Abstract
Background
The primary lung function endpoint in clinical trials in adolescent and adult patients with asthma is usually forced expiratory volume in one second (FEV1). The objective of our analysis was to assess whether peak expiratory flow (PEF) is a suitable alternative primary lung function endpoint.
Methods
For this assessment, we calculated post hoc the correlation between pre-dose FEV1 and pre-dose PEF measured under supervision in the clinic and, for both lung function parameters, the correlations between supervised clinic and unsupervised home measurements, using the results from the 8 Phase III parallel-group trials of the global clinical development programme with tiotropium Respimat® in patients with asthma aged 12 to 75 years.
Results
Across all 8 trials included in this analysis, changes in lung function from baseline correlated well between pre-dose FEV1 and pre-dose PEF when both were measured under supervision in the clinic. Correlation between supervised in-clinic and unsupervised home measurements was stronger for pre-dose PEF than for pre-dose FEV1.
Conclusions
Pre-dose PEF measured at home could be an alternative primary lung function endpoint for trials in adolescent and adult patients with asthma. Using home-measured PEF could facilitate trial conduct and improve the convenience for patients by relocating scheduled assessments from the clinic to the patient’s home.
Trial registration
Adolescents aged 12 to 17 years: RubaTinA-asthma® (NCT01257230), PensieTinA-asthma® (NCT01277523).
Adults aged 18 to 75 years: GraziaTinA-asthma® (NCT01316380), MezzoTinA-asthma® (NCT01172808/NCT01172821), CadenTinA-asthma® (NCT01340209), PrimoTinA-asthma® (NCT00772538/NCT00776984).
All from Clinicaltrials.gov (https://clinicaltrials.gov/).
Similar content being viewed by others
Background
Spirometry is one of the fundamental outcome measures used in asthma studies. It provides an objective and highly reproducible measure of airflow limitation caused by smooth muscle contraction or structural changes [1]. Forced expiratory volume in 1 s (FEV1) is recommended as the primary endpoint for studies of bronchodilator therapy by the American Thoracic Society (ATS) and the European Respiratory Society (ERS) in their official statement on asthma control and exacerbations [1]. Pre-bronchodilator FEV1, i.e. the FEV1 recorded after withholding bronchodilators for their duration of action, is a strong, independent predictor of future exacerbation risk, and has been used in the majority of asthma clinical trials as the primary lung function endpoint in recent decades [1]. This is in line with regulatory recommendations for clinical trials in asthma that also consider pre-bronchodilator FEV1 as the most suitable variable [2].
Peak expiratory flow (PEF) is also an accepted spirometric measure that provides information about the level of airflow obstruction, both initially and in clinical trials to monitor asthma control and treatment responses [3]; however, it is generally considered more appropriate for home monitoring of lung function [2].
Both FEV1 and PEF can be measured under supervision in the clinic or unsupervised at home. In clinical trials, home measurements could increase the convenience and reduce the time and logistical burden for trial participants. So far, home-measured FEV1 or PEF have mainly been used in studies to provide complementary information to symptom diaries or clinic FEV1 [1].
The main objective of our analysis was to assess whether PEF, measured either at home or in the clinic, could be used as an alternative lung function endpoint in asthma clinical trials. In addition, the suitability of home-measured FEV1 as a lung function endpoint was investigated. For this assessment, we calculated post hoc the correlation between pre-dose FEV1 and pre-dose PEF measured under supervision in the clinic and, for both lung function parameters, the correlations between supervised clinic and unsupervised home measurements, using the results from the 8 Phase III parallel-group trials of the global clinical development programme with tiotropium Respimat® in patients with asthma aged 12–75 years. Tiotropium Respimat® has demonstrated improvements in lung function, asthma exacerbation risk and asthma control, is approved in the European Union [4] and in the United States [5], and is indicated as an add-on maintenance bronchodilator treatment in patients aged 6 years and older with severe asthma who experienced one or more severe asthma exacerbations in the preceding year.
Methods
Trial design and trial population
This exploratory post hoc analysis included lung function data from all Phase III parallel-group trials of the global Boehringer Ingelheim programme of tiotropium Respimat® in asthma in patients aged 12 years and older [6,7,8,9,10,11]. These were 8 randomised, double-blind, placebo-controlled trials of between 12 and 52 weeks’ duration. All trials included once-daily tiotropium Respimat® 5 μg and placebo, 6 trials also included once-daily tiotropium Respimat® 2.5 μg, and 2 trials included twice-daily salmeterol as a fourth treatment arm. All trial medication was administered as add-on to ICS, with or without other controller medications such as long-acting β2-agonists (LABAs) or leukotriene receptor antagonists (LTRAs). Out of a total of 4550 treated patients aged 12 to 75 years with symptomatic persistent asthma of different severities, 4525 patients had baseline and at least 1 on-treatment efficacy measurement, and were evaluated for efficacy. Further details on the trial design, the required minimum maintenance therapy and the treatment groups are summarised in Table 1.
Lung function assessments
Supervised measurements of FEV1 and PEF at clinic visits were performed for all trials, except PrimoTinA-asthma®, using MasterScope® computed tomography spirometers (eResearch Technology [ERT]). For PrimoTinA-asthma®, only FEV1 was measured under supervision, using the sites’ own equipment; PEF was not measured under supervision. For all trials, spirometers and their use, including daily calibration, had to meet the ATS/ERS criteria [12]. Pulmonary function tests were to be performed at approximately the same time of the day before administration of maintenance ICS therapy and trial medication.
Unsupervised measurements of FEV1 and PEF at home were performed using an electronic peak flow meter (Asthma Monitor® [ERT]). All patients were trained in the use of the device at the screening and randomisation visits in the clinic. For all trials, pulmonary function tests were to be performed at approximately the same time of the day, prior to administration of maintenance ICS therapy and trial medication.
For both supervised and unsupervised measurements, the highest FEV1 and PEF values out of 3 acceptable manoeuvres (not necessarily from the same manoeuvre) were used for the evaluation.
Correlation analyses
We analysed the correlations between pre-dose FEV1 and pre-dose PEF measured under supervision in the clinic and between supervised in-clinic and unsupervised home measurements for both PEF and FEV1. For the correlation analyses, data of the two pairs of replicate trials in adults with moderate (MezzoTinA-asthma®) or severe (PrimoTinA-asthma®) asthma were pooled; the other analyses were performed by trial. Since in PrimoTinA-asthma® no in-clinic measurement of pre-dose PEF was performed, correlation analyses in these pooled trials were limited to pre-dose FEV1.
For the calculation of the correlation coefficients, the response values (i.e. the change from baseline of all treatment groups of the respective trial) were considered. As tiotropium is a long-acting bronchodilator with once-daily dosing, the lung function parameters measured at the end of the dosing interval are relevant to support efficacy. Therefore, the pre-dose values were used for the correlation assessment, although, in most of the trials, both FEV1 peak and trough were included as primary and key secondary lung function endpoints. For the in-clinic measured values, the pre-dose values included in the calculation were those assessed just prior to the next dose. For the home-measured values, the weekly means of the values assessed daily prior to dosing were used, i.e. morning FEV1 and morning PEF for the trials with morning dosing, and evening FEV1 and evening PEF for the trials with evening dosing.
The statistical measures used for the correlation analyses are summarised in Fig. 1. For the two different lung function parameters, pre-dose FEV1 and pre-dose PEF, the Pearson correlation (PCC) was calculated [13]. For the different assessments (in-clinic vs. home) of the same variable (either pre-dose FEV1 or pre-dose PEF), the intraclass correlation (ICC) was calculated [13].
To assess the extent of correlation, the correlation coefficients (PCC or ICC) were interpreted as follows: > 0.9 to 1.0 very high, > 0.7 to 0.9 high, and > 0.5 to 0.7 moderate correlation.
Results
Across the trials, patients had a broad range of asthma severities. Key baseline demographics and disease characteristics are summarised by trial in Table 2. Within each trial, baseline demographics and disease characteristics were comparable between the treatment groups (see published manuscripts [6,7,8,9,10,11]). Most of the adolescent or adult patients were White or Asian and had never smoked. Mean duration of asthma was about 8 years in the trials in adolescents and from around 16 years in adult patients with mild persistent asthma to around 30 years in adult patients with severe persistent asthma. Lung function in terms of FEV1 and PEF at baseline was in line with the different ranges of asthma severity. In summary, the patients were representative of adolescent and adult patients with different severities of persistent asthma in the real-world setting.
