependent manner.
TNF-α strengthened cell cycle arrest induced by docetaxel and cisplatin
We then investigated whether the chemotherapy sensitization effect could involve the regulation on cell cycle distribution. As shown in Fig. 4a, docetaxel induced G2/M cell arrest and decreased population of cells at G0/G1 plus S phases, consisting with its microtubule structure stability role [23]. Co-treatment with TNF-α increased G2/M cell cycle arrest which was induced by docetaxel.
Cisplatin induced accumulation of cells at S phase in Fig. 4b, due to its DNA crosslinking role [24]. Co-treatment with TNF-α increased S phase cell cycle arrest induced by cisplatin.
Therefore, TNF-α drove cells out of G0/G1 phase, leading more cells entering S and G2/M phase and these proportions were further arrested by cisplatin and docetaxel at S and G2/M phases respectively.
TNF-α increased necroptosis induced by 5-FU
Figure 5a revealed that 5-FU caused G0/G1 cell arrest through its inhibition of thymidylate synthase [25]. Therefore, TNF-α might not be able to drove cells out of G0/G1 phase in this circumstance. However, combined application of TNF-α with 5-FU induced a significant sub G0/G1 peak, suggesting necrosis and/or apoptosis induction might be involved in the TNF-α-induced 5-FU sensitization.
Co-treatment of TNF-α and 5-FU induced necroptosis in MDA-MB-231 breast cancer cells. a Cell cycle distribution after treatment with TNF-α (5 ng/ml) and/or 5-FU (12 μM). b Apoptosis/necrosis rate after treatment with TNF-α and/or 5-FU. c Protein level of necroptosis marker, RIP3, after treatment with TNF-α and/or 5-FU. d Confirmation of knockdown of RIP3 by using real-time PCR. e Effect of RIP3 knockdown on the synergetic effect of TNF-α and 5-FU. **P < 0.01 indicated significant differences from the respective control groups; ## P < 0.01 indicated significant differences from the TNF-α (5 ng/ml) treated groups; & P < 0.05, && P < 0.01 and @@ P < 0.01 indicated significant differences between folds induction
Therefore, we performed apoptosis assays to further investigate the mechanisms involved. The percentages of cell populations at various stages of apoptosis are shown in Fig. 5b. After co-treatment with TNF-α and 5-FU, the numbers of cells that underwent later apoptosis and/or necrosis (Annexin V+/PI+) increased significantly. These data suggested that the enhanced growth-inhibition effect of TNF-α combined with antitumor drugs could be due to induction of later apoptosis and/or necrosis.
It has been reported that necrosis induced by TNF-α is distinguished from traditional necrosis, and termed as necroptosis (programmed necrosis) [26]. Therefore, we evaluated the activation of necroptosis marker, receptor-interacting protein kinase 3 (RIP3), by using Western blot. As shown in Fig. 5c, TNF-α treatment induced phosphorylation, the active form of RIP3 [26]. 5-FU had no effect on RIP3 phosphorylation, but up-regulated expression of RIP3. Therefore, co-treatment of TNF-α and 5-FU led to significant activation of RIP3, suggesting the involvement of necroptosis in the 5-FU sensitization effect of TNF-α.
To verify whether the chemotherapy sensitization effect of TNF-α on 5-FU was executed through a RIP3-dependent manner, we applied siRNAs targeting RIP3 (Fig. 5d). As expected, knockdown of RIP3 attenuated the synergetic effect of TNF-α and 5-FU (Fig. 5e), indicating TNF-α strengthened cytotoxicity of 5-FU through RIP3-dependent necroptosis.
Discussion
Resistance to anticancer drugs and radiotherapy is the primary reason for treatment failure in cancer [27]. Cytotoxic agents and radiotherapy killed only proliferating cancer cells and, in contrast, had little effect on quiescent cancer cells [28]. After cessation of chemotherapy and radiotherapy, these therapeutic-resistant quiescent cancer cells restarted cycling, leading to a relapse situation. Therefore, drugs that target quiescent cancer cells are urgently needed. In our present study, we proved that, TNF-α preserved the radiotherapy-sensitizing ability and strengthened cytotoxicity of chemotherapeutics, including docetaxel, cisplatin and 5-FU, making TNF-α a promising candidate for further clinical application.
Mitotic cellular division requires the cell to leave the resting state and proceed through phases of DNA synthesis and mitosis. Well-organized progression of dividing cells through the G0, G1, S, G2, and M phases of the cell cycle in eukaryotic cells relies on a series of cell-cycle regulatory proteins, such as Cyclin A, B, D and E, and these cyclins exert their functions via binding to and activating a variety of specific cyclin-dependent kinases (CDKs) [29]. Furthermore, there are a number of kinases and phosphatases which modulate CDK respectively through a way of phosphorylation and dephosphorylation. For instance, the regulation of the CDKs activity is associated with many CDK inhibitors (CKIs), and cell cycle checkpoint proteins. Cell division is conceived as a passage through these checkpoints. The earliest of these is the ‘‘restriction point’’ late in G1, when the cell becomes irreversibly transformed to DNA synthesis and mitosis. Cyclin D1 is a key regulator of G1/S checkpoint control, which forms a holoenzyme complex with CDK4 and CDK6, to phosphorylate pRb (retinoblastoma protein) [30]. When phosphorylated, pRB releases transcription facctor E2F to promote cell cycle [29, 31]. Previous studies have shown that the Cyclin D1 promoter region includes binding sites for NF-κB [32], and NF-κB is required to induce Cyclin D1 expression and pRb hyperphosphorylation to promote G1→S transition. There are other two D group of cyclins, Cyclin D2 and Cyclin D3. Cyclin D2 abundance peaks in late G1-phase. And cyclin D3 peaks during S-phase. Like cyclin D1, both Cyclin D2 and Cyclin D3 associate with CDK4 and CDK6, forming holoenzyme complexes that phosphorylate pRB [33]. Cyclin E, which forms a complex with CDK2, reaches abundance peak in late G1-phase [34]. The Cyclin E/CDK2 complex also phosphorylates pRB, although some phosphorylation sites are distinct from those targeted by kinases associating with D group cyclins. Consistent with our results in Fig. 3, apparently, the activation of NF-κB by the pre-treatment of TNF-α stimulates transcriptions of Cyclin D1, Cyclin D2, Cyclin E, CDK4 and CDK6 to accelerate the progression of cell cycle from G0/G1 phase to S phase. As a result, TNF-α drove quiescent cancer cells out of G0/G1 phase, entering treatment sensitive proliferating phases. By using cell cycle analysis, we further confirmed that TNF-α strengthened the G2/M phase and S phase cell cycle arresting ability of docetaxel and cisplatin respectively.
Actually, TNF-α is a pleiotropic cytokine that plays a vital role in a wide variety of physiological processes [35]. The pleiotropic nature of TNF-α response results from the sequential formation of different signaling complexes via binding of TNF-α to its receptor. So far, there are two identified TNF-α receptors, TNF-α receptor 1 (TNFR1) and TNF-α receptor 2 (TNFR2), both of which belong to the TNFR superfamily. TNFR1 has a single intracellular death domain (DD), whereas TNFR2 has a TNF receptor-associated factor (TRAF)-binding area [36]. There is a broad body of evidences that TNF-induced cell death is largely mediated via TNFR1 [6, 8, 10, 23]. Binding to TNFR1 can cause apoptosis by caspase 8 or necroptosis by receptor-interacting protein kinase 1 (RIP1)- and RIP3-dependent mechanisms [36,37,38]. Necroptosis, or programmed necrosis, refers to a caspase-8-independent death mechanism triggered by RIP1 and RIP3. RIP1 bears a DD that can interact with TNFR1, upon binding of TNF-α, RIP1 is recruited to TNFR1 either directly via its DD or indirectly by TRADD, to form a complex on the cytoplasmic domain of TNFR1 [39]. The complex is released from TNFR1 and RIP3 to cause necroptosis. If cells have high level of RIP3, RIP1 recruits RIP3 to form necrosome containing FADD [40, 41], caspase-8, RIP1, and RIP3, and the cells undergo necroptosis. Necrosome also suppresses apoptosis but the underlying mechanism has not been described yet. Mixed-lineage kinase domain-like (MLKL) is downstream of RIP3 [42, 43], the phosphorylated RIP3 recruits MLKL, and phosphorylation of MLKL is required for subsequent execution of necroptosis. Therefore, RIP3 is conceived as a key regulator of necroptosis.
5-FU can suppress thymidylate synthase and block DNA synthesis, causing a restraint of the transition from G1 phase to S phase [25]. Although the G0/G1 cell cycle arrest effect of 5-FU may antagonise the G1→S transition accelerating effect of TNF-α, co-treatment with TNF-α still strengthened the cytotoxicity of 5-FU, suggesting mechanisms beyond cell cycle regulation might be involved. It was noteworthy that there was a significant peak in sub G0/G1 phase in the co-treating group. Therefore, we carried out the apoptosis assays and detected a remarkble Annexin V+/PI+ population in the combination group of TNF-α and 5-FU, suggesting an apoptosis and/or necroptosis mechanism might be involved. We further demonstrated that 5-FU was able to up-regulate RIP3 expression. Since TNF-α activates RIP3 and induce necroptosis, the synergistic effect of co-treatment of TNF-α and 5-FU might be executed through elevated necroptosis. In consisting with this assumption, RIP3 siRNA apparently attenuated the synergistic effect by the combination of TNF-α and 5-FU.
In conclusion, we presently proved that, TNF-α present radiotherapy- and chemotherapy-sensitizing effect against breast cancer cells. TNF-α drove cells out of quiescent G0/G1 phase, entering vulnerable proliferating phases. As a result, TNF-α brought more DNA damage after irradiation and strengthened G2/M and S phase cell cycle arrest induced by docetaxel and cisplatin respectively. Moreover, the up-regulation of RIP3 by 5-FU, and the activation of RIP3 by TNF-α, synergistically triggered necroptosis. Taking together, our present investigation brought new strategies for breast cancer treatment through sensitized chemotherapy and radiotherapy by TNF-α.
Conclusions
TNF-α presented radiotherapy- and chemotherapy-sensitizing effects against breast cancer cells.
Abbreviations
- PCD:
-
programmed cell death
- DOC:
-
docetaxel
- 5-FU:
-
5-flurouracil
- DDP:
-
cisplatin
- MTT:
-
methyl thiazolyl tetrazolium
- DMSO:
-
dimethyl sulfoxide
- PI:
-
propidium iodide
- LTα:
-
lymphotoxin-α
- FasL:
-
fas ligand
- CD40L:
-
CD40 ligand
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- TNFR1:
-
TNF-α receptor 1
- TRAF:
-
TNF receptor-associated factor
- TRADD:
-
TNF receptor-associated death domain
- RIP1:
-
receptor-interacting protein 1
- NF-κB:
-
nuclear factor of kappa light polypeptide gene enhancer in B-cells
- CDKs:
-
cyclin-dependent kinases
- CKI:
-
cyclin-dependent kinase inhibitor
- CDC:
-
cell division cycle protein
- DD:
-
death domain
- FADD:
-
Fas-associated with death domain protein
- DISC:
-
death-inducing signaling complex
- pRb:
-
retinoblastoma protein
- RIP:
-
receptor-interacting protein kinase
- MLKL:
-
mixed-lineage kinase domain-like
References
Sandoo A, Kitas GD, Carmichael AR. Breast cancer therapy and cardiovascular risk: focus on trastuzumab. Vasc Health Risk Manag. 2015;11:223–8.
