Abstract
Background
Alpha-glucosidase inhibitors (AGIs) have been shown to reduce incident type 2 diabetes but their impact on cardiovascular (CV) disease remains controversial. We sought to identify the overall impact of AGIs with respect to incident type 2 diabetes in individuals with impaired glucose tolerance (IGT), and CV outcomes in those with IGT or type 2 diabetes.
Methods
We used PubMed and SCOPUS to identify randomized controlled trials reporting the incidence of type 2 diabetes and/or CV outcomes that had compared AGIs with placebo in populations with IGT or type 2 diabetes, with or without established CV disease. Eligible studies were required to have ≥ 500 participants and/or ≥ 100 endpoints of interest. Meta-analyses of available trial data were performed using random effects models to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for incident type 2 diabetes and CV outcomes.
Results
Of ten trials identified, three met our inclusion criteria for incident type 2 diabetes and four were eligible for CV outcomes. The overall HR (95% CI) comparing AGI with placebo for incident type 2 diabetes was 0.77 (0.67–0.88), p < 0.0001, and for CV outcomes was 0.98 (0.89–1.10), p = 0.85. There was little to no heterogeneity between studies, with I2 values of 0.03% (p = 0.43) and 0% (p = 0.79) for the two outcomes respectively.
Conclusions
Allocation of people with IGT to an AGI significantly reduced their risk of incident type 2 diabetes by 23%, whereas in those with IGT or type 2 diabetes the impact on CV outcomes was neutral.
Similar content being viewed by others
Background
Alpha-glucosidase inhibitors (AGIs) such as acarbose, miglitol and voglibose are oral drugs used in the management of diabetes, primarily to reduce post-prandial glucose concentrations. Their use in individuals with impaired glucose tolerance (IGT) has been shown to delay progression to diabetes, but their effects on cardiovascular (CV) outcomes remain uncertain [1]. A previous systematic review evaluated the effects of acarbose on various outcomes, including CV, but it did not include a meta-analysis of randomized controlled trials [2].
STOP-NIDDM was a placebo-controlled randomized trial conducted in centres across Canada, Germany, Austria, Norway, Denmark, Sweden, Finland, Israel, and Spain that randomly allocated participants with IGT to 100 mg acarbose or placebo three times daily [3]. The trial showed a 25% reduction in the incidence of type 2 diabetes with acarbose, compared with placebo (hazard ratio (HR) 0.75, 95% confidence interval (CI) 0.63–0.90, p = 0.001) over a mean follow-up of 3.3 years. A pre-specified secondary analysis, also showed that there was a 49% reduction in cardiovascular outcomes over the same time period (HR 0.51 [0.28–0.95], p = 0.03) suggesting that acarbose might confer cardio protection, although this finding was based on only 47 CV events in total. Similarly, a meta-analysis of seven studies conducted between 1987 and 1999 reported a reduction in myocardial infarction (MI) in individuals assigned acarbose compared with placebo, but was based on only 28 events [4]. When a composite CV outcome (MI, stroke, CV death, angina or coronary revascularization) was examined, a 35% reduction in events was demonstrated based on 167 events in total [4].
The recently completed Acarbose Cardiovascular Evaluation (ACE) trial [5] was a randomized, double-blind, placebo-controlled, phase 4 study conducted in China that recruited patients with coronary heart disease and IGT. Participants were randomly allocated to acarbose (50 mg three times daily) or placebo, given in addition to fully-optimized CV secondary prevention therapy. The ACE trial showed that acarbose delayed progression to type 2 diabetes by 18% (odds ratio 0.82 (95% CI 0.71–0.94, p = 0.005) but was neutral with respect to major adverse cardiovascular events (HR 0.98, 0.86–1.11, p = 0.73).
The aim of this analysis was to identify the overall impact of AGIs with respect to incident type 2 diabetes specifically in individuals with IGT, and on combined CV outcomes in those with IGT or type 2 diabetes.
Methods
Data sources and searches
We conducted a meta-analysis of randomized placebo-controlled trials of AGIs in populations with IGT or type 2 diabetes, with or without established CV disease. Guidelines published by the Cochrane Collaboration for the conduct of a meta-analysis and the PRISMA checklist [6] for reporting were followed to ensure best practice. This meta-analysis was registered with PROSPERO, an international prospective register of systematic reviews. PROSPERO registration provides transparency in the review process and helps counter publication bias by providing a permanent record of prospectively registered reviews, whether they are eventually published or not. Our PROSPERO submission included publication of key information relating to the design and conduct of the meta-analysis [7].
Two reviewers (RLC, CABS) independently screened titles/abstracts and full texts for eligibility, assessed risk of bias, and collected data from each eligible study. Any reviewer disagreements were resolved by discussion. Ethics approval and patient consent were not required for these analyses.
We used PubMed and SCOPUS to conduct literature searches to identify relevant studies, with no language restrictions for trials from inception up to the 28th of February 2018. An initial search of published systematic reviews/meta-analyses concerning AGIs was performed to identify commonly used terms relating to AGIs. Pre-defined search terms (plus spelling variations) included “alphaglucosidase inhibitor”, “acarbose”, “voglibose”, “miglitol”, “cardiovascular outcomes”, “type 2 diabetes”, “impaired glucose tolerance”, “postprandial hyperglycaemia”, “dysglycemia” and “randomized controlled trials”. For these analyses, only published articles were considered.
Study selection
The search results were filtered to include only those randomized controlled trials comparing an AGI with placebo on progression to type 2 diabetes and/or CV outcomes in individuals with IGT or type 2 diabetes, with or without a history of CV disease. To avoid the inclusion of short-term small-scale trials with little or no outcome data, we specified in advance that those selected should have a ≥ 500 human participants and/or ≥ 100 pre-defined cardiovascular/diabetes events, with at least 1 year of follow up. Trials reporting CV outcomes were required to include as a minimum all three components of a major adverse cardiovascular event (MACE-3) composite outcome defined as CV death, non-fatal MI or non-fatal stroke. Trials reporting diabetes outcomes were required to specify type 2 diabetes diagnosed by two successive glucose values (fasting plasma glucose [FPG] ≥ 7.0 mmol/l or 2-h post challenge plasma glucose [2hrPG] ≥ 11.1 mmol/l).
Data extraction and quality assessment
Sources of reporting bias, e.g. publication bias, language bias, citation bias, were examined using funnel plots.
Data synthesis and analysis
Study-level data for all eligible trials identified were extracted from their corresponding published papers. We performed a random effects meta-analysis, with each study weighted according to the inverse variance method. HRs and 95% CIs were calculated for both incident diabetes and CV outcomes. Possible heterogeneity between studies was examined using Cochrane’s Q-test and the I2 inconsistency index used to quantify the percentage of total variation across all the studies. A Q test P-value of < 0.05 indicates significant heterogeneity. I2 heterogeneity thresholds are defined as low (≤ 25%), moderate (26–49%) or high (≥ 50%) [8]. The same statistical methodologies were applied separately to incident diabetes and to CV outcomes. All analyses were performed using SAS version 9.4 and/or R version 3.4.0 [9].
Results
Incident diabetes
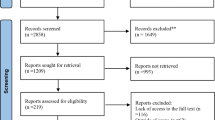
Our literature search identified 157 articles, of which there were ten trials that met the criteria for possible inclusion in the incident diabetes meta-analysis (Fig. 1), but only three remained eligible after detailed review. These were the Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM), [3] the voglibose Ph-3 Study, [10] and the ACE trial [5] (Table 1). Participants in these trials were at high risk of developing type 2 diabetes, but people experiencing a CV event in the pre-screening period (3 months for ACE, 6 months for the other 2 studies) were excluded. All three trials reported statistically significant relative risk reductions for incident diabetes in the treatment group, compared with the placebo group, of 25%, 40% and 18% for STOP-NIDDM, voglibose Ph-3 and ACE respectively. This equated to an overall 23% reduction for AGIs (HR 0.77, 95% CI 0.67–0.88, p < 0.0001), with an I2 value of 0.03% (p = 0.43) suggesting minimal heterogeneity between studies (Fig. 2), and the funnel plot (Additional file 1: Figure S1) indicating that publication bias is unlikely.
Cardiovascular outcomes
Of the 187 articles identified in the literature search, there were ten trials that met the criteria for possible inclusion in the CV outcomes meta-analysis (Fig. 1), but only four of these remained eligible after detailed review. These were the UK Prospective Diabetes Study (UKPDS), [11] STOP-NIDDM, [3] the Alpha-glucosidase-inhibitor Blocks Cardiac Events in Patients with Myocardial Infarction and Impaired Glucose Tolerance (ABC) trial, [12] and the ACE trial [5] (Table 1).
The CV outcome definition used in all four trials was MACE-3: plus unstable angina and congestive heart failure for UKPDS and ACE; plus angina, coronary revascularization, congestive heart failure and peripheral vascular disease for STOP-NIDDM; plus unstable angina and coronary revascularization for ABC. UKPDS, ABC and ACE showed no difference between treatment groups with respect to their CV outcome, with HRs ranging from 0.98 to 1.24 for AGI compared with placebo). STOP-NIDDM, however, showed a 49% reduction (HR 0.51, 95% CI 0.28–0.95, p = 0.03) but with only 47 composite CV events in total, compared with 100 in UKPDS and 949 in ACE. Overall, our meta-analysis showed no reduction in CV events for AGIs compared with placebo (HR 0.98, 95% CI 0.89–1.10, p = 0.85), with an I2 value of 0% (p = 0.79) showing no heterogeneity between studies (Fig. 3), and the funnel plot (Additional file 1: Figure S2) indicating that publication bias is unlikely.
Discussion
This meta-analysis shows an overall statistically significant 23% reduced risk of new-onset diabetes with AGIs in people with IGT (p < 0.0001). These results affirm the benefit of AGIs in reducing the risk of new-onset diabetes, and with acarbose licensed in China and 52 other countries for the treatment of IGT affords a pharmacological opportunity to help in the battle to contain the worldwide diabetes epidemic of type 2 diabetes [13].
AGIs, however, showed no overall impact of on CV outcomes, suggesting that the 49% risk reduction reported for the STOP-NIDDM [3] was most likely a chance finding, particularly with so few events for this secondary outcome, although this was a primary CVD prevention population mostly not taking cardioprotective medications such as statins and renin-angiotensin system inhibitors. An earlier meta-analysis of randomised controlled trials of alpha-glucosidase inhibitors suggested that they may prevent the progression if carotid intima thickness in patients with IGT or type 2 diabetes, [14] but our CV meta-analysis suggests this does not translate into fewer CV events. Our analysis was driven by the neutral outcome results reported by ACE [5] and UKPDS [11], with 949 and 200 events respectively, suggests that AGIs neither increase nor decrease the risk of major adverse cardiovascular events in people with IGT or diabetes, although given the mean follow-up for this analysis is 4.0 years the possibility that AGI use might reduce CV risk in the longer term cannot be excluded. Aggressive treatment of other cardiovascular risk factors, such as hypertension and dyslipidemia, as well as the use of antiplatelet therapy and inhibitors of the renin angiotensin system have resulted in significant reductions in CVD events in populations with [15] or without diabetes [16]. However, the impact of glycaemic reduction on CV disease is modest, [17] possibly making it difficult to detect the effect of some glucose-lowering agents, especially when added to optimized CV risk therapy. Recent studies have demonstrated cardio protection with sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists [18, 19] probably via non-glycaemic mechanisms. AGIs, however, might reduce CV risk indirectly in the longer term by delaying the onset of type 2 diabetes in people with IGT [20]. Such an effect was seen in the Chinese Da Qing study where a 6-year lifestyle intervention program which delayed the onset of type 2 diabetes was shown to be associated with an 11.9% reduction in CV death and a 28.1% reduction in all-cause mortality after 23 years follow-up [21].
Strengths of these analyses include the use of data from all randomized controlled trials reporting outcomes that could be compared, with a minimum 1-year follow-up to enable collection and adjudication of the required endpoints (>1600 incident diabetes events and > 1200 CV events). Our meta-analysis has several limitations. We used study-level rather than patient-level data, which is considered the gold standard for meta-analysis and which restricts our ability to investigate further any subgroups of interest.
Only a small number of eligible trials could be identified, although it is noteworthy that for CV outcomes no other trials with published CV outcomes would have been excluded by our selection criteria. For incident diabetes, a further two small-scale trials were identified but not included. These were the Early Diabetes Intervention programme (EDIP), which reported 62 events of progression to type 2 diabetes defined as FPG ≥ 7.8 mmol/l in 196 subjects followed for 5 years, [22] and the Dutch acarbose intervention study in persons with impaired glucose tolerance (DAISI) which reported 25 events of progression to diabetes in 118 individuals followed for 3 years based on a single glucose measurement [23]. Although these trials did not meet our inclusion criteria, we conducted a sensitivity analysis for incident diabetes which yielded a similar result (HR 0.74 [95% CI 0.67–0.82], p < 0.0001). In addition, the Cochrane collaboration recently published the results of trials reporting the incidence of type 2 diabetes, and separately CV mortality, non-fatal MI and non-fatal stroke in all populations without normal glucose levels (impaired fasting glucose, IGT or elevated HbA1c) [24], which yielded similar results to our analysis showing a type 2 diabetes incidence risk ratio of 0.73 (95% CI 0.59–0.90), but the combined MACE-3 endpoint was not reported.
Conclusion
This meta-analysis demonstrates that the overall impact of AGIs on CV outcomes is neutral, it is clear that they cannot be indicated for CV secondary prevention. To date, although many countries have licensed AGIs for use in IGT, very few currently approve any medication for diabetes prevention. Given that this meta-analysis demonstrates that allocation of people with IGT to an AGI can significantly reduce their risk of incident diabetes, AGIs should be considered as one approach to delaying or preventing new-onset diabetes in people with or without pre-existing CV disease.
Availability of data and materials
Not applicable.
Abbreviations
- AGI:
-
alpha glucosidase inhibitor
- IGT:
-
impaired glucose tolerance
- CV:
-
cardiovascular
- HR:
-
hazard ratio
- CI:
-
confidence interval
- MI:
-
myocardial infarction
- ACE:
-
acarbose cardiovascular evaluation
- MACE:
-
major adverse cardiovascular event
- FPG:
-
fasting plasma glucose
- 2hrPG:
-
two-hour post challenge plasma glucose
- STOP-NIDDM:
-
study to Prevent Non-Insulin-Dependent Diabetes Mellitus
- UKPDS:
-
uK Prospective Diabetes Study
- ABC:
-
alpha-glucosidase-inhibitor Blocks Cardiac Events in Patients with Myocardial Infarction and Impaired Glucose Tolerance
References
Xu J, Rajaratnam R. Cardiovascular safety of non-insulin pharmacotherapy for type 2 diabetes. Cardiovasc Diabetol. 2017;16:18.
Standl E, Theodorakis MJ, Erbach M, Schnell O, Tuomilehto J. On the potential of acarbose to reduce cardiovascular disease. Cardiovasc Diabetol. 2014;13:81.
Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, Group S-NTR. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–7.
Hanefeld M, Cagatay M, Petrowitsch T, Neuser D, Petzinna D, Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J. 2004;25:10–6.
Holman RR, Coleman RL, Chan JCN, Chiasson JL, Feng H, Ge J, Gerstein HC, Gray R, Huo Y, Lang Z, McMurray JJ, Ryden L, Schroder S, Sun Y, Theodorakis MJ, Tendera M, Tucker L, Tuomilehto J, Wei Y, Yang W, Wang D, Hu D, Pan C, Group ACES. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:877–86.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Holman R, Scott C, Coleman R. Effects of alpha-glucosidase inhibitors on cardiovascular (CV) outcomes and on progression to type 2 diabetes: a meta-analysis. PROSPERO 2018 CRD42018080127.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. https://www.R-project.org/.
Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Kaku K, Voglibose Ph-3 Study G. Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet. 2009;373:1607–14.
Holman RR, Cull CA, Turner RC. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (U.K. Prospective Diabetes Study 44). Diabetes Care. 1999;22:960–4.
Asakura M, Kim J, Asanuma H, Hamasaki T, Tsukahara K, Higashino Y, Ishikawa T, Nakama Y, Koba S, Maruyama Y, Tsujimoto M, Himeno H, Ohkusa T, Fujino S, Shimizu M, Endo T, Yoda S, Muroya T, Murohara T, Ohte N, Suzuki H, Kohno T, Fukui K, Shiono T, Takase H, Uzui H, Nagai Y, Hashimoto Y, Ikeda S, Mizuno S, Tamita K, Fujita M, Satake K, Kinoshita Y, Nunohiro T, Sakagami S, Higaki J, Morii I, Sawada R, Hiasa Y, Shigemasa T, Nakahama M, Sata M, Doi O, Ueda T, Yamada T, Yamanouchi T, Yamaguchi H, Morita Y, Hayashi H, Kitakaze M, Investigators ABC. Does treatment of impaired glucose tolerance improve cardiovascular outcomes in patients with previous myocardial infarction? Cardiovasc Drugs Ther. 2017;31:401–11.
Zimmet PZ. Diabetes and its drivers: the largest epidemic in human history? Clin Diabetes Endocrinol. 2017;3:1.
Geng DF, Jin DM, Wu W, Fang C, Wang JF. Effect of alpha-glucosidase inhibitors on the progression of carotid intima-media thickness: a meta-analysis of randomized controlled trials. Atherosclerosis. 2011;218:214–9.
Szummer K, Wallentin L, Lindhagen L, Alfredsson J, Erlinge D, Held C, James S, Kellerth T, Lindahl B, Ravn-Fischer A, Rydberg E, Yndigegn T, Jernberg T. Improved outcomes in patients with ST-elevation myocardial infarction during the last 20 years are related to implementation of evidence-based treatments: experiences from the SWEDEHEART registry 1995–2014. Eur Heart J. 2017;38:3056–65.
Rawshani A, Rawshani A, Franzen S, Sattar N, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, McGuire DK, Rosengren A, Gudbjornsdottir S. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379:633–44.
Control G, Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, Duckworth WC, Evans GW, Gerstein HC, Holman RR, Moritz TE, Neal BC, Ninomiya T, Patel AA, Paul SK, Travert F, Woodward M. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52:2288–98.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E-RO. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–288.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB, Committee LS, Investigators LT. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22.
Holman RR. What does the acarbose cardiovascular evaluation (ACE) trial tell us? J Diabetes. 2018;10:683–5.
Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, Yang W, Zhang B, Shuai Y, Hong J, Engelgau MM, Li H, Roglic G, Hu Y, Bennett PH. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014;2:474–80.
Kirkman MS, Shankar RR, Shankar S, Shen C, Brizendine E, Baron A, McGill J. Treating postprandial hyperglycemia does not appear to delay progression of early type 2 diabetes: the Early Diabetes Intervention Program. Diabetes Care. 2006;29:2095–101.
Nijpels G, Boorsma W, Dekker JM, Kostense PJ, Bouter LM, Heine RJ. A study of the effects of acarbose on glucose metabolism in patients predisposed to developing diabetes: the Dutch acarbose intervention study in persons with impaired glucose tolerance (DAISI). Diabetes Metab Res Rev. 2008;24:611–6.
Moelands SV, Lucassen PL, Akkermans RP, De Grauw WJ, Van de Laar FA. Alpha-glucosidase inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2018;12:CD005061.
Acknowledgements
RRH is an Emeritus NIHR Senior Investigator.
Funding
The ACE study was funded by Bayer AG.
Author information
Authors and Affiliations
Contributions
RLC contributed to the literature search, design of the study and wrote the first draft of the manuscript. CABS contributed to the literature search and performed the analyses. ZL, MAB, JT and RRH contributed to the study design and editing of the manuscript. All authors reviewed the manuscript. RRH is the guarantor of this work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests with respect to the research, authorship, and/or publication of this review. ZL is an employee of Bayer Healthcare, China.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
Funnel plot of included incident diabetes trials. Figure S2. Funnel plot of included cardiovascular outcome trials.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Coleman, R.L., Scott, C.A.B., Lang, Z. et al. Meta-analysis of the impact of alpha-glucosidase inhibitors on incident diabetes and cardiovascular outcomes. Cardiovasc Diabetol 18, 135 (2019). https://doi.org/10.1186/s12933-019-0933-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-019-0933-y