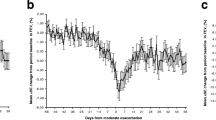
Correlation between pre-dose FEV1 and pre-dose PEF, when both parameters were measured under supervision in the clinic, was consistently high for all trials: the PCCs ranged from 0.773 to 0.852 across all trials at all time points; see Table 3 and Additional file 1: Table S1 for details. The scatter plots in Fig. 2 visualise the strong correlation between supervised pre-dose FEV1 and supervised pre-dose PEF at the time of primary efficacy evaluation across all trials; for CadenTinA-asthma®, which did not have a primary efficacy evaluation, Week 24 was used for the analysis.
Correlation analysis results between pre-dose FEV1 and pre-dose PEF, measured either under supervision in the clinic or unsupervised at home, at the time of primary efficacy evaluation. Phase III trials with tiotropium Respimat® in patients aged 12–75 years with persistent asthma – all patients analysed for efficacy. (a) In-clinic pre-dose FEV1 vs. in-clinic pre-dose PEF; (b) in-clinic pre-dose FEV1 vs. home-measured pre-dose FEV1a; (c) in-clinic pre-dose PEF vs. home-measured pre-dose PEFb. aHome-measured pre-dose FEV1: weekly mean morning FEV1 in the trials with morning dosing (PrimoTinA-asthma®) and weekly mean evening FEV1 in the trials with evening dosing (RubaTinA-asthma®, PensieTinA-asthma®, GraziaTinA-asthma®, MezzoTinA-asthma®, CadenTinA-asthma®), measured with AM device. bHome-measured pre-dose PEF: weekly mean morning PEF in the trials with morning dosing (PrimoTinA-asthma®) and weekly mean evening PEF in the trials with evening dosing (RubaTinA-asthma®, PensieTinA-asthma®, GraziaTinA-asthma®, MezzoTinA-asthma®, CadenTinA-asthma®), measured with AM device. PEF was not measured at clinic visits in the PrimoTinA-asthma® study. FEV1 = forced expiratory volume in one second; PEF = peak expiratory flow
Correlation between supervised in-clinic and unsupervised home measurements was generally higher for pre-dose PEF than for pre-dose FEV1. For pre-dose FEV1, the ICCs between in-clinic responses and home-measured weekly mean responses ranged from 0.558 to 0.840 across all trials and all time points (Table 3 and Additional file 1: Table S2). For pre-dose PEF, the ICCs between in-clinic responses and home-measured weekly mean responses ranged from 0.683 to 0.846 across all trials and all time points (Table 3 and Additional file 1: Table S3). For both variables, correlations between supervised in-clinic and unsupervised home measurements were higher in the trials in adults (ICCs pre-dose FEV1: 0.741 to 0.840, ICCs pre-dose PEF: 0.780 to 0.846) than in the trials in adolescents (ICCs pre-dose FEV1: 0.558 to 0.691, ICCs pre-dose PEF: 0.683 to 0.794); see Table 3 and Additional file 1: Tables S2 and S3. For the scatter plots that visualise the correlation at the time of primary efficacy evaluation (for CadenTinA-asthma®, Week 24 was used for the analysis) across all trials, see Fig. 2.
Discussion
The correlation analyses of pre-dose FEV1 and pre-dose PEF, measured under supervision in the clinic or unsupervised at home, were based on the data from 4525 patients aged 12 to 75 years who were evaluated for efficacy in the 8 Phase III parallel-group trials of the global clinical development programme with tiotropium Respimat® in asthma. Major strengths of these analyses are that the data originated from one clinical development programme, offering a high degree of consistency of trial design, and included a large number of patients, representing broad ranges of age, race, and asthma severities. However, the different trial designs and study durations mean that correlation data are not available for all time points for all studies.
Our results indicated a strong association between pre-dose FEV1 and pre-dose PEF when both parameters were measured under supervision in the clinic, with the PCC being greater than 0.773 across all trials and all time points. Although other studies have found only moderate correlations between PEF values and FEV1 [14], our results are consistent with another analysis based on data from more than 1,500 patients with asthma aged ≥15 years from two 1-year trials with montelukast, which found a mean PCC of 0.85 for the relationship between in-clinic FEV1 and in-clinic PEF [15]. These results support using PEF as a suitable lung function endpoint in clinical trials with asthma and a possible alternative to the more established endpoint of FEV1. Compared with FEV1, PEF has the advantage of being more broadly available to clinicians. A potential weakness of PEF, however, is that it lacks accurate reference values for many populations [1], and that reference values are specific to each brand of peak flow meter [3]. Both lung function parameters can be used to derive important information about the level of airflow obstruction initially, and in response to treatment [3].
In clinical trials, home measurements could simplify procedures and reduce the logistical burden for participating patients by decreasing the number of clinic visits required. A downside of home measurements could, however, be the dependency of the lung function values on the patient’s effort. A clear strength of ambulatory recordings of FEV1 or PEF is that these data provide objective and very frequent day-to-day measures of airway obstruction [1], and their weekly mean values offer robust data on patients’ lung function. When assessing the association between supervised in-clinic and unsupervised home measurements, the correlation was stronger for pre-dose PEF (ICC ≥0.683) than for pre-dose FEV1 (ICC ≥0.558) and for both parameters higher in adults (PEF: ICC ≥0.780, FEV1: ICC ≥0.741) than in adolescents (PEF: ICC ≥0.683, FEV1: ICC ≥0.558). This indicates that, as a lung function endpoint for self-measurement at home, PEF may be more suitable than FEV1. Home-measured PEF as an appropriate lung function endpoint for asthma trials is supported by the finding that longitudinal correlations between changes in asthma diary scores were stronger for average daily PEF than for weekly clinic FEV1 [16].
Trials in children aged < 12 years were not included in this analysis because, even with careful training, results from home spirometry in children may be less consistent [1]. However, it should be noted that home-measured PEF has been successfully used as a primary outcome measure in children previously [17].
Our results support the use of home-measured PEF in clinical asthma trials in adolescent and adult patients, potentially not only as a secondary or further outcome variable as recommended for National Institutes of Health-initiated clinical research [14], but also for consideration as a primary outcome variable. This could improve patients’ acceptance and willingness to participate in clinical trials by facilitating procedures and reducing the logistical burden for them by relocating scheduled assessments from the clinic to their home. It also supports respiratory clinical trials that are more geared towards patient involvement or follow a real-world pragmatic approach, with the potential opportunity to recruit patients who would not have been able to participate otherwise. This finding would have to be implemented in regulatory guidelines.
Conclusions
In conclusion, this post hoc analysis supports pre-dose PEF, measured under supervision in the clinic or unsupervised at home, as an alternative primary lung function endpoint for trials in adolescent and adult patients with asthma.
Availability of data and materials
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ATS:
-
American Thoracic Society
- ERS:
-
European Respiratory Society
- ERT:
-
eResearch Technology
- FEV1 :
-
forced expiratory volume in 1 s
- ICC:
-
intraclass correlation coefficient
- ICS:
-
inhaled corticosteroid
- PCC:
-
Pearson correlation coefficient
- PEF:
-
peak expiratory flow
References
Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99.
European Medicines Agency. Guideline on the clinical investigation of medicinal products for the treatment of asthma (CHMP/EWP/2922/01 Rev. 1). 2015. https://www.ema.europa.eu/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-asthma_en.pdf. Accessed 28 June 2019.
US Department of Health and Human Services, National Institutes of Health, National Heart, Lung and Blood Institute. Expert panel report 3: guidelines for the diagnosis and management of asthma. 2007. https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf. Accessed 28 June 2019.
Boehringer Ingelheim Limited. Summary of product characteristics – Spiriva Respimat 2.5 microgram, inhalation solution. October 2018. https://mri.cts-mrp.eu/human/downloads/NL_H_0718_001_FinalPI.pdf. Accessed June 28, 2019.
U.S. Food and Drug Administration. Prescribing information for Spiriva® Respimat® (tiotropium bromide) inhalation spray, for oral inhalation. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021936s007lbl.pdf. Accessed 22 Oct 2018.
Hamelmann E, Bateman ED, Vogelberg C, Szefler SJ, Vandewalker M, Moroni-Zentgraf P, et al. Tiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trial. J Allergy Clin Immunol. 2016;138(2):441–50.e8.
Hamelmann E, Bernstein JA, Vandewalker M, Moroni-Zentgraf P, Verri D, Unseld A, et al. A randomised controlled trial of tiotropium in adolescents with severe symptomatic asthma. Eur Respir J. 2017;49:1601100.
Paggiaro P, Halpin DM, Buhl R, Engel M, Zubek VB, Blahova Z, et al. The effect of tiotropium in symptomatic asthma despite low- to medium-dose inhaled corticosteroids: a randomized controlled trial. J Allergy Clin Immunol Pract. 2016;4(1):104–13.e2.
Kerstjens HA, Casale TB, Bleecker ER, Meltzer EO, Pizzichini E, Schmidt O, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med. 2015;3(5):367–76.