Lian L, Li W, Li ZY, Mao YX, Zhang YT, Zhao YM, Chen K, Duan WM, Tao M. Inhibition of MCF-7 breast cancer cell-induced platelet aggregation using a combination of antiplatelet drugs. Oncol Lett. 2013;5(2):675–80.
Li W, Liang RR, Zhou C, Wu MY, Lian L, Yuan GF, Wang MY, Xie X, Shou LM, Gong FR, et al. The association between expressions of Ras and CD68 in the angiogenesis of breast cancers. Cancer Cell Int. 2015;15(1):17.
Shou LM, Zhang QY, Li W, Xie X, Chen K, Lian L, Li ZY, Gong FR, Dai KS, Mao YX, et al. Cantharidin and norcantharidin inhibit the ability of MCF-7 cells to adhere to platelets via protein kinase C pathway-dependent downregulation of alpha2 integrin. Oncol Rep. 2013;30(3):1059–66.
Majeed W, Aslam B, Javed I, Khaliq T, Muhammad F, Ali A, Raza A. Breast cancer: major risk factors and recent developments in treatment. Asian Pacific J Cancer Prev: APJCP. 2014;15(8):3353–8.
Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114(2):181–90.
Zheng L, Bidere N, Staudt D, Cubre A, Orenstein J, Chan FK, Lenardo M. Competitive control of independent programs of tumor necrosis factor receptor-induced cell death by TRADD and RIP1. Mol Cell Biol. 2006;26(9):3505–13.
Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278(51):51613–21.
Tomita A, Fuchino Y, Otsuka K, Shinohara T, Tanaka SN, Umeno T, Ikeda S. Clinical effects of exogenous/endogenous TNF therapy on metastatic lesions of 34 colorectal cancer patients. Anticancer Res. 1998;18(5D):3937–9.
Watanabe N, Niitsu Y, Sone H, Neda H, Urushizaki I, Yamamoto A, Nagamuta M, Sugawara Y. Therapeutic effect of endogenous tumor necrosis factor on ascites Meth A sarcoma. J Immunopharmacol. 1986;8(2):271–83.
Bertrand F, Rochotte J, Colacios C, Montfort A, Tilkin-Mariame AF, Touriol C, Rochaix P, Lajoie-Mazenc I, Andrieu-Abadie N, Levade T, et al. Blocking tumor necrosis factor alpha enhances CD8 T-cell-dependent immunity in experimental melanoma. Cancer Res. 2015;75(13):2619–28.
Jayasooriya RG, Moon DO, Park SR, Choi YH, Asami Y, Kim MO, Jang JH, Kim BY, Ahn JS, Kim GY. Combined treatment with verrucarin A and tumor necrosis factor-alpha sensitizes apoptosis by overexpression of nuclear factor-kappaB-mediated Fas. Environ Toxicol Pharmacol. 2013;36(2):303–10.
Moon DO, Kim MO, Lee JD, Choi YH, Kim GY. Rosmarinic acid sensitizes cell death through suppression of TNF-alpha-induced NF-kappaB activation and ROS generation in human leukemia U937 cells. Cancer Lett. 2010;288(2):183–91.
Li W, Xie L, Chen Z, Zhu Y, Sun Y, Miao Y, Xu Z, Han X. Cantharidin, a potent and selective PP2A inhibitor, induces an oxidative stress-independent growth inhibition of pancreatic cancer cells through G2/M cell-cycle arrest and apoptosis. Cancer Sci. 2010;101(5):1226–33.
Yasui H, Adachi M, Imai K. Combination of tumor necrosis factor-alpha with sulindac augments its apoptotic potential and suppresses tumor growth of human carcinoma cells in nude mice. Cancer. 2003;97(6):1412–20.
Yasukawa M, Fujihara H, Fujimori H, Kawaguchi K, Yamada H, Nakayama R, Yamamoto N, Kishi Y, Hamada Y, Masutani M. Synergetic effects of PARP inhibitor AZD2281 and cisplatin in oral squamous cell carcinoma in vitro and in vivo. Int J Mol Sci. 2016;17(3):272.
