Abstract
In the face of rising prevalence of antibiotic resistance, susceptibility testing to provide personalized treatment is recommended prior to eradication therapy for Helicobacter pylori (H. pylori). Yet, population specific treatment according to the local prevalence of antibiotic resistance may be an alternative if susceptibility testing is not available. In this article, we reviewed the global prevalence of primary antibiotic resistance and the efficacies of commonly used regimens in antibiotic susceptible and resistance strains. We then constructed a model to predict the efficacies of these regimens and proposed an algorithm to choose the optimal first-line and rescue therapies according to the prevalence of antibiotic resistance. Clarithromycin-based therapy (triple, sequential, concomitant, and hybrid therapies) for 14 days remains the treatment of choice in regions with low clarithromycin resistance (≤15%) and bismuth quadruple therapy may be an alternative therapy. In regions with high clarithromycin resistance (> 15%), bismuth quadruple therapy is the treatment of choice and non-bismuth quadruple therapy may be an alternative. Either levofloxacin-based therapy or bismuth quadruple therapy may be used as second-line rescue therapy for patients fail after clarithromycin-based therapies, whereas levofloxacin-based therapy may be used for patients fail after bismuth quadruple therapy. Susceptibility testing or genotypic resistance should be determined after two or more eradication failures. However, empirical therapy according to prior medication history to avoid the empirical reuse of levofloxacin and clarithromycin may be an acceptable alternative after consideration of cost, patient preference, and accessibility. Rifabutin-based therapy for 14 days may serve as the fourth-line therapy. New antibiotics specific for H. pylori are highly anticipated.
Similar content being viewed by others
Background
Helicobacter pylori (H. pylori) infection is a causal factor of peptic ulcer disease, gastric cancer (adenocarcinoma) and mucosal associated lymphoid tissue lymphoma [1]. Eradication of H. pylori may reduce the recurrence rate of peptic ulcer and may reduce the risk of gastric cancer [1,2,3]. However, the efficacy of standard triple therapy containing a proton pump inhibitor (PPI), clarithromycin, with amoxicillin or metronidazole has been declining in many countries [4, 5]. Factors that might lead to treatment failure include the presence of antibiotic resistance, lack of good compliance, inadequate treatment length, and inadequate suppression of gastric acid secretion [6, 7]. Of these, the presence of antibiotic resistance is the most important factor [6, 7]. Therefore, the best strategy to increase the eradication rate is to provide individualized treatment according to antibiotic susceptibility testing (personalized treatment) [8]. However, endoscopy with biopsy and culture for H. pylori are costly and time consuming (2–4 weeks). Besides, the successful rate of culture and susceptibility testing ranges from 75 to 90% [9, 10]. Therefore, susceptibility testing guided therapy is not widely applicable for the first-line therapy and is not easily accessible even for refractory H. pylori infection [11, 12]. Development of less invasive and less costly tests, such as genotyping of antibiotic resistance genes using gastric biopsy, gastric juice or fecal samples might be an alternative [10]. Yet, the accuracies of these tests using fecal samples are still less than perfect. Another strategy is to choose the best regimen for a population according to the prevalence of antibiotic resistance (population specific treatment) [13,14,15,16]. The efficacy of a regimen for H. pylori eradication can be predicted as long as its efficacies in susceptible and resistant strains and the prevalence of antibiotic resistance in the population are known [17, 18]. Therefore, we reviewed the global prevalence of antibiotic resistance and the efficacies of different regimens in antibiotic susceptible and resistant strains and constructed prediction models to predict the efficacies of these regimens in regions with different prevalence of antibiotic resistance in this article. Finally, we proposed an algorithm to choose the optimal first-line and rescue therapies according to the prevalence of antibiotic resistance.
Updated prevalence of primary antibiotic resistance worldwide [19,20,21,22,23,24]
The prevalence of primary antibiotic resistance varies from country to country and changes with time. The updated global prevalence of antibiotic resistance was as follows (Fig. 1).
Clarithromycin resistance
The overall prevalence of primary clarithromycin resistance was 10% (95% CI 4–16) in America’s region [22], 17% (95% CI 15–18) in Asia-Pacific [5], and 18% (95% CI 16–20) in Europe [22]. However, there were trends of rising clarithromycin resistance in these regions. The pooled resistance rates of clarithromycin resistance after 2011 were 21% (95% CI 18–25%) in Asia-Pacific, 20% (95% CI 12–28%) in America, and 28% (95% CI 25–31%) in Europe, as shown in Table 1. In Asia-Pacific region [5], clarithromycin resistance was higher than 15% in 13 countries: Bangladesh, China, India, Iran, Japan, Nepal, New Zealand, Pakistan, Saudi Arabia, Singapore, South Korea, Turkey, and Vietnam. In contrast, frequency of resistance was less than 15% in eight countries: Bhutan, Indonesia, Laos, Malaysia, Myanmar, Russia (data were specifically from eastern Russia), Taiwan, and Thailand (Fig. 1).
Metronidazole resistance
The overall prevalence of primary clarithromycin resistance was 23% (95% CI 2–44) in Americas [22], 32% (95% CI 27–36) in Europe [22], and 44% (95% CI 39–48) in Asia-Pacific [5]. Although there were no remarkable changes in metronidazole resistance over time compared to clarithromycin, the pooled prevalence of primary metronidazole resistance after 2011 was greater 25% in these regions (Table 1). According to data for 2006–15 in Asia-Pacific, metronidazole resistance was higher than 40% in most countries, except Japan, Myanmar, South Korea, Taiwan, and Turkey [5].
Levofloxacin resistance
The overall prevalence of primary levofloxacin resistance was 11% (95% CI 9–13) in Europe [22], 15% (95% CI 5–16) in Americas [22], and 18% (95% CI 15–22) in Asia-Pacific [5]. Prevalence of resistance to levofloxacin in America and Asia-Pacific rose significantly over time during the period investigated. The pooled prevalence of primary levofloxacin resistance after 2011 was 19% (95% CI 5–16%) in America, 12% (95% CI 8–15%) in Europe, and 27% (95% CI 21–34%) in Asia-Pacific (Table 1). In Asia-Pacific regions, resistance to levofloxacin increased over time in all included countries, except in Iran. The levofloxacin resistance rates were significantly higher in Eastern Asia (including China, Hong Kong, Japan, South Korea, and Taiwan) than in western Asia (including Israel, Saudi Arabia, and Turkey) and southeastern Asia (including Indonesia, Laos, Malaysia, Myanmar, Singapore, Thailand, and Vietnam) [5]. Megraud et al. [19] and Liou et al. [21] showed that fluoroquinolone resistance correlated with consumption of fluoroquinolones in Europe and Taiwan, respectively. The global consumption of fluoroquinolones has significantly increased since 2000 [23], which might be explained by the recommendation in 2004 guidelines to use fluoroquinolone monotherapy as an alternative first-line therapy for community-acquired pneumonia [24].
Amoxicillin resistance
The overall prevalence of primary amoxicillin resistance was 0% (95% CI 0–0) in Europe [22], 3% (95% CI 2–4) in Asia-Pacific [5], and 10% (95% CI 2–19) in Americas [22]. The trend in amoxicillin resistance was only available in Asia-Pacific region and country-specific data showed no remarkable changes in resistance over time [5]. Although amoxicillin resistance was uncommon in the Asia-Pacific region, resistance to amoxicillin was higher than 10% in Pakistan and India.
Tetracycline resistance
The overall prevalence of primary tetracycline resistance was 0% (95% CI 0–0) in Europe, [23] 4% (95% CI 2–5) in Asia-Pacific [5], and 4% (95% CI 1–11) in Americas [22]. The trend in tetracycline resistance was only available in Asia-Pacific region and no remarkable changes over time [5]. The prevalence of resistance to tetracycline was < 10% in all countries, except Pakistan and India, where tetracycline resistance was higher than 10%.
Strategies to improve the efficacy of first-line therapy
The dosages and frequencies of PPI, bismuth, and antibiotics of the commonly used regimens are shown in Table 2. There are several strategies to improve the efficacy of first-line therapy, including extending the length of treatment to 14 days, the use of vonoprazan or higher dosage of PPI, the use of four drug regimens (bismuth quadruple therapy, concomitant therapy, sequential therapy, or hybrid therapy), susceptibility testing (or genotypic resistance) guided therapy, and supplementation with probiotics (Table 3) [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
Extending the treatment length of triple therapy to 14 days
Clarithromycin-based triple therapy remains one of the treatment options in countries where the prevalence of clarithromycin resistance is lower than 15% [13,14,15,16, 25]. A Cochrane meta-analysis of 59 randomized trials showed that the efficacy of triple therapy may be increased by extending its treatment length from 7 days to 10 days (75.7% vs 79.9%, RR 0.80, 95% CI 0.72 to 0.89), from 7 or 14 days (72.9% vs 81.9%, RR 0.66, 95% CI 0.60 to 0.74), or from 10 days to 14 days (78.5% vs 84.4%, RR 0.72, 95% CI 0.58 to 0.90) [26]. Therefore, extending the treatment length of triple therapy to 14 days is recommended in several international consensus reports [13,14,15,16, 25].
Use of higher dosage of PPI or vonoprazan
The minimum inhibitory concentrations (MICs) of amoxicillin, clarithromycin, and levofloxacin are higher in acidic environment [7, 9]. Therefore, increasing the gastric pH values through the use of higher dosage of PPI may increase the efficacy of eradication therapy for H. pylori [7]. The standard dosages of PPI used for H. pylori eradication were omeprazole 20 mg, esomeprazole 20 mg, pantoprazole 40 mg, lansoprazole 30 mg, and rabeprazole 20 mg given twice daily. Meta-analysis of 6 randomized trials (N = 1703) showed that the use of higher dosage of PPI may increase the eradication rate of standard triple therapy [30, 31]. However, only two trials compared the same PPI of different dosage [30, 31]. Vonoprazan, a potassium-competitive acid blocker (P-CAB), is a novel gastric acid secretion suppressant. A randomized trial showed that vonoprazan-based triple therapy is superior to lansoprazole-based triple therapy in Japan, especially for clarithromycin resistant strains [32]. It’s efficacy against clarithromycin resistant strains has been confirmed in several retrospective or prospective non-randomized studies in Japan. However, the finding needs to be validated in more trials outside Japan.
Use of four drug regimen
Clarithromycin based triple therapy is not recommended in countries where the prevalence of clarithromycin resistance is higher than 15% in international consensus reports [13,14,15,16, 25]. Bismuth quadruple therapy or non-bismuth quadruple therapies (concomitant therapy, sequential therapy, hybrid therapy) are recommended in these regions [13,14,15,16, 25, 27,28,29, 33,34,35,36,37]. Recent meta-analysis of randomized trials showed that 14-day sequential therapy, but not 10-day sequential therapy, was superior to 14-day triple therapy [13]. A recent randomized trial showed that 14-day sequential therapy was not inferior to 10-day bismuth quadruple therapy [33]. Therefore, extending the treatment length of sequential therapy to 14 days is recommended [27,28,29, 33]. Our recent systematic review and meta-analysis showed that concomitant therapy for 5, 7 or 10 days was superior to triple therapy for 7 or 10 days, but was not superior to 14-day triple therapy [38]. A non-randomized trial showed that 14-day concomitant therapy was superior to 14-day triple therapy [39]. Therefore, the treatment length of concomitant therapy is 14 days in several international consensus reports [13,14,15,16]. Although the Maastricht V and the Toronto Consensus recommended that bismuth quadruple therapy should be given for 14 days, the evidence level supporting the recommendation is low [13, 14]. Our recent trials showed that bismuth quadruple therapy given for 10 days was superior to 14-day triple therapy and its efficacy was greater than 90% in Taiwan [36]. Therefore, 10-day bismuth quadruple therapy is an acceptable regimen in Taiwan.
Susceptibility testing guided therapy
Meta-analysis of 9 randomized trials including 1958 subjects showed that susceptibility testing guided therapy was more effective than empirical triple therapy for 7 or 10 days in the first-line treatment of H. pylori infection [8]. However, most of these trials randomize patients after endoscopy and/or culture which is not similar to that in clinical practice because patients might decline endoscopy, the yield rate of culture is only 70–90%, and the accuracy of susceptibility testing is not 100% [8]. Besides, whether susceptibility testing guided therapy is superior to 14-day triple therapy or bismuth quadruple therapy are still unknown.
Supplementation with probiotics
A recent meta-analysis showed that probiotics may induce a significant reduction in delta values of urea breath test than placebo (8.61% with a 95%CI: 5.88–11.34, vs 0.19% for placebo, P < 0.001) [40]. However, only about 10–15% of H. pylori infection was eradicated with probiotic monotherapy [40]. Earlier studies showed that supplementation of probiotics may increase the eradication rate of triple therapy, probably through the alleviation of adverse effects of triple therapy [41]. However, more recent meta-analysis of 21 randomized control trials showed that standard therapy plus probiotics may reduce the frequency of adverse effect compared to standard therapy with or without a placebo, but does not increase the eradication rate of standard therapy [42]. Yet, another meta-analysis of randomized trial showed that adjunctive use of some multi-strain probiotics may increase the eradication rate and reduce the risk of adverse events but not all mixtures were effective [43]. Therefore, routine supplementation of probiotics is not recommended in the Toronto and the Asean Consensus Reports considering the controversial results and the cost [14, 15].
Efficacies of different eradication regimens in susceptible and resistant strains
The efficacies of six commonly used regimens in susceptible and resistant strains in the first-line treatment of H. pylori infection were reviewed in this article. Pooled analyses of efficacies of the six different regimens in antibiotic susceptible and resistant strains according to the length of treatment were shown in Table 4 and in Additional file 1: Tables S1-S6 [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30, 33,34,35,36,37,38]. Except for 5-day concomitant therapy and 7-day bismuth quadruple therapy, the eradication rates of the other regimens were greater than 90% in clarithromycin susceptible strains (Table 4). However, the efficacy of levofloxacin triple therapy was only 87.5% in the first-line therapy, even for levofloxacin susceptible strains. The efficacies of triple therapy, sequential therapy, concomitant therapy, and hybrid therapy were significantly lower in clarithromycin resistant strains, especially when the treatment length were 10 days or less (Table 4). The efficacies of bismuth quadruple therapy were not affected by clarithromycin resistance. However, the efficacy of bismuth quadruple therapy was affected by metronidazole resistance when it was given for 7 days.
Prediction of different regimens in regions with different prevalence of antibiotic resistance
The efficacy of a regimen which contains antibiotic A (drug A) and antibiotic B (drug B) in a region can be predicted if the prevalence of antibiotic resistance in that region and the efficacy of this regimen in susceptible and resistant strains are known [17, 18] . Assuming the prevalence of antibiotic resistance for drug A and drug B are p and q, respectively, the prevalence of dual drug resistance and dual susceptible strains would be p*q and (1-p)*(1-q), respectively. Therefore, the estimated eradication rate of that regimen would be 【ERSS* (1-p)*(1-q)】 + 【ERSR* (1-p)*q】 + 【ERRS *P*(1-q)】 + 【ERRR* P*q】, where ERSS, ERSR, ERRS, and ERRR are eradication rates in dual susceptible, susceptible to drug A but resistant to drug B, resistant to drug A but susceptible to drug B, and dual resistant strains, respectively. Based on this prediction model and the efficacies of different regimens in antibiotic susceptible and resistant strains, the efficacies of these regimens in regions with different prevalence of antibiotic resistance can be predicted, as shown in Fig. 2. For example, the predicted efficacy of 7-day standard triple therapy according to the prevalence of clarithromycin resistance would be 0.885(1-p) + 0.258p (p is the prevalence of clarithromycin resistance). Comparing to other regimens, the eradication rates of 7-day, 10-day, 14-day triple therapy and 5-day concomitant therapy would be lower than 80% in regions where the prevalence of clarithromycin resistance is higher than 20% (Fig. 2). Among these regimens, the efficacy of bismuth quadruple therapy would remain higher than 90% in regions with high prevalence of primary clarithromycin resistance (Fig. 2). The efficacies of metronidazole-containing regimens, including sequential therapy, concomitant therapy, hybrid therapy and bismuth quadruple therapy were also affected by metronidazole resistance, but the effect size was relatively smaller (Fig. 2). The efficacy of levofloxacin triple therapy for treatment-naïve patients would be lower than 80% when the levofloxacin-resistant rate higher than 15%.
Predicted efficacies of different regimens according to prevalence of (a) clarithromycin resistance and (b) metronidazole resistance. T7: triple therapy for 7 days; T10: triple therapy for 10 days; T14: triple therapy for 14 days; S10: sequential therapy for 10 days; S14: sequential therapy for 14 days; C5: concomitant therapy for 5 days; C7: concomitant therapy for 7 days; C10: concomitant therapy for 10 days; H14: hybrid therapy for 14 days; BQ10: bismuth quadruple therapy for 10 days; BQ14: bismuth quadruple therapy for 14 days
Based on the Hp-normogram in Fig. 2, bismuth quadruple therapy and non-bismuth quadruple therapy (14-day sequential therapy, 14-day concomitant therapy, and 14-day hybrid therapy) are the preferred regimens for the first-line treatment of H. pylori infection in regions with higher prevalence of clarithromycin resistance. Standard triple therapy given for 14 day may still be an option in regions where the prevalence of clarithromycin resistance is lower than 15%. Levofloxacin triple therapy is not recommended in the first- line treatment of H. pylori infection due to its low efficacy.
Second-line therapy
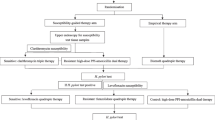
After failure of one eradication therapy, the choice of second-line eradication regimen can be empirical or guided by susceptibility testing [13,14,15,16, 25]. A recent meta-analysis of 4 randomized trials failed to show the superiority of susceptibility testing guided therapy over empirical therapy in the second-line therapy, probably attributed to the small sample size and the heterogeneity among the trials [8]. Therefore, the majority of these patients were treated empirically in clinical practice. Antibiotics used in previous eradication therapy are important and helpful to guide the second-line rescue therapy (Fig. 3). The Taiwan Consensus Report recommended the avoidance of empirical reuse of clarithromycin and levofloxacin without susceptibility testing because the secondary resistance rates of clarithromycin and levofloxacin are high for patients who fail after clarithromycin-based and levofloxacin-based therapies, respectively [25]. Bismuth quadruple therapy and levofloxacin based therapy are the most commonly used second-line rescue regimens for patients who fail after clarithromycin-based therapies [13,14,15,16, 25]. An earlier systematic review and meta-analysis showed similar efficacies of levofloxacin triple therapy and bismuth quadruple therapy in the second-line therapy [44]. However, the frequency of adverse effects was higher for bismuth quadruple therapy than levofloxacin triple therapy [44]. Yet, the prevalence of levofloxacin resistance is rising in recent years in many parts of the world [5, 19,20,21,22]. Therefore, Chen et al. found that the efficacy of levofloxacin triple therapy was only 74% in the second-line therapy in a recent systematic review and meta-analysis [45]. Liou et al. further showed that levofloxacin sequential therapy for 10 days was superior to levofloxacin triple therapy for 10 days in the second-line treatment in Taiwan [46, 47]. Levofloxacin concomitant therapy given for 5 days has been shown to be similarly effective as levofloxacin sequential therapy for 10 days in the first-line therapy, but its efficacy in the second-line therapy remains unknown [48]. In another randomized trial in Taiwan, Hsu et al. showed that modified bismuth quadruple therapy containing bismuth, a PPI, tetracycline, and levofloxacin for 10 days was superior to levofloxacin triple therapy for 10 days in the second-line therapy [49]. Non-bismuth quadruple therapy (preferably concomitant therapy) may be a second-line rescue therapy for patients who fail after bismuth quadruple therapy, but the level of evidence is low for this recommendation [13,14,15,16].
Treatment of refractory H. pylori infection
Refractory H. pylori infection is defined as failure after two or more eradication therapies. Earlier Maastricht Consensus Reports recommended that susceptibility testing should be done after failure of two eradication therapies whenever possible [50] . However, susceptibility testing for H. pylori is not widely available because of it is costly (endoscopy required), time consuming (2–4 weeks) and the successful culture rate varies from 70 to 90%. Besides, the reported efficacies of susceptibility testing guided therapy were not satisfactory, ranging from 36 to 91% in some published retrospective or prospective case series [11, 12]. Therefore, the majority of patients are treated empirically in routine clinical practice. Bismuth quadruple therapy and levofloxacin-based therapy are commonly used as third-line rescue therapy, whereas rifabutin-based therapy is usually reserved as fourth-line rescue therapy [13,14,15,16, 25]. Bismuth quadruple therapy may be used as the third-line rescue therapy for patients fail after clarithromycin-based therapy and levofloxacin-based therapy in previous eradication therapies [13,14,15,16]. Levofloxacin-based therapy may be used as the third-line rescue therapy for patients fail after clarithromycin-based therapy and bismuth quadruple therapy. 23S rRNA mutations and gyrase A mutations correlate well with clarithromycin and levofloxacin resistance, respectively [10]. Our previous pilot trial showed that genotypic resistance guided therapy may achieve 80% eradication rate in the third line treatment [51]. Therefore, we further conducted a multicenter randomized trial to compare the efficacies of genotypic resistance guided therapy vs. empirical therapy for refractory H. pylori infection [52]. We found that H. pylori was eradicated in 160/205 patients receiving genotypic resistance-guided therapy (78%) and 148/205 patients receiving empirical therapy 72.2% (P = 0.170) [52]. This is the first randomized trial to show that properly designed empirical therapy is an acceptable alternative to genotypic resistance-guided therapy for eradication of refractory H. pylori infection after consideration of cost, patient preference, and accessibility [52]. However, further studies are warranted to compare the efficacy of susceptibility testing guided therapy to genotypic resistance guided therapy or empirical therapy according to medication history.
Conclusion
The rising prevalence of primary clarithromycin and levofloxacin resistance of H. pylori is a global problem. However, the prevalence of antibiotic resistance varies greatly in different countries and regions. We proposed an algorithm to choose the optimal first-line and rescue therapies according to the prevalence of antibiotic resistance in this article (Fig. 3). Clarithromycin-based therapy (triple, sequential, concomitant, and hybrid therapies) given for 14 days remains the treatment of choice in regions with low clarithromycin resistance (≤15%). Bismuth quadruple therapy may be an alternative therapy in this region. In regions with high clarithromycin resistance (> 15%), bismuth quadruple therapy is the treatment of choice. Non-bismuth quadruple therapy may be an alternative if the prevalence of dual clarithromycin and metronidazole resistance is lower than 10%. Either levofloxacin-based therapy or bismuth quadruple therapy may be used as second-line rescue therapy for patients fail after clarithromycin-based therapies, whereas levofloxacin-based therapy may be used for patients fail after bismuth quadruple therapy. Susceptibility testing or genotypic resistance should be determined after two or more eradication failures. However, empirical therapy according to prior medication history to avoid the empirical reuse of levofloxacin and clarithromycin may be an acceptable alternative after consideration of cost, patient preference, and accessibility. Rifabutin-based therapy given for 14 days may be used as the fourth-line rescue therapy. New antibiotics specific for H. pylori are highly anticipated.
Abbreviations
- BQ10:
-
Bismuth quadruple therapy for 10 days
- BQ14:
-
Bismuth quadruple therapy for 14 days
- C10:
-
Concomitant therapy for 10 days
- C5:
-
Concomitant therapy for 5 days
- C7:
-
Concomitant therapy for 7 days
- CIs:
-
Confidence intervals
- CLA:
-
Clarithromycin
- H. pylori :
-
Helicobacter pylori
- H14:
-
Hybrid therapy for 14 days
- LEV:
-
Levofloxacin
- MET:
-
Metronidazole
- PPI:
-
Proton pump inhibitor
- S10:
-
Sequential therapy for 10 days
- S14:
-
Sequential therapy for 14 days
- T10:
-
Triple therapy for 10 days
- T14:
-
Triple therapy for 14 days.
- T7:
-
Triple therapy for 7 days
References
Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347(15):1175–86.
Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, et al. Association between Helicobacter pylori eradication and gastric Cancer incidence: a systematic review and meta-analysis. Gastroenterology 2016;150(5):1113–1124 e5.
Chen LT, Lin JT, Tai JJ, Chen GH, Yeh HZ, Yang SS, et al. Long-term results of anti-Helicobacter pylori therapy in early-stage gastric high-grade transformed MALT lymphoma. J Natl Cancer Inst. 2005;97(18):1345–53.
Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015;148(4):719–31. e3
Kuo YT, Liou JM, El-Omar EM, Wu JY, Leow AHR, Goh KL, et al. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2(10):707–15.
Megraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53(9):1374–84.
Vakil N, Megraud F. Eradication therapy for Helicobacter pylori. Gastroenterology. 2007;133(3):985–1001.
Lopez-Gongora S, Puig I, Calvet X, Villoria A, Baylina M, Munoz N, et al. Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother. 2015;70(9):2447–55.
Megraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev. 2007;20(2):280–322.
Liou JM, Chang CY, Sheng WH, Wang YC, Chen MJ, Lee YC, et al. Genotypic resistance in Helicobacter pylori strains correlates with susceptibility test and treatment outcomes after levofloxacin- and clarithromycin-based therapies. Antimicrob Agents Chemother. 2011;55(3):1123–9.
Gisbert JP. “rescue” regimens after Helicobacter pylori treatment failure. World J Gastroenterol. 2008;14(35):5385–402.
Puig I, Lopez-Gongora S, Calvet X, Villoria A, Baylina M, Sanchez-Delgado J, et al. Systematic review: third-line susceptibility-guided treatment for Helicobacter pylori infection. Therap Adv Gastroenterol. 2016;9(4):437–48.
Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2017;66(1):6–30.
Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151(1):51–69 e14.
Mahachai V, Vilaichone RK, Pittayanon R, Rojborwonwitaya J, Leelakusolvong S, Maneerattanaporn M, et al. Helicobacter pylori management in ASEAN: the Bangkok consensus report. J Gastroenterol Hepatol. 2018;33(1):37–56.
Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112(2):212–39.
Liou JM, Wu MS, Lin JT. Treatment of Helicobacter pylori infection: where are we now? J Gastroenterol Hepatol. 2016;31(12):1918–26.
Graham DY. Hp-normogram (normo-graham) for assessing the outcome of H. pylori therapy: effect of resistance, duration, and CYP2C19 genotype. Helicobacter. 2016;21(2):85–90.
Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, Hirschl AM, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62(1):34–42.
Camargo MC, Garcia A, Riquelme A, Otero W, Camargo CA, Hernandez-Garcia T, et al. The problem of Helicobacter pylori resistance to antibiotics: a systematic review in Latin America. Am J Gastroenterol. 2014;109(4):485–95.
Liou JM, Chang CY, Chen MJ, Chen CC, Fang YJ, Lee JY, et al. The primary resistance of Helicobacter pylori in Taiwan after the National Policy to restrict antibiotic consumption and its relation to virulence factors-a Nationwide study. PLoS One. 2015;10(5):e0124199.
Savoldi A, Carrara E, Graham Prof DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology. 2018. Jul 7; https://doi.org/10.1053/j.gastro.2018.07.007.
Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14(8):742–50.
Niederman MS. Review of treatment guidelines for community-acquired pneumonia. Am J Med. 2004;117(Suppl 3A):51S–7S.
Sheu BS, Wu MS, Chiu CT, Lo JC, Wu DC, Liou JM, et al. Consensus on the clinical management, screening-to-treat, and surveillance of Helicobacter pylori infection to improve gastric cancer control on a nationwide scale. Helicobacter. 2017;22(3) https://doi.org/10.1111/hel.12368.
Yuan Y, Ford AC, Khan KJ, Gisbert JP, Forman D, Leontiadis GI, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013;12:CD008337.
Liou JM, Chen CC, Chen MJ, Chen CC, Chang CY, Fang YJ, et al. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 2013;381(9862):205–13.
Liou JM, Chen CC, Lee YC, Chang CY, Wu JY, Bair MJ, et al. Systematic review with meta-analysis: 10- or 14-day sequential therapy vs. