Abstract
Background
The biosynthesis of high value-added compounds using metabolically engineered strains has received wide attention in recent years. Myo-inositol (inositol), an important compound in the pharmaceutics, cosmetics and food industries, is usually produced from phytate via a harsh set of chemical reactions. Recombinant Escherichia coli strains have been constructed by metabolic engineering strategies to produce inositol, but with a low yield. The proper distribution of carbon flux between cell growth and inositol production is a major challenge for constructing an efficient inositol-synthesis pathway in bacteria. Construction of metabolically engineered E. coli strains with high stoichiometric yield of inositol is desirable.
Results
In the present study, we designed an inositol-synthesis pathway from glucose with a theoretical stoichiometric yield of 1 mol inositol/mol glucose. Recombinant E. coli strains with high stoichiometric yield (> 0.7 mol inositol/mol glucose) were obtained. Inositol was successfully biosynthesized after introducing two crucial enzymes: inositol-3-phosphate synthase (IPS) from Trypanosoma brucei, and inositol monophosphatase (IMP) from E. coli. Based on starting strains E. coli BW25113 (wild-type) and SG104 (ΔptsG::glk, ΔgalR::zglf, ΔpoxB::acs), a series of engineered strains for inositol production was constructed by deleting the key genes pgi, pfkA and pykF. Plasmid-based expression systems for IPS and IMP were optimized, and expression of the gene zwf was regulated to enhance the stoichiometric yield of inositol. The highest stoichiometric yield (0.96 mol inositol/mol glucose) was achieved from recombinant strain R15 (SG104, Δpgi, Δpgm, and RBSL5-zwf). Strain R04 (SG104 and Δpgi) reached high-density in a 1-L fermenter when using glucose and glycerol as a mixed carbon source. In scaled-up fed-batch bioconversion in situ using strain R04, 0.82 mol inositol/mol glucose was produced within 23 h, corresponding to a titer of 106.3 g/L (590.5 mM) inositol.
Conclusions
The biosynthesis of inositol from glucose in recombinant E. coli was optimized by metabolic engineering strategies. The metabolically engineered E. coli strains represent a promising method for future inositol production. This study provides an essential reference to obtain a suitable distribution of carbon flux between glycolysis and inositol synthesis.
Similar content being viewed by others
Background
Myo-inositol (inositol), a water-soluble vitamin B group compound with a six-carbon ring, is widely used in many industries such as food, pharmaceuticals and cosmetics [1,2,3,4]. Inositol is also a precursor of various biofunctional compounds containing inositol phosphate and lipid [5, 6]. Inositol has attracted the attention of the food and feed industries, where it is mainly used as an additive in drinks, in milk powder as a nutritional supplement, and in animal feed to prevent diseases [7, 8]. Inositol has been proved to treat some diseases such as diabetes and fatty liver [9,10,11]. Furthermore, inositol is an important intermediate used for the production of uronic acid and inositol derivatives. There are a few reports describing the use of whole-cell biocatalysis to produce glucuronic acid, glucaric acid and scyllo-inositol from inositol or glucose [12,13,14,15,16,17].
Microbial production of inositol is regarded as a promising alternative to conventional methods of synthesis such as hydrolysis of phytate [18, 19]. The synthesis pathway of inositol from the precursor glucose-6-phosphate (G-6-P) involves two crucial enzymes, inositol-3-phosphate synthase (IPS) and inositol monophosphatase (IMP). Research applying G-6-P to produce inositol via a two-step reaction has been extensive. IPS, the key rate-limiting enzyme in biosynthesis of inositol, can convert G-6-P to the intermediate inositol-3-phosphate (inositol-3-P). The final product inositol is reached by IMP-catalyzed dephosphorylation of inositol-3-P [20,21,22]. There are some reports concerning in vitro enzymatic production of inositol using G-6-P, and G-6-P was obtained from starch through two enzymes, alpha-glucan (or maltodextrin) phosphorylase and phosphoglucomutase [21, 22]. We have previously reported a trienzymatic cascade system of polyphosphate glucokinase, IPS and IMP to produce inositol from glucose, giving a molar conversion of 90% [20]. However, enzymatic production of inositol had many problems, including a complicated process of isolation, high cost, and instability of enzymes [23]. Inositol has been produced from glucose by using metabolically engineered Escherichia coli strains. Nevertheless, the yield was low, mainly because of the competitive relationship between cell growth and inositol synthesis [24]. The low yield indicates that only a small amount of G-6-P is distributed to inositol production. Therefore, an inositol biosynthesis pathway in recombinant E. coli strains needs to be metabolically optimized. Our laboratory has developed an E. coli strain (SG104) in which the glucose utilization system was modified by replacing native ptsG (gene ID: 945651) and galR (gene ID: 947314) with glk (gene ID: 946858) and zglf (galactose:H+ symporter from Zymomonas mobilis) respectively in the chromosome of E. coli BW25113, and the accumulation of acetic acid was reduced by deleting poxB (gene ID: 946132) and overexpressing acs (gene ID: 948572) for high-density fermentation [25].
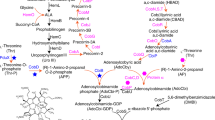
In the present study, our aim was to achieve high stoichiometric yields of inositol, by guiding the carbon flux of precursor G-6-P to inositol synthesis. A series of metabolic engineering strategies was performed in the inositol synthesis pathway (Fig. 1). First, a biosynthesis pathway for inositol from G-6-P was designed by overexpressing two crucial enzymes—IPS and IMP—and the plasmid expression systems of IPS and IMP were optimized. Second, to enhance metabolic flux to G-6-P, pgi (G-6-P isomerase, gene ID: 948535), pfkA (6-phosphofructokinase I, gene ID: 948412), pykF (pyruvate kinase II, gene ID: 946179), and pgm (phosphoglucomutase, gene ID: 945271) were deleted. Thereafter, the gene zwf (gene ID: 946370), encoding G-6-P dehydrogenase in the pentose phosphate pathway (PPP), was regulated by replacement of the promoter or ribosome binding site (RBS) to further improve inositol production. The highest stoichiometric yield (0.96 mol inositol/mol glucose) was obtained from strain R15 (SG104, Δpgi, Δpgm, RBSL5-zwf). Third, four recombinant strains (R04, R12, R14 and R15) with high stoichiometric yields (> 0.7 mol inositol/mol glucose) were chosen for high-density fermentation using glucose and glycerol as a mixed carbon source. Finally, strain R04 (SG104 and Δpgi) reached the density OD600 = 135 after 48 h, and scaled-up production of inositol through bioconversion in situ was accomplished to obtain a titer of 106.3 g/L (590.5 mM), corresponding to a stoichiometric yield of 0.82 mol inositol/mol glucose.
Results
Production of inositol by slowing carbon flux in glycolysis pathway
The intake of glucose in E. coli involves two key enzymes: glucose kinase (glk) and protein-Npi-phosphohistidine-d-glucose phosphotransferase (ptsG). Glucose kinase converts glucose to G-6-P with high catalytic efficiency [26,27,28]. Our laboratory has developed an E. coli strain, SG104, by deleting ptsG and enhancing glk to increase glucose intake [25]. E. coli BW25113 and SG104 were chosen as starting strains to construct host strains that slow carbon flux to glycolysis to enhance the supply of inositol precursor G-6-P. The key genes involved—pgi, pfkA and pykF—were respectively deleted (Fig. 1). Deletion of pgi directly enhances precursor G-6-P accumulation by blocking glycolysis. The pfkA redirected carbon flux in glycolysis pathway, and showed most of 6-phosphofructokinase activity [24]. Previous study has shown that pykF, the major pyruvate kinase, is a regulatory factor of glycolysis [29]. Our previous study indicated that TbIPS from Trypanosoma brucei and EcIMP from E. coli have high specific activity [20]. As such, plasmid p01 expressing TbIPS and p02 expressing EcIMP were co-transformed into E. coli strains BW25113, SG104, R01, R02, R03, R04, R05 and R06 respectively to construct recombinant strains for inositol production.
The expression of IPS and IMP in different host strains was shown as Fig. 2a. Whole-cell bioconversions were performed using different strains to select an appropriate host strain for inositol production. After 10 h of bioconversion, 29.6 mM inositol was obtained using strain R04; a stoichiometric yield of 0.6 mol inositol/mol glucose was reached, and no residual glucose was observed (Fig. 2b). Compared with strain R01 in which pgi is deleted to block glycolysis, strain R04 derived from strain SG104 showed increased inositol production. The results showed that deletion of pgi was effective for accumulation of precursor G-6-P. Strains R05 and R06 showed faster glucose consumption than R02 and R03, but low inositol concentration was achieved (Fig. 2b).
Host strain selection. a SDS-PAGE showing the expression of inositol-3-phosphate synthase (IPS; approximately 60 kDa) and inositol monophosphatase (IMP; approximately 30 kDa) in different chassis strains. b Production of inositol in different chassis strains. The recombinant strains transformed with plasmids p01 and p02 were induced and then suspended in a bioconversion mixture containing 50 mM glucose. The bioconversions were performed for 10 h at 37 °C and 220 rpm
Improvement of inositol production by optimizing plasmid expression systems
Plasmid expression systems are useful for the reconstruction of biosynthesis pathways and usually give a high yield of a target product [25]. To investigate the effect of expression of TbIPS and EcIMP from a single plasmid, plasmids pR01 and pR02 were constructed (Fig. 3a) and respectively transformed into host strain R04 to produce inositol. The results showed that pR01 improved the production of inositol; 0.62 mol inositol/mol glucose was produced with a titer of 31.1 mM (Fig. 3b). The expression of key enzymes (TbIPS and EcIMP) is shown in Additional file 1: Fig. S1a.
Effects of different plasmid combinations in host strain R04 on inositol production. a Schematic diagram of pR01 and pR02 in which the genes encoding TbIPS and EcIMP are arranged in different sequences. rbs, ribosome binding site; pBAD, an araBAD promoter. b Inositol production using different plasmids singly or in combination. c Effect of IPS expression on introducing ScIPS from Saccharomyces cerevisiae
The activity of IPS in the metabolic pathway is one of the most important factors [20]. Therefore, ScIPS from Saccharomyces cerevisiae was introduced to enhance IPS activity in the inositol-biosynthesis pathway. Plasmids pR03 and p03 were constructed, and plasmid combinations pR01 + p03, and pR03 were respectively transformed into host strain R04 to produce inositol. The cells transformed with pR01 + p03 produced 0.71 mol inositol/mol glucose with a titer of 35.5 mM (Fig. 3c). The expression of TbIPS, ScIPS, and EcIMP is shown in Additional file 1: Fig. S1b.
Further strain optimization by regulating zwf and deleting pgm
To further enhance the stoichiometric yield of inositol, the strength of expression of gene zwf from the PPP was adjusted by replacement of its promoter or RBS. Strain R04 was chosen as the platform strain to construct seven host strains (R7 to R13), which were used as controls to evaluate the effects of blocking and enhancing the PPP, respectively. Expression of zwf was decreased in strains R08 to R12 by using different RBS strengths (RBSL1 to RBSL5).
The resulting host strains were then transformed with the plasmid combination pR01 + p03. The expression of key enzymes (TbIPS, ScIPS, and EcIMP) in host strains (R04, and R07 to R15) is shown in Fig. 4a. Strain R12 exhibited inositol production of 44.7 mM after 10 h of bioconversion, corresponding to a stoichiometric yield of 0.9 mol inositol/mol glucose (Fig. 4b). The gene pgm, encoding phosphoglucomutase, which acts in isomerization of G-6-P and glucose-1-phosphate, was deleted to construct host strains R14 and R15. The highest stoichiometric yield (0.96 mol inositol/mol glucose) was achieved in strain R15, corresponding to a concentration of 48 mM inositol and consumption of 50 mM glucose (Fig. 4b).
Effects of regulating zwf and deleting pgm on inositol production. a SDS-PAGE showing the expression of IPS (approximately 60 kDa) and IMP (approximately 30 kDa). b Production of inositol in different host strains. The recombinant strains were induced and harvested, then suspended in a bioconversion mixture containing 50 mM glucose. The bioconversions were performed at 37 °C and 220 rpm for 10 h
High-density fermentation through synergetic utilization of glucose and glycerol
To evaluate the fermentation capacity of recombinant strains, host strains R04, R12, R14 and R15 transformed with plasmids pR01 + p03 were chosen for high-density fermentation using a modified inorganic salts medium containing glucose and glycerol. The stoichiometric yields produced by the four strains in shaken flasks were all > 0.7 mol inositol/mol glucose (Fig. 4b). E. coli follows the glycolysis pathway as the central metabolic system for cell growth. However, glycolysis was blocked by deletion of pgi; thus glycerol was selected as a carbon source to enable cell growth.
The four recombinant strains were cultivated in modified inorganic salts medium with glycerol and glucose as a mixed carbon source. Strain R04 reached a density of OD600 = 135 after 48 h. However, the other three strains could not grow, or grew slowly, in our modified inorganic salts medium (Fig. 5a). The expression of key enzymes (TbIPS, ScIPS, and EcIMP) in strain R04 in the high-density fermentation is shown in Additional file 1: Fig. S2. In both shaken flasks and high-density fermentation, strain R04 showed approximately equivalent stoichiometric yields (> 0.7 mol inositol/mol glucose) without glucose residue (Fig. 5b). The other three strains (R12, R14 and R15) showed poor expression of key enzymes and low stoichiometric inositol yield (Additional file 1: Fig. S3). The results indicated that the high-density fermentation of strain R04 could be improved through synergetic use of glucose and glycerol as carbon sources.