Kutty RV, Chia SL, Setyawati MI, Muthu MS, Feng SS, Leong DT. In vivo and ex vivo proofs of concept that cetuximab conjugated vitamin E TPGS micelles increases efficacy of delivered docetaxel against triple negative breast cancer. Biomaterials. 2015;63:58–69.
Li W, Chen Z, Zong Y, Gong F, Zhu Y, Lv J, Zhang J, Xie L, Sun Y, Miao Y, et al. PP2A inhibitors induce apoptosis in pancreatic cancer cell line PANC-1 through persistent phosphorylation of IKKalpha and sustained activation of the NF-kappaB pathway. Cancer Lett. 2011;304(2):117–27.
Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137(6):1112–23.
Matsudaira H, Furuno I, Ueno AM, Shinohara K, Yoshizawa K. Induction and repair of strand breaks and 3′-hydroxy terminals in the DNA of mammalian cells in culture following gamma-ray irradiation. Biochim Biophys Acta. 1977;476(2):97–107.
Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol: CB. 2000;10(15):886–95.
Podhorecka M, Skladanowski A, Bozko P. H2AX phosphorylation: its role in DNA damage response and cancer therapy. J nucleic acids. 2010;2010.
Hu Q, Rijcken CJ, Bansal R, Hennink WE, Storm G, Prakash J. Complete regression of breast tumour with a single dose of docetaxel-entrapped core-cross-linked polymeric micelles. Biomaterials. 2015;53:370–8.
Plaimee P, Weerapreeyakul N, Barusrux S, Johns NP. Melatonin potentiates cisplatin-induced apoptosis and cell cycle arrest in human lung adenocarcinoma cells. Cell Prolif. 2015;48(1):67–77.
Grem JL, Fischer PH. Enhancement of 5-fluorouracil’s anticancer activity by dipyridamole. Pharmacol Ther. 1989;40(3):349–71.
Chang YJ, Hsu SL, Liu YT, Lin YH, Lin MH, Huang SJ, Ho JA, Wu LC. Gallic acid induces necroptosis via TNF-alpha signaling pathway in activated hepatic stellate cells. PLoS ONE. 2015;10(3):e0120713.
Miwa S, Yano S, Kimura H, Yamamoto M, Toneri M, Matsumoto Y, Uehara F, Hiroshima Y, Murakami T, Hayashi K, et al. Cell-cycle fate-monitoring distinguishes individual chemosensitive and chemoresistant cancer cells in drug-treated heterogeneous populations demonstrated by real-time FUCCI imaging. Cell Cycle. 2015;14(4):621–9.
Miwa S, Yano S, Kimura H, Yamamoto M, Toneri M, Murakami T, Hayashi K, Yamamoto N, Fujiwara T, Tsuchiya H, et al. Heterogeneous cell-cycle behavior in response to UVB irradiation by a population of single cancer cells visualized by time-lapse FUCCI imaging. Cell Cycle. 2015;14(12):1932–7.
Shackelford RE, Kaufmann WK, Paules RS. Cell cycle control, checkpoint mechanisms, and genotoxic stress. Environ Health Perspect. 1999;107(Suppl 1):5–24.
Pestell RG, Albanese C, Reutens AT, Segall JE, Lee RJ, Arnold A. The cyclins and cyclin-dependent kinase inhibitors in hormonal regulation of proliferation and differentiation. Endocr Rev. 1999;20(4):501–34.
van de Loosdrecht AA, Ossenkoppele GJ, Beelen RH, Broekhoven MG, Langenhuijsen MM. Cell cycle specific effects of tumor necrosis factor alpha in monocyte mediated leukemic cell death and the role of beta 2-integrins. Cancer Res. 1993;53(18):4399–407.
