Abstract
Background
This study assessed the cost-effectiveness of indacaterol/glycopyrronium (IND/GLY) versus salmeterol/fluticasone (SFC) in chronic obstructive pulmonary disease (COPD) patients with moderate to very severe airflow limitation and ≥1 exacerbation in the preceding year.
Methods
A previously published and validated patient-level simulation model was adapted using clinical data from the FLAME trial and real-world cost data from the ARCTIC study. Costs (total monetary costs comprising drug, maintenance, exacerbation, and pneumonia costs) and health outcomes (life-years (LYs), quality-adjusted life-years (QALYs)) were projected over various time horizons (1, 5, 10 years, and lifetime) from the Swedish payer’s perspective and were discounted at 3% annually. Uncertainty in model input values was studied through one-way and probabilistic sensitivity analyses. Subgroup analyses were also performed.
Results
IND/GLY was associated with lower costs and better outcomes compared with SFC over all the analysed time horizons. Use of IND/GLY resulted in additional 0.192 LYs and 0.134 QALYs with cost savings of €1211 compared with SFC over lifetime. The net monetary benefit (NMB) was estimated to be €8560 based on a willingness-to-pay threshold of €55,000/QALY. The NMB was higher in the following subgroups: severe (GOLD 3), high risk and more symptoms (GOLD D), females, and current smokers.
Conclusion
IND/GLY is a cost-effective treatment compared with SFC in COPD patients with mMRC dyspnea grade ≥ 2, moderate to very severe airflow limitation, and ≥1 exacerbation in the preceding year.
Similar content being viewed by others
Summary
Indacaterol/glycopyrronium is more effective and cost saving vs salmeterol/fluticasone in Swedish COPD patients with a history of exacerbations.
Background
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease characterised by persistent respiratory symptoms and airflow limitation and is a major cause of morbidity and mortality throughout the world [1,2,3,4]. In the European Union, the total direct and indirect cost for COPD amounts to nearly €48 billion [5]. In Sweden, the prevalence of COPD was reported to be 16.2%; 6.8% men and 6.6% women aged ≥40 years had spirometric stage II and higher COPD [6]. The societal costs of COPD in Sweden are high, with total annual costs estimated to be €1.5 bn (SEK 13.9 bn) in 2010, where 35% accounted for direct costs and 65% for indirect costs [7]. A survey reported that subjects with moderate and severe/very severe COPD accounted for 37% and 3% of the studied population (subjects with COPD aged 39–84 years living in northern Sweden), but contributed to 80% of the total COPD costs in Sweden (66% and 14%, respectively) [7].
According to the international Global Initiative for Chronic Obstructive Lung Disease (GOLD) report, COPD treatment aims to reduce exacerbations and improve quality of life [1]. For that purpose, besides non-pharmacologic treatments, several medications are available including bronchodilators and inhaled corticosteroids. The 2017 GOLD report recommends the first line use of dual bronchodilators, such as combination of the long-acting β2-adrenergic agonist (LABA) indacaterol and the long-acting muscarinic antagonist (LAMA) glycopyrronium (IND/GLY), in the treatment of symptomatic COPD patients, regardless of their exacerbation risk [1]. In contrast, the use of inhaled corticosteroid (ICS)-containing combination therapies, such as salmeterol/fluticasone (SFC) may only be a first choice therapy in COPD patients with features of asthma [1]. Key evidence for this recent GOLD strategy comes from the FLAME trial which demonstrated the superiority of IND/GLY in significantly reducing the rate of moderate or severe COPD exacerbations by 17% vs salmeterol/fluticasone (SFC) and increasing time-to-first moderate or severe exacerbation in patients with dyspnoea modified Medical Research Council (mMRC) scale grade ≥ 2 and a documented history of ≥1 moderate or severe COPD exacerbations during the previous year [8]. In addition, the incidence of pneumonia was significantly lower in patients on IND/GLY than in those on SFC (3.2% vs. 4.8%, p = 0.02). This, along with the results demonstrated in the ILLUMINATE and LANTERN trial, indicates that IND/GLY addresses needs for both exacerbating and non-exacerbating patients, with a lower risk of pneumonia (the clinical significance of reduced incidence of pneumonia remains to be elucidated) than ICS-containing regimens [9, 10].