High-density fermentation of recombinant strains with high stoichiometric yields and bioconversion by strain R04. a High-density fermentation of strains R04, R12, R14, and R15. Cells were initially cultured in Luria–Bertani medium at 37 °C. Then, this seed culture was inoculated into 500 mL inorganic salts medium with glycerol and glucose as a mixed carbon source in a 1-L fermenter. b Comparison of bioconversion by strain R04 cultured in shaken flasks (1) and high-density fermentation (2). Cells were suspended in a mixture with 50 mM glucose for 10 h at 37 °C and 220 rpm
Scaled-up production of inositol using strain R04
Strain R04 reached high-density through synergetic use of glucose and glycerol. To comprehensively evaluate the overall production performance of inositol, scaled-up bioconversion using strain R04 was carried out in a 1-L fed-batch fermenter. After 25 h of bioconversion ex situ, 375 mM inositol was obtained, while 580 mM glucose was consumed. The stoichiometric yield was 0.65 mol inositol/mol glucose (Fig. 6a). 0.82 mol inositol/mol glucose was produced after 23 h of bioconversion in situ, while 720.5 mM glucose was consumed. The concentration of inositol reached 590.5 mM, corresponding to a titer of 106.3 g/L (Fig. 6b).
Scaled-up production of inositol by bioconversion by strain R04 ex situ (a) and in situ (b). The bioconversion ex situ was in a 1-L fermenter containing biomass (OD600 = 80), 1 mM MgSO4, and 1 × M9 salts buffer. Glucose was added in batches; 200 mM glucose was added at 0, 4.5, and 11 h respectively. The bioconversion in situ in a 1-L fermenter was started when the cells grew to the density of OD600 = 80. Glucose was added in batches; 200 mM glucose was added at 0, 3, 6, and 9 h, respectively
Discussion
Inositol and its derivatives are widely distributed in nature, displaying a variety of biological activities and metabolic functions; they are also valuable as nutrients and pharmaceuticals [30,31,32]. The tools of metabolic engineering and synthetic biology have been combined in recent years to design microorganisms with desirable functions. This has motivated many studies to examine the production of high-value products from glucose, which is cheap [33,34,35]. Efficient production of inositol via biotechnological approaches is urgently needed. E. coli is considered a “generally regarded as safe” organism and possesses a glycolysis pathway that synthesizes the inositol precursor G-6-P. Inositol has been produced from glucose by using metabolically engineered E. coli, but low yields have restricted industrial production of inositol by this method [24]. Metabolic engineering of E. coli to efficiently supply G-6-P would enhance the production of inositol.
Numerous studies have explored various approaches for reallocating the metabolic flux in E. coli to attenuate glycolysis and accumulate G-6-P [24, 29]. In this study, the key targets pgi, pfkA, pykF, and pgm were deleted to enhance the supply of G-6-P. Thereafter, the key gene zwf in the PPP was modified by replacement of its promoter or RBS to further accumulate G-6-P (Fig. 1). It is not surprising that this study found evidence that the accumulation of precursor G-6-P was beneficial for production of inositol. This was substantiated by the significant increase in stoichiometric yield observed upon knockout of pgi (Fig. 2). In addition, the intake of glucose is also a key issue to be addressed, and the SG104 background was also benefit inositol production (Additional file 1: Fig. S4a). Furthermore, the SG104 background promoted cell growth in high-density fermentation (Additional file 1: Fig. S4b). The activity of IPS is important to the effective synthesis of inositol from G-6-P. Increasing the activity of IPS by introducing two such enzymes from different sources (i.e., TbIPS and ScIPS) showed great potential for boosting the production of inositol. In the present inositol biosynthesis pathway, 1 mol of glucose is, in principle, metabolized to 1 mol of inositol via a three-step reaction. It is important to design the metabolic pathway toward the compound(s) of interest by considering the stoichiometric yield. The highest yield in our study was 0.96 mol inositol/mol glucose in strain R15, approaching the theoretical stoichiometric yield (Fig. 4b).
Suitable distribution of carbon flux between cell growth and inositol synthesis is the second challenge to be addressed for inositol production from glucose using microbial cell factories. However, here, the growth of recombinant strains was restricted because glycolysis was blocked to supply G-6-P. The strains in which pgi is deleted almost could not grow in organic salts (i.e., M9 salts) medium with glucose. This seriously impedes the efficient production of inositol. Glycerol is a valuable carbon source for cell growth in microbial fermentation. Hence, the use of glucose and glycerol as a mixed carbon source may be a promising strategy for high-density fermentation. In our study, four recombinant strains (R4, R12, R14, and R15) were cultivated in modified inorganic salts medium containing glucose and glycerol as a mixed carbon source. Disappointingly, only strain R04 reached high-density (OD600 = 135), while the growth of strains R12, R14, R15 was poor (Fig. 5a). Ex situ and in situ bioconversions for inositol production by strain R04 were carried out in a 1-L fed-batch fermenter. A high stoichiometric yield (0.82 mol inositol/mol glucose) was achieved, and 720.5 mM glucose was consumed, corresponding to production of 590.5 mM inositol (Fig. 6b). The titer of inositol, 106.3 g/L, was higher than that in most previous reports, and is promising for scaling up for commercial applications. Balancing cell growth and inositol production is expected to be a powerful strategy for industrial production of inositol.
Besides metabolic engineering approaches, optimization of cultivation conditions and bioconversion processes will be important to improve inositol production. Considering that appropriate coupling between cell growth and inositol synthesis is critical for the overall inositol production, high-density fermentation could be further optimized to enhance inositol yield. It is also generally accepted that recombinant strains (strain R12, R14, R15) should be considered to evolve.
Conclusions
In the present study, we performed inositol production using different engineering strategies to construct E. coli host strains. Enhancement of the supply of precursor G-6-P in the inositol-biosynthesis pathway, and optimization of high-density fermentation by using glucose and glycerol as a mixed carbon source, were key strategies to enhance inositol production. The combination of these approaches increased the stoichiometric yield to 0.96 mol inositol/mol glucose. Glycolysis in E. coli was blocked by inactivating the gene pgi to redirect the carbon flux toward inositol production, but this resulted in restriction of cell growth. Fortunately, recombinant strain R04 reached high-density through synergetic use of glucose and glycerol. Bioconversions for inositol production were carried out ex situ and in situ in a 1-L fermenter by using cells at high density. Finally, we achieved 590.5 mM inositol (a titer of 106 g/L) by using the bioconversion in situ, corresponding to a stoichiometric yield of 0.82 mol inositol/mol glucose. The coupling of high-density fermentation of engineered strains and bioconversion in situ may therefore be a promising strategy for efficient production of inositol with high stoichiometric yield and titer.
Methods
Chemicals and media
T4 DNA ligase, Gibson kits and restriction enzymes were purchased from New England Biolabs (USA). Plasmid extraction and gel purification kits were purchased from Omega (Beijing, China). DNA polymerase (2× High Fidelity Master Mix) was bought from TSINGKE (Beijing, China). Media components were bought from Becton–Dickinson (Beijing, China). Standards of Glucose, G-6-P, inositol, and other chemicals were obtained from Sigma-Aldrich (Shanghai, China).
All E. coli strains were grown in Luria–Bertani (LB) medium containing 10 g/L tryptone, 10 g/L NaCl, and 5 g/L yeast extract. Modified inorganic salts medium was used for high-density fermentation of engineered strains. Seed culture was inoculated into modified inorganic salts medium containing mixed carbon sources (10 g/L glucose and 10 g/L glycerol), KH2PO4 14 g/L, (NH4)2PO4 4 g/L, Na2SO4 2 g/L, citric acid 1.8 g/L, MgSO4·7H2O 0.6 g/L, and trace elements [8.4 mg/L EDTA, 2.5 mg/L CoCl2·6H2O, 0.15 mg/L MnCl2·4H2O, 1.5 mg/L CuCl2·2H2O, 3 mg/L H3BO3, 2.5 mg/L Na2MoO4·2H2O, 13 mg/L Zn(CH3COO)2·2H2O, and 10 g/L Fe3+citrate]. The feeding medium contained 600 g/L mixed carbon sources, 2 g/L MgSO4·7H2O, and trace elements.
Strains and plasmids
The bacterial strains and plasmids used in this study are listed in Table 1. Primers used in the study are listed in Additional file 1: Table S1. The plasmids pYB1s and pLB1a used for expression of genes are derived from our laboratory’s vectors, which have the origin of replication, streptomycin and ampicillin resistance genes, an araBAD promoter (pBAD), multiple cloning sites, and a rrnB terminator. The IPS genes from T. brucei and S. cerevisiae were codon-optimized and synthesized by General Biosystems Co., Ltd. (Anhui, China). The encoding nucleotide sequences of the enzymes used for producing inositol were amplified by PCR and ligated into the vectors between XhoI and SpeI sites by T4 ligation and Gibson assembly [36]. E. coli transT1 was used for molecular cloning. E. coli BW25113 was used as the parental strain for genetic modification and inositol production. Gene knockout strains were obtained from the KEIO collection (National BioResource Project) [37, 38]. P1 virus-mediated transfection was used for integration into the chromosome [39, 40]. The Crispr-Cas9 system was used for gene knockout, replacement, and change of RBS or promoter [41].
Culture conditions
For expression of proteins, strains were cultured in LB medium with appropriate antibiotic (streptomycin 50 mg/L) at 37 °C and 220 rpm. The strains were incubated at 30 °C and 220 rpm and induced by adding l-arabinose (2 g/L). For high-density fermentation of recombinant strains, strains were initially cultured in LB medium at 37 °C and 220 rpm to produce a seed culture. Then, the seed culture was inoculated into 500 mL modified inorganic salts medium in a 1-L fermenter (Shanghai Bailun Biotechnology Co., Ltd.). The fermentation was conducted at 37 °C and pH 7.0, and initially 10 g/L glucose and 10 g/L glycerol were added. The dissolved oxygen (DO) level was maintained at 30–50% (v/v) by air flow at 1 L/min and changing the agitation speed from 500 to 1100 rpm. Feeding was started when the initial glucose concentration decreased below 1 g/L and was adjusted to maintain the residual glucose level below 1 g/L. The cells were induced by adding l-arabinose (2 g/L) at 30 °C when strains grew to suitable biomass (OD600 = 30).
Bioconversion conditions
The recombinant strains were induced and harvested, then suspended in a bioconversion mixture containing 50 mM glucose. The bioconversions were performed for 10 h at 37 °C and 220 rpm in 1 × M9 salts buffer (Na2HPO4·7H2O 12.8 g/L, KH2PO4 3 g/L, NaCl 0.5 g/L, NH4Cl 1 g/L) containing 1 mM MgSO4. The cell densities (OD600) at the starting and end points of the bioconversions were all almost 30. For the scaled-up production of inositol, ex situ and in situ bioconversions were used for inositol production by strain R04. For ex situ bioconversions, induced cells were harvested by centrifugation and suspended in 500 mL buffer containing 1 × M9 salts with 1 mM MgSO4 in a 1-L fermenter. Cells (OD600 = 80) were incubated with 30–50% DO at 37 °C. When the bioconversion starts, glucose is added in batches. The bioconversion in situ in a 1-L fermenter was started when the cells grew to the density of OD600 = 80, glucose is added in batches. The supply from the alkali pump is changed from NH3·H2O to NaOH, resulting in the transformation of cell growth to bioconversion. Cells were incubated with 30–50% DO at 37 °C.
Analytical methods
Cell density was estimated by measuring optical density at 600 nm with a spectrophotometer. Recombinant enzyme expression was compared and analyzed by SDS-PAGE. For the preparation of SDS-PAGE samples, induced cells were harvested and suspended in 50 mM phosphate buffer (pH 7.0) with cell density OD600 = 10. The cells were lysed by ultrasonic disruption. The mixture was centrifuged, and the supernatant was mixed with isometric 2× protein loading buffer. After boiling for 10 min, equal volumes of sample were loaded onto gels. Concentrations of glucose, glycerol and inositol in culture supernatant were measured by high-performance liquid chromatography (HPLC) with a Bio-Rad Aminex HPX-87 H column (7.8 × 300 mm; Hercules, CA, USA) and a refractive index detector. Samples taken from bioconversions were centrifuged, and HPLC samples were obtained by filtration–sterilization of the supernatants. The analysis was performed with a flow rate of 0.5 mL/min using 5 mM H2SO4 as the mobile phase at 40 °C. The retention times of G-6-P, glucose and inositol were 7.621, 10.633, and 11.188 min respectively (Additional file 1: Fig. S5).
Availability of data and materials
Not applicable.
Abbreviations
- OD600 :
-
The optical density of cells at 600 nm
- IPS:
-
Inositol 1-phosphate synthase
- IMP:
-
Inositol monophosphatase
- G-6-P:
-
Glucose-6-phosphate
- PPP:
-
Pentose phosphate pathway
- RBS:
-
Ribosome binding site
- pBAD:
-
An araBAD promoter
- SDS-PAGE:
-
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- HPLC:
-
High-performance liquid chromatography
References
Salway JG, Whitehead L, Finnegan JA, Karunanayaka A, Barnett D, Payne RB. Effect of myo-inositol on peripheral-nerve function in diabetes. Lancet. 1978;2:1282–4.
Clements RS Jr, Vourganti B, Kuba T, Oh SJ, Darnell B. Dietary myo-inositol intake and peripheral nerve function in diabetic neuropathy. Metabolism. 1979;28:477–83.
Michell RH. Do inositol supplements enhance phosphatidylinositol supply and thus support endoplasmic reticulum function? Br J Nutr. 2018;120:1–16.
Benvenga S, Antonelli A. Inositol(s) in thyroid function, growth and autoimmunity. Rev Endocr Metab Disord. 2016;17:471–84.
Ruf JC, Ciavatti M, Gustafsson T, Renaud S. Effect of D-myo-inositol on platelet function and composition and on cataract development in streptozotocin-induced diabetic rats. Biochem Med Metab Biol. 1992;48:46–55.
Armand M, Tarascon JM. Building better batteries. Nature. 2008;451:652–7.
Li SA, Jiang WD, Feng L, Liu Y, Wu P, Jiang J, Kuang SY, Tang L, Tang WN, Zhang YA, et al. Dietary myo-inositol deficiency decreased intestinal immune function related to NF-kappaB and TOR signaling in the intestine of young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2018;76:333–46.
Li SA, Jiang WD, Feng L, Liu Y, Wu P, Jiang J, Kuang SY, Tang L, Tang WN, Zhang YA, et al. Dietary myo-inositol deficiency decreased the growth performances and impaired intestinal physical barrier function partly relating to nrf2, jnk, e2f4 and mlck signaling in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2017;67:475–92.
Sacchinelli A, Venturella R, Lico D, Di Cello A, Lucia A, Rania E, Cirillo R, Zullo F. The efficacy of inositol and n-acetyl cysteine administration (Ovaric HP) in improving the ovarian function in infertile women with PCOS with or without insulin resistance. Obstet Gynecol Int. 2014;2014:141020.
Gerli S, Mignosa M, Di Renzo GC. Effects of inositol on ovarian function and metabolic factors in women with PCOS: a randomized double blind placebo-controlled trial. Eur Rev Med Pharmacol Sci. 2003;7:151–9 PMID: 15206484.
Colodny L, Hoffman RL. Inositol–clinical applications for exogenous use. Altern Med Rev. 1998;3:432–47 PMID: 9855568.
Lagana AS, Barbaro L, Pizzo A. Evaluation of ovarian function and metabolic factors in women affected by polycystic ovary syndrome after treatment with D-Chiro-Inositol. Arch Gynecol Obstet. 2015;291:1181–6.
Luo Y, Qin G, Zhang J, Liang Y, Song Y, Zhao M, Tsuge T, Aoyama T, Liu J, Gu H, Qu LJ. D-myo-inositol-3-phosphate affects phosphatidylinositol-mediated endomembrane function in Arabidopsis and is essential for auxin-regulated embryogenesis. Plant Cell. 2011;23:1352–72.
Teng F, You R, Hu M, Liu W, Wang L, Tao Y. Production of D-glucuronic acid from myo-inositol using Escherichia coli whole-cell biocatalyst overexpressing a novel myo-inositol oxygenase from Thermothelomyces thermophila. Enzyme Microb Technol. 2019;127:70–4.
Tanaka K, Takanaka S, Yoshida K. A second-generation Bacillus cell factory for rare inositol production. Bioengineered. 2014;5:331–4.
Tanaka K, Natsume A, Ishikawa S, Takenaka S, Yoshida KI. A new-generation of Bacillus subtilis cell factory for further elevated scyllo-inositol production. Microb Cell Fact. 2017;16:67.
Liu Y, Gong X, Wang C, Du G, Chen J, Kang Z. Production of glucaric acid from myo-inositol in engineered Pichia pastoris. Enzyme Microb Technol. 2016;91:8–16.
Magala M, Kohajdova Z, Karovicova J. Degradation of phytic acid during fermentation of cereal substrates. J Cereal Sci. 2015;61:94–6.
Febles CI, Arias A, Hardisson A, Rodriquez-Alvarez C, Sierra A. Phytic acid level in wheat flours. J Cereal Sci. 2002;36:19–23.
Lu Y, Wang L, Teng F, Zhang J, Hu M, Tao Y. Production of myo-inositol from glucose by a novel trienzymatic cascade of polyphosphate glucokinase, inositol 1-phosphate synthase and inositol monophosphatase. Enzyme Microb Technol. 2018;112:1–5.
Fujisawa T, Fujinaga S, Atomi H. An in vitro enzyme system for the production of myo-inositol from starch. Appl Environ Microbiol. 2017;83:e00550.
You C, Shi T, Li YJ, Han PP, Zhou XG, Zhang YHP. An in vitro synthetic biology platform for the industrial biomanufacturing of myo-inositol from starch. Biotechnol Bioeng. 2017;114:1855–64.
Zhang YP, Sun J, Ma Y. Biomanufacturing: history and perspective. J Ind Microbiol Biotechnol. 2017;44:773–84.
Brockman IM, Prather KLJ. Dynamic knockdown of E. coli central metabolism for redirecting fluxes of primary metabolites. Metab Eng. 2015;28:104–13.
Zhang S, Yang W, Chen H, Liu B, Lin B, Tao Y. Metabolic engineering for efficient supply of acetyl-CoA from different carbon sources in Escherichia coli. Microb Cell Fact. 2019;18:130.
Lunin VV, Li Y, Schrag JD, Iannuzzi P, Cygler M, Matte A. Crystal structures of Escherichia coli ATP-dependent glucokinase and its complex with glucose. J Bacteriol. 2004;186:6915–27.
Hernandez-Montalvo V, Martinez A, Hernandez-Chavez G, Bolivar F, Valle F, Gosset G. Expression of galP and glk in a Escherichia coli PTS mutant restores glucose transport and increases glycolytic flux to fermentation products. Biotechnol Bioeng. 2003;83:687–94.
Fraenkel DG, Falcoz-Kelly F, Horecker BL. The utilization of glucose 6-phosphate by Glucokinaseless and wild-type strains of Escherichia Coli. Proc Natl Acad Sci USA. 1964;52:1207–13.
Keseler IM, Collado-Vides J, Santos-Zavaleta A, Peralta-Gil M, Gama-Castro S, Muniz-Rascado L, Bonavides-Martinez C, Paley S, Krummenacker M, Altman T, et al. EcoCyc: a comprehensive database of Escherichia coli biology. Nucleic Acids Res. 2011;39:D583–90.
Ghosh A, Charalampous F, Sison Y, Borer R. Metabolic function of myo-inositol. I. Cytological and chemical alterations in yeast resulting from inositol deficiency. J Biol Chem. 1960;235:2522–8.
Lehle L, Tanner W. The function of myo-inositol in the biosynthesis of raffinose. Purification and characterization of galactinol:sucrose 6-galactosyltransferase from Vicia faba seeds. Eur J Biochem. 1973;38:103–10.
Holub BJ. The nutritional significance, metabolism, and function of myo-inositol and phosphatidylinositol in health and disease. Adv Nutr Res. 1982;4:107–41.
Wu G, Yan Q, Jones JA, Tang YJ, Fong SS, Koffas MAG. Metabolic burden: cornerstones in synthetic biology and metabolic engineering applications. Trends Biotechnol. 2016;34:652–64.
Lee SY, Kim HU. Systems strategies for developing industrial microbial strains. Nat Biotechnol. 2015;33:1061–72.
Yadav VG, De Mey M, Lim CG, Ajikumar PK, Stephanopoulos G. The future of metabolic engineering and synthetic biology: towards a systematic practice. Metab Eng. 2012;14:233–41.
Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6:343–U341.
Yamamoto N, Nakahigashi K, Nakamichi T, Yoshino M, Takai Y, Touda Y, Furubayashi A, Kinjyo S, Dose H, Hasegawa M, et al. Update on the Keio collection of Escherichia coli single-gene deletion mutants. Mol Syst Biol. 2009;5:335.
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2006(2):0008. https://doi.org/10.1038/msb4100050.
Thomason LC, Costantino N, Court DL. E. coli genome manipulation by P1 transduction. Curr Protoc Mol Biol. 2007. https://doi.org/10.1002/0471142727.mb0117s79.
Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–5.
Jiang Y, Chen B, Duan C, Sun B, Yang J, Yang S. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl Environ Microbiol. 2015;81:2506–14.
Acknowledgements
This work was supported by National Natural Science Foundation of China Grant 31670051. We thank James Allen, DPhil, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
National Natural Science Foundation of China Grant 31670051.
Author information
Authors and Affiliations
Contributions
RY and LW carried out the main work and analyzed the data. MH and CS supervised the work and analyzed the data. LW and YT participated in the conception and design of the study and finalized the manuscript. HC and SZ participated in the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1
Primers used in this study. Fig. S1. SDS-PAGE for expressions of IPS and IMP by optimizing plasmid expression systems. Fig. S2. The expressions of key enzymes of engineered strain R04 in 1-L fermenter for high-density fermentation. Fig. S3. Inositol production by strains R12, R14 and R15. a SDS-PAGE showing the expression of IPS (approximately 60 kDa) and IMP (approximately 30 kDa). b Production of inositol by strains R14, R12 and R15 cultivated in high-density fermentation and in shaken flasks, respectively. The columns correspond to “concentration of inositol”, and the lines and symbols correspond to “stoichiometric yield”. The recombinant strains were induced and harvested, then suspended in a bioconversion mixture containing 50 mM glucose. The bioconversions were performed at 37 °C and 220 rpm for 10 h. Fig. S4. Comparison of strains R01 and R04. a Production of inositol in strains R01 and R04 transformed with plasmids p01 and p02. The recombinant strains were induced and harvested, then suspended in a bioconversion mixture containing 50 mM glucose. The bioconversions were performed at 37 °C and 220 rpm for 10 h. b High-density fermentation of strains R01 and R04. Cells were cultured in LB medium at 37 °C. Then, this seed solution was inoculated into 500 mL inorganic medium with glycerol and glucose as mixed carbon source in a 1-L fermenter. Fig. S5. HPLC chromatograms of G-6P, glucose and inositol. The x-axis shows retention time and the y-axis the refractive index detector (RID) signal.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
You, R., Wang, L., Shi, C. et al. Efficient production of myo-inositol in Escherichia coli through metabolic engineering. Microb Cell Fact 19, 109 (2020). https://doi.org/10.1186/s12934-020-01366-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-020-01366-5