Joyce D, Bouzahzah B, Fu M, Albanese C, D’Amico M, Steer J, Klein JU, Lee RJ, Segall JE, Westwick JK, et al. Integration of Rac-dependent regulation of cyclin D1 transcription through a nuclear factor-kappaB-dependent pathway. J Biol Chem. 1999;274(36):25245–9.
Matsushime H, Roussel MF, Ashmun RA, Sherr CJ. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65(4):701–13.
Mumberg D, Wick M, Burger C, Haas K, Funk M, Muller R. Cyclin ET, a new splice variant of human cyclin E with a unique expression pattern during cell cycle progression and differentiation. Nucleic Acids Res. 1997;25(11):2098–105.
Tracey KJ, Cerami A. Tumor necrosis factor: an updated review of its biology. Crit Care Med. 1993;21(10 Suppl):S415–22.
Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3(9):745–56.
Degterev A, Zhou W, Maki JL, Yuan J. Assays for necroptosis and activity of RIP kinases. Methods Enzymol. 2014;545:1–33.
Wei B, Yue P, Qingwei L. The molecular mechanism of necroptosis. Yi chuan=Hereditas/Zhongguo yi chuan xue hui bian ji. 2014;36(6):519–24.
Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4(5):313–21.
Zhang DW, Zheng M, Zhao J, Li YY, Huang Z, Li Z, Han J. Multiple death pathways in TNF-treated fibroblasts: RIP3- and RIP1-dependent and independent routes. Cell Res. 2011;21(2):368–71.
Lu JV, Weist BM, van Raam BJ, Marro BS, Nguyen LV, Srinivas P, Bell BD, Luhrs KA, Lane TE, Salvesen GS, et al. Complementary roles of Fas-associated death domain (FADD) and receptor interacting protein kinase-3 (RIPK3) in T-cell homeostasis and antiviral immunity. Proc Natl Acad Sci USA. 2011;108(37):15312–7.
Xie T, Peng W, Yan C, Wu J, Gong X, Shi Y. Structural insights into RIP3-mediated necroptotic signaling. Cell reports. 2013;5(1):70–8.
Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39(3):443–53.
Authors’ contributions
XW, MYW, MJ and QZ designed, performed experiments, and participated in drafting the manuscript. XB, MDX and FRG participated in performing experiments and evaluating data. JH and MT participated in the design of the study and performed the statistical analysis. MT, LMS and WD helped to design the experiments and revise the manuscript. KC, MS and WL conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81472296, 81101867, 81602091, 81272542, 81200369, 81572992, 81402176, 81402093, 81402093 and 81501970), the Six Major Talent Peak Project of Jiangsu Province (Grant No. 2015-WSN-022), the China International Medical Foundation (Grant No. CIMF-F-H001-057), the Special Foundation of Clinical Medicine of Jiangsu Provincial Bureau of Science and Technology (Grant No. BL2014039), the Scientific Research Project of Jiangsu Provincial Bureau of Traditional Chinese Medicine (Grant No. L213236), the Medical Scientific Research Project of Jiangsu Provincial Bureau of Health (Grant No. Z201206), the Special Foundation of Wu Jieping Medical Foundation for Clinical Scientific Research (Grant Nos. 320.6753.1225 and 320.6750.12242), the Natural Science Foundation of Jiangsu Province (Grant No. BK20140288), the Natural Science Foundation of Jiangsu Higher Education Institutions of China (Grant No. 14KJB320011), the Science and Education for Health Foundation of Suzhou for Youth (Grant Nos. kjxw2015003, SWKQ1003 and SWKQ1011), the Science and Technology Project Foundation of Suzhou (Grant Nos. SYS201112, SYSD2012137, SYSD201464 and SYS201335).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiao Wu, Meng-Yao Wu and Min Jiang authors contributed equally to this work
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wu, X., Wu, MY., Jiang, M. et al. TNF-α sensitizes chemotherapy and radiotherapy against breast cancer cells. Cancer Cell Int 17, 13 (2017). https://doi.org/10.1186/s12935-017-0382-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-017-0382-1