Ohta K, Ichinose M, Tohda Y, Engel M, Moroni-Zentgraf P, Kunimitsu S, et al. Long-term once-daily tiotropium Respimat® is well tolerated and maintains efficacy over 52 weeks in patients with symptomatic asthma in Japan: a randomised, placebo-controlled study. PLoS One. 2015;10(4):e0124109.
Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367(13):1198–207.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38.
Liu J, Tang W, Chen G, Lu Y, Feng C, Tu XM. Correlation and agreement: overview and clarification of competing concepts and measures. Shanghai Arch Psychiatry. 2016;28(2):115–20.
Tepper RS, Wise RS, Covar R, Irvin CG, Kercsmar CM, Kraft M, et al. Asthma outcomes: pulmonary physiology. J Allergy Clin Immunol. 2012;129(Suppl 3):S65–87.
Shingo S, Zhang J, Reiss TF. Correlation of airway obstruction and patient-reported endpoints in clinical studies. Eur Respir J. 2001;17(2):220–4.
Santanello NC, Barber BL, Reiss TF, Friedman BS, Juniper EF, Zhang J. Measurement characteristics of two asthma symptom diary scales for use in clinical trials. Eur Respir J. 1997;10(3):646–51.
Meltzer EO, Pearlman DS, Eckerwall G, Uryniak T, DePietro M, Lampl K. Efficacy and safety of budesonide administered by pressurized metered-dose inhaler in children with asthma. Ann Allergy Asthma Immunol. 2015;115(6):516–22.
Acknowledgements
This study was supported by Boehringer Ingelheim.
Funding
This study was funded by Boehringer Ingelheim.
Author information
Authors and Affiliations
Contributions
All of the authors have made substantial contributions to study design, data acquisition, analysis or interpretation, drafting the article, or critically revising the content; and provided final approval of the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All studies included in this analysis were performed in accordance with the provisions of the Declaration of Helsinki (1996 version), in accordance with the International Conference on Harmonization Tripartite Guideline for Good Clinical Practice, and in accordance with applicable regulatory requirements and Boehringer Ingelheim Standard Operating Procedures. All patients provided written informed consent. This article does not report individual patient data; all data presented here are anonymised. The clinical trial protocols and the informed consent and patient information forms were reviewed and received approval/favorable opinion from a constituted local Institutional Review Board or an Independent Ethics Committee at each center prior to the start of the study.
Competing interests
D. M. G. H. reports personal fees from AstraZeneca, Chiesi and Pfizer, and grants and personal fees from Boehringer Ingelheim, GlaxoSmithKline and Novartis, outside the submitted work. T. C. reports grants and consultancy fees from AstraZeneca, Sanofi/Roche and Novartis, personal fees and consultancy fees from Genentech, and grants from Genentech, outside the submitted work. J. M. F. reports grants from Boehringer Ingelheim, paid directly to UBC, during the conduct of the study; and personal fees from Boehringer Ingelheim, outside the submitted work. E. O. M. reports personal fees from ALK, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Stallergenes, Meda, Merck, Mylan, Sanofi/Regeneron and Teva, outside the submitted work. W. P.-R., P. M.-Z. and M. E. are employees of Boehringer Ingelheim. L. Z.-P. was employed as a contractor by Boehringer Ingelheim during the conduct of the study and is now a contractor at Elderbrook Solutions GmbH.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Table S1. Correlation analysis results (Pearson correlation coefficient) at different time points between pre-dose FEV1 and pre-dose PEF, both measured under supervision in the clinic. Phase III trials with tiotropium Respimat® in patients aged 12–75 years with persistent asthma – all patients evaluated for efficacy. Table S2. Correlation analysis results (intraclass correlation coefficient) at different time points between supervised measurement in the clinic and unsupervised measurement at home for pre-dose FEV1. Phase III trials with tiotropium Respimat® in patients aged 12–75 years with persistent asthma – all patients evaluated for efficacy. Table S3. Correlation analysis results (intraclass correlation coefficient) at different time points between supervised measurement in the clinic and unsupervised measurement at home for pre-dose PEF. Phase III trials with tiotropium Respimat® in patients aged 12–75 years with persistent asthma – all patients evaluated for efficacy. (DOCX 20 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Halpin, D.M.G., Meltzer, E.O., Pisternick-Ruf, W. et al. Peak expiratory flow as an endpoint for clinical trials in asthma: a comparison with FEV1. Respir Res 20, 159 (2019). https://doi.org/10.1186/s12931-019-1119-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-019-1119-6