The Swedish health care system is financed by a social insurance that provides all citizens with subsidised healthcare through the government. For prescribed drugs fees to the user are capped at 2200 Swedish Krona (SEK) or around €230 per annum. The dual bronchodilator IND/GLY is approved and reimbursed in Sweden for the maintenance treatment for COPD patients remaining symptomatic on long-acting bronchodilator monotherapy [11].
Because COPD carries a significant health and economic burden, available therapies should be critically evaluated for their costs and benefits when making treatment decisions. Indeed, two previously conducted cost-effectiveness analyses (CEAs) have shown favourable cost-effectiveness of IND/GLY compared with SFC in patients with moderate-to-severe COPD and a history of one or no exacerbation in the previous year [12, 13]. Given changing drug treatment costs and the growing role of LABA/LAMAs in the GOLD 2017 strategy new economic evaluations are needed.
This analysis therefore aimed to determine the health economic impact of IND/GLY and SFC as competing treatment options in COPD patients with moderate to very severe airflow limitation and a history of ≥1 exacerbation in the preceding year.
Methods
Study design
A previously published and validated microsimulation model [14], was employed to assess the cost-effectiveness (a type of economic evaluation that compares relative costs and outcomes of two or more treatments) of IND/GLY compared with SFC, and was adapted for the present analysis by incorporating clinical data from the FLAME study and real-world Swedish cost data.
Perspective
The analysis was conducted from a Swedish payer’s perspective. Only direct costs were considered for the analysis.
Patient population
The FLAME study was a 52-week, phase IIIB, multi-centre, randomised, double-blind, double-dummy, parallel-group, non-inferiority trial that included adults aged ≥40 years, with a clinical diagnosis of COPD, with a mMRC score ≥ 2, a post-bronchodilator forced expiratory volume in 1 s (FEV1) of ≥25% predicted to <60% predicted, and a post-bronchodilator ratio of FEV1 to forced vital capacity (FVC) of <0.70 [8]. In addition, patients had a documented history of ≥1 COPD exacerbation during the previous year for which they had received treatment with systemic glucocorticoids, antibiotic agents, or both. Table 1 represents the baseline characteristics of the FLAME study population.
Model structure
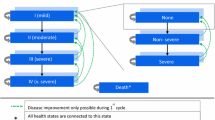
A patient-level simulation model was chosen over a cohort model because it is better suited to simultaneously account for different aspects of a patient’s profile such as smoking status, GOLD FEV1 status and exacerbation history, and it better reflects the heterogeneous disease progression in COPD patients [15]. Figure 1 shows the structure of the model. In-depth model mechanics and validation have been previously published and the model has been used in an earlier assessment of dual bronchodilation by IND/GLY [12,13,14]. This model was adapted to the Swedish setting using exacerbation and maintenance costs from the ARCTIC study, a large, real-world retrospective Swedish cohort study of 18,586 eligible primary care COPD patients [16,17,18]. Other inputs such as costs data, utilities and mortality data were derived from publicly available sources to compare IND/GLY with SFC.
Model structure. Figure notes: BMI: body mass index; FEV1: forced expiratory volume in 1 s; ICER: incremental cost-effectiveness ratio; ICUR: incremental cost-utility ratio; IND/GLY: indacaterol/glycopyrronium; NNT: number needed to treat; QALY: quality-adjusted life-year; SFC: salmeterol/fluticasone
Disease progression
A simulated cohort of 100,000 patients was assigned baseline characteristics derived from the FLAME trial. The model then generated patients based on the mean values and variance-covariance matrices derived from patient-level trial data. In the simulation, a generated patient moved through the model in cycles of 6 months, experiencing disease progression and clinical events based on their characteristics and pre-defined probabilities of experiencing events until death or the end of the time horizon. These clinical events included FEV1 decline, exacerbations and pneumonia events. A disease severity level was estimated at each cycle. The patient’s disease status was represented by their percent predicted FEV1 score, which was generated for each patient according to their baseline characteristics. Treatment-specific FEV1 improvements as reported in the FLAME trial were added to each treatment group. Increase over baseline FEV1 was assumed to be maintained over time. The patient’s FEV1 declined over time at a rate described for the general population studied by Falaschetti et al. [19]. As FEV1 declines, patients move into GOLD airflow limitation stages of increasing severity.
Exacerbations and pneumonia
An annualised rate of moderate and severe exacerbations adjusted for cycle length was applied, with a probability that a patient experienced either a moderate or severe exacerbation at each cycle (proportion calculation based on number of exacerbations/ total exacerbations as reported in the FLAME study). Though the FLAME study assessed all exacerbations including mild exacerbations, the present analyses only focused on moderate and severe exacerbations (see Additional file 1 for definitions). Pneumonia incidence rates and costs were calculated considering inclusion of an ICS comparator and the established evidence of risk of pneumonia with ICS use [20].
Time horizon
Cumulative costs and health outcomes for both IND/GLY and SFC were estimated using a lifetime horizon (considered to be 78 years when ~100% patients are dead) according to Swedish guidelines [21]. Treatment effects were assumed to be constant over the lifetime horizon. To better inform healthcare policies on the short-term, different time horizons (1, 5 and 10 years) were used in the scenario analyses.
Model input parameters
Efficacy
The efficacy inputs used in the analysis were derived from the FLAME trial for the modified intention-to-treat population (Table 2). The FLAME trial demonstrated superior efficacy of IND/GLY over SFC in reducing the annual rate of moderate and severe exacerbations, improvement in trough FEV1, health-related quality-of-life, and decrease in the use of rescue medication [8]. Definitions of exacerbation and pneumonia are provided in the Additional file 1.
The annualised rates of pneumonia-related events were derived from the incidence of pneumonia reported at 52 weeks and the total number of treatment years in the FLAME study by the following equation (Table 2).
Annualized rate = number of events/person years, where person years = ~N/2.
Costs
The cost items considered were drugs for COPD treatment, maintenance/monitoring, exacerbations (moderate and severe) and pneumonia events. Daily drug costs were derived from the Swedish Pharmaceutical Benefits Agency (TLV) [22]. Table 2 shows the drug costs (per day) used in the analysis. Both maintenance and exacerbation costs were sourced from a burden of illness analysis in the ARCTIC study (Table 2) [16]. The cost inputs used and their definitions are provided in the Additional file 1.
Moderate exacerbation costs comprised the following costs during 14 days after the exacerbation occurrence: outpatient visits, nurse visits, physician visits, oral steroids, and antibiotics targeted at respiratory diseases [16]. Moderate exacerbation costs are low and independent of the severity of airflow limitation. Severe exacerbation costs comprised all the components of moderate exacerbation costs and costs of hospital admissions [16]. There may be outpatient costs in patients hospitalized for exacerbations, corresponding to healthcare expenses occurring between discharge and day 14 after exacerbation onset.
Maintenance costs were defined as non-exacerbation related cost after the exclusion of COPD drug costs [16].
Pneumonia costs were based on three diagnosis-related group (DRG) codes (D47A, D47C and D47E) describing lung inflammation with three levels of complications [23]. As no case mix information was available, an average of all three was assumed (Table 2). Costs were inflated to the year 2015 where necessary using the Harmonized Indices of Consumer Prices [24] and are expressed in 2015 euros (€) using European Central Bank foreign exchange reference rates (2015 annual average SEK/€ rate, 9.35:1 or 1:0.107) [25].
Utilities
Utilities, which represent the strength of a society’s preference for specific health-related outcomes, were calculated at the end of each cycle depending on disease severity status and other characteristics, based on a regression model by Rutten-van Mölken et al. [26] (see Additional file 1). The co-variate values were informed by the characteristics of simulated patients in the model. Baseline characteristics, including gender, body mass index etc. were derived from the FLAME trial baseline data [8]. FEV1% predicted over the time horizon of the model is described under disease progression. ER visits and hospitalisation admissions were linked to the incidence of moderate exacerbations and severe exacerbations, respectively, predicted by the model for each comparator.
Both costs and health benefits were discounted annually at the rate of 3% according to Swedish guidelines [27].
Mortality
Swedish life tables from Statistics Sweden for 2015 were used to generate background all-cause mortality [28]. Overall mortality was calculated by applying a COPD specific hazard ratio of 1.02 based on the Obstructive Lung Disease in Northern Sweden COPD study [29]. Supplementary details can be found in the Additional file 1. This hazard ratio was adjusted by the predicted decline in FEV1 for an individual patient, using the following equation:
Probability of death = (general population risk for the appropriate age and gender) * 1.02^ (the decline in FEV1 percent predicted).
Exacerbations themselves, in fact, did not affect mortality in this model. Both the rate of exacerbations and COPD-related mortality rate were based upon FEV1 status.
Model outputs
The model outputs analysed in terms of health benefit were: life-years (LYs) and quality-adjusted life-years (QALYs). QALYs were calculated as a product of the quantity (LYs) and quality (utilities) of life lived. The model output in terms of cost was the total monetary cost which comprised drug, maintenance, exacerbation, and pneumonia costs. Net monetary benefit (NMB) was also estimated using the following formula [30]: NMB = (WTP*∆E) - ∆C, where WTP is the willingness to pay (per QALY) threshold, ΔE is the difference in effectiveness (e.g. number of QALYs) and ΔC is the difference in costs. NMB >0 would indicate that IND/GLY was cost-effective at the given WTP threshold.
Number needed to treat (NNT), which represents the average number of patients who need to be treated to prevent one patient from having an exacerbation was estimated for exacerbations. NNTs were calculated based on the following equation:
NNT = 1/ ((proportion benefiting from an intervention)-(proportion benefiting from a control)).
The NNT to prevent one severe exacerbation and one case of repeat exacerbation were also calculated and data are presented in the Additional file 1.
Sensitivity analyses
Both one-way sensitivity analyses and probabilistic sensitivity analyses (PSA) were performed to acknowledge the uncertainty of key model input values and to test the robustness of the results.
One-way sensitivity analyses were conducted by changing each model input value discretely by 25%, keeping other model input values constant to identify key parameters affecting the results. The model inputs studied in the one-way sensitivity analyses were: exacerbation rate ratio, exacerbation severity, drug costs, FEV1 benefit, exacerbation costs, baseline rate of exacerbations, maintenance costs, pneumonia costs, and discount rates (0, 2.5, 3.5, 4, 5, 7, and 10%, for both costs and effects).
Details of the PSA can be found in the Additional file 1.
Subgroup analyses
Subgroup analyses were also performed with respect to smoking (yes vs no), severity status (severity of airflow limitation (GOLD stages 1, 2, 3 and 4), and GOLD 2015-A, B, C and D categories based on symptoms, airflow limitation and risk of exacerbations) and gender (male vs female).
Results
Base case
IND/GLY was associated with lower costs and better outcomes compared with SFC over all the analysed time horizons of 1, 5, 10 years and lifetime. Compared with SFC, treatment with IND/GLY resulted in the addition of 0.192 LYs and 0.134 QALYs as well as cost savings of €1211 per patient over lifetime. Table 3 presents the results for the other time horizons (1, 5, and 10 years). As compared to the findings at 1 year, greater cost savings and more benefits were observed at the extended time horizons.
Fewer moderate and severe exacerbations were reported with the use of IND/GLY compared with SFC over all the time horizons (Table 4). Since severe exacerbations are defined as those requiring hospitalization in addition to treatment with systemic corticosteroids and/or antibiotics, the results imply that hospitalization rates tend to be lower with IND/GLY compared with SFC.
Furthermore, the incidence of pneumonia was lower among patients receiving IND/GLY compared to those receiving SFC over the lifetime horizon (0.39 vs. 0.58).
The NMB was estimated to be €8560 based on a WTP threshold of €55,000/QALY.
The NNT to prevent one moderate or severe exacerbation was estimated to be 5 (4.76), i.e., for every ~5 patients treated over 12 months with IND/GLY rather than SFC, on average, one exacerbation was avoided. Or, in other words, if ~5 patients with moderate to very severe airflow limitation and ≥1 exacerbation in the preceding year are treated with IND/GLY instead of SFC, one exacerbation can be prevented.
Uncertainty analyses
NMB was also positive after variation in the values of the following parameters: SFC exacerbation rate (1.07, 1.30), SFC cost per day (1.25, 2.08), IND/GLY FEV1 benefit (0.00, 0.03), exacerbation costs (25% variation) and pneumonia costs (25% variation) (Fig. 2) and when different discount rates were applied (data not shown). This indicates IND/GLY continues to be cost-effective under each of these alternative scenarios.
Results for the PSA are presented in the Additional file 1.
Subgroup analyses
At a WTP threshold of €55,000/QALY and considering the variables- severity, gender and smoking status, the NMB increased further in the following subgroups: severe (GOLD 3, 2011–2014 criteria), high risk and more symptoms (GOLD D, 2015 criteria), female gender, and current smokers (Fig. 3).
Discussion
Results of this analysis indicated that IND/GLY is cost saving with respect to reduction in exacerbation and pneumonia costs, and associated with favourable clinical effects such as reduced rate of moderate and severe exacerbations and lower incidence of pneumonia compared with SFC. These results were robust in uncertainty analyses. With various time horizons assessed, both immediate and lifelong benefits were observed with IND/GLY over SFC implying the beneficial use of IND/GLY for short-term or long-term policy planning. To highlight, the decrease in cost savings from 10 years to lifetime was due to increasing maintenance costs. At 10 years, IND/GLY is delaying transition to GOLD state IV and therefore is saving cost, but by lifetime almost all patients (who don’t die of comorbidities or other reasons) progress to GOLD IV so the cumulative savings in maintenance costs is less pronounced. Hence, greater drug costs for IND/GLY over this more distal time period results in overall costs being slightly higher for IND/GLY compared to SFC. Results from the pre-specified subgroup analyses also suggest that even in much targeted use, IND/GLY is the preferable treatment option compared to SFC.
Results of this study are in line with the previously conducted CEA in the Swedish setting, which suggested that IND/GLY is associated with cost savings and is more effective than SFC in moderate to severe COPD patients with no history of exacerbations [13]. Recent studies have also demonstrated cost-effectiveness of dual bronchodilators over monotherapies in patients with COPD [31, 32].
To the best of our knowledge, this is the first study to assess the cost-effectiveness of IND/GLY versus SFC utilising clinical data from the FLAME trial, which assessed COPD patients with moderate to very severe airflow limitation and ≥1 exacerbation in the preceding year. Results from the present analyses become more relevant with the recent recommendations from GOLD suggesting the first-line option of dual bronchodilators in many symptomatic COPD patients, regardless of their exacerbation risk. The subgroup analyses highlight specific populations in which IND/GLY can be an economic advantage over the use of SFC in settings with restrained budgets. The current analysis not only focused on the cost-effectiveness outcomes, but also on outcomes relevant for clinical practice such as exacerbations, pneumonia and NNT.
There are also several limitations to this study. Results from this analysis are relevant to patients resembling the studied population in the FLAME trial i.e. COPD patients with mMRC dyspnea grade ≥ 2, moderate to very severe airflow limitation and ≥1 exacerbation in the preceding year. We can only speculate that inhaler technique and lower adherence in real-life patients as compared to a clinical trial population would influence treatment effectiveness in a similar way for all maintenance treatments, hence not affecting differences between them; however, we acknowledge the lack of data on this specific point in the literature. The modelling approach used is largely in line with previously published models in COPD. However, despite recent recommendations, costs related to comorbidities and some adverse effects could not be included for IND/GLY and SFC separately due to lack of corresponding data [15]. While the most significant and relevant adverse events (exacerbations and pneumonia) were included in the model as reported in the FLAME study [8], other adverse events were omitted as the rates were small and similar between the two treatment groups while corresponding costs are low. Thus their inclusion would not meaningfully inform the cost-effectiveness analysis. Lastly, the study selected the Swedish implicit threshold of €55,000 (dependent on the unmet need and severity of the disease), for which no official reference is available.
Since the applicability of findings from a controlled environment to real-world settings is uncertain hence future research is recommended to follow-up clinical and economic implications of these findings in real life.
In patients with moderate to very severe COPD, LABA/ICS fixed-dose combination is known to reduce the frequency of COPD exacerbations [33, 34], and it is also no doubt that some patients may actually benefit from the addition of ICS. However, an indiscriminate and long-term use of ICS in these patients may expose them to an increased risk of developing pneumonia leading to increased associated healthcare costs [35]. Therefore, as recommended by GOLD, most patients with moderate to very severe airflow limitation and a history of exacerbations should be treated with LABA/LAMA combination before using LABA/ICS.
Until now, the cost-effectiveness of LABA/LAMA combination vs. LABA/ICS combination in COPD population with moderate to very severe airflow limitation and ≥1 exacerbation in the preceding year was uncertain. This study provides an answer to this so far unaddressed question, showing that from a health economic perspective, IND/GLY is not only cost-effective but should be the preferred treatment option compared to SFC in COPD patients with moderate to very severe airflow limitation and ≥1 exacerbation in the preceding year.
Conclusion
Under the current WTP threshold in Sweden, IND/GLY is a cost-effective treatment compared with SFC in COPD patients with mMRC dyspnea grade ≥ 2, moderate to very severe airflow limitation, and a history of exacerbations. Cost savings were observed with respect to reduction in exacerbation and pneumonia costs, and superior efficacy in terms of reduced rate of moderate and severe exacerbations and lower incidence of pneumonia. Sensitivity analyses performed considering key parameters also confirm the results for their robustness.
Abbreviations
- CEAs:
-
cost-effectiveness analyses
- COPD:
-
chronic obstructive pulmonary disease
- DRG:
-
diagnosis-related group
- FEV1:
-
forced expiratory volume in 1 s
- FVC:
-
forced vital capacity
- ICS:
-
inhaled corticosteroid
- IND/GLY:
-
indacaterol/glycopyrronium
- LABA:
-
long-acting β2-adrenergic agonist
- LAMA:
-
long-acting muscarinic antagonist
- mMRC:
-
modified Medical Research Council
- NMB:
-
net monetary benefit
- NNT:
-
Number needed to treat
- PSA:
-
probabilistic sensitivity analyses
- QALYs:
-
quality-adjusted-life-years
- SEK:
-
Swedish krona
- SFC:
-
salmeterol/fluticasone
References
Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2017. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/. Accessed 16 Nov 2016.
Chronic obstructive pulmonary disease (COPD) 2015. http://www.who.int/mediacentre/factsheets/fs315/en/. Accessed 17 Aug 2015.
Anzueto A, Sethi S, Martinez FJ. Exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4:554–64.
Lopez-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21:14–23.
ERS: The economic burden of lung disease. EUROPEAN LUNG White Book.
Danielsson P, Olafsdottir IS, Benediktsdottir B, Gislason T, Janson C. The prevalence of chronic obstructive pulmonary disease in Uppsala, Sweden--the burden of obstructive lung disease (BOLD) study: cross-sectional population-based study. Clin Respir J. 2012;6:120–7.
Jansson SA, Backman H, Stenling A, Lindberg A, Ronmark E, Lundback B. Health economic costs of COPD in Sweden by disease severity--has it changed during a ten years period? Respir Med. 2013;107:1931–8.
Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT, Thach C, Fogel R, Patalano F, Vogelmeier CF, Investigators F. Indacaterol-Glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374:2222–34.
Vogelmeier CF, Bateman ED, Pallante J, Alagappan VK, D'Andrea P, Chen H, Banerji D. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1:51–60.
Zhong N, Wang C, Zhou X, Zhang N, Humphries M, Wang L, Thach C, Patalano F, Banerji D. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:1015–26.
TLV Decision 2014. http://www.tlv.se/upload/beslut_2014/bes140227-ultibro-breezhaler.pdf. Accessed Nov 2016.
Reza Maleki-Yazdi M, Molimard M, Keininger DL, Gruenberger JB, Carrasco J, Pitotti C, Sauvage E, Chehab S, Price D. Cost Effectiveness of the Long-Acting beta2-Adrenergic Agonist (LABA)/Long-Acting Muscarinic Antagonist Dual Bronchodilator Indacaterol/Glycopyrronium Versus the LABA/Inhaled Corticosteroid Combination Salmeterol/Fluticasone in Patients with Chronic Obstructive Pulmonary Disease: Analyses Conducted for Canada, France, Italy, and Portugal. Appl Health Econ Health Policy. 2016;14:579–94.
Price D, Keininger D, Costa-Scharplatz M, Mezzi K, Dimova M, Asukai Y, Stallberg B. Cost-effectiveness of the LABA/LAMA dual bronchodilator indacaterol/glycopyrronium in a Swedish healthcare setting. Respir Med. 2014;108:1786–93.
Asukai Y, Baldwin M, Fonseca T, Gray A, Mungapen L, Price D. Improving clinical reality in chronic obstructive pulmonary disease economic modelling : development and validation of a micro-simulation approach. PharmacoEconomics. 2013;31:151–61.
van der Schans S, Goossens LM, Boland MR, Kocks JW, Postma MJ, van Boven JF, Rutten-van Molken MP. Systematic review and quality appraisal of cost-effectiveness analyses of pharmacologic maintenance treatment for chronic obstructive pulmonary disease: methodological considerations and recommendations. Pharmacoeconomics. 2016;
The economic burden of COPD in a Swedish cohort: The ARCTIC study 2016. http://www.nature.com/article-assets/npg/npjpcrm/abstracts/npjpcrm201622.pdf. Accessed 3 Aug 2016.
ICS and risk of pneumonia in Swedish COPD patients: ARCTIC 2016. http://www.nature.com/article-assets/npg/npjpcrm/abstracts/npjpcrm201622.pdf. Accessed 3 Aug 2016.
The prevalence of comorbidities in Swedish COPD and non-COPD patients: The ARCTIC study 2016. http://www.nature.com/article-assets/npg/npjpcrm/abstracts/npjpcrm201622.pdf. Accessed 3 Aug 2016.
Falaschetti E, Laiho J, Primatesta P, Purdon S. Prediction equations for normal and low lung function from the health survey for England. Eur Respir J. 2004;23:456–63.
Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68:1029–36.
Application for pharmaceutical benefits scheme. 2017. https://www.tlv.se/Upload/Foretag/Handbok_foretag_ansokan_subvention_pris_lakemedel.pdf. Accessed Aug 2017.
TLV 2016. http://www.tlv.se/beslut/sok/lakemedel/. Accessed 22 June 2016.
Regionala priser och ersättningar för södra sjukvårdsregionen 2016. http://sodrasjukvardsregionen.se/avtal-priser/regionala-priser-och-ersattningar-foregaende-ar/. Accessed 7 Dec 2017.
Eurostat 2016. http://ec.europa.eu/eurostat/web/hicp/data/database. Accessed 9 Nov 2016.
FLAME News release November 2015. https://www.novartis.com/news/news-archive?type=press_release&language=en&page=2. Accessed 24 Mar 2016.
Rutten-van Molken MP, Oostenbrink JB, Tashkin DP, Burkhart D, Monz BU. Does quality of life of COPD patients as measured by the generic EuroQol five-dimension questionnaire differentiate between COPD severity stages? Chest. 2006;130:1117–28.
Swedish Agency for Health Technology Assessment and Assessment of Social Services 2016. http://www.sbu.se/en/. Accessed Dec 2016.
Swedish life tables for 2015. 2015. http://www.scb.se/. Accessed 22 June 2016.
Lindberg A, Larsson LG, Muellerova H, Ronmark E, Lundback B. Up-to-date on mortality in COPD - report from the OLIN COPD study. BMC Pulm Med. 2012;12:1.
Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW: Methods for the economic evaluation of health care programmes. Oxford university press; 2015.
Miravitlles M, Galdiz JB, Huerta A, Villacampa A, Carcedo D, Garcia-Rio F. Cost-effectiveness of combination therapy umeclidinium/vilanterol versus tiotropium in symptomatic COPD Spanish patients. Int J Chron Obstruct Pulmon Dis. 2016;11:123–32.
van Boven JF, Kocks JW, Postma MJ. Cost-effectiveness and budget impact of the fixed-dose dual bronchodilator combination tiotropium-olodaterol for patients with COPD in the Netherlands. Int J Chron Obstruct Pulmon Dis. 2016;11:2191–201.
Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012:CD006829.
Nannini LJ, Poole P, Milan SJ, Kesterton A. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013:CD006826.
Price D, Yawn B, Brusselle G, Rossi A. Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Prim Care Respir J. 2013;22:92–100.
Acknowledgements
The authors would like to thank Purnima Pathak (Novartis) for managing and providing writing assistance in the development of this manuscript. The authors would also like to thank Colin Burke (Health Economic Modeller; Novartis) for the modelling support.
Funding
This study was funded by Novartis Pharma AG (Basel, Switzerland).
Availability of data and materials
Please refer the additional file.
Authors’ contributions
All authors have been involved in reviewing and approving the manuscript. In addition, all authors were responsible for the adaptation of the cost-effectiveness model.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Leif Bjermer has attended advisory board and received lecture fees from ALK, AIrsonette, AZ, Boehringer, Chiesi, GSK, Novartis, Takeda and Teva. Job F. M. van Boven has received grants from AstraZeneca, GSK, Boehringer Ingelheim, consultancy fees from AstraZeneca and lecture fees from Menarini, and reports non-financial support from Respiratory Effectiveness Group and European COPD Coalition, outside the submitted work. Madlaina Costa-Scharplatz, Dorothy L. Keininger, Florian S. Gutzwiller, Ronan Mahon, and Petter Olsson are employees of Novartis. Karin Lisspers has received personal fees from AstraZeneca, Novartis, GlaxoSmithKline, and Teva for lectures, educational activities, and scientific committee for studies outside the submitted work. Nicolas Roche has received grants and personal fees from Boehringer Ingelheim, Novartis, Teva, GSK, AstraZeneca, Chiesi, Mundipharma, Cipla, Pfizer, Sanofi, Sandoz, 3 M, and Zambon, outside the submitted work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Methods and Results (additional details) (ZIP 535 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bjermer, L., van Boven, J.F.M., Costa-Scharplatz, M. et al. Indacaterol/glycopyrronium is cost-effective compared to salmeterol/fluticasone in COPD: FLAME-based modelling in a Swedish population. Respir Res 18, 206 (2017). https://doi.org/10.1186/s12931-017-0688-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-017-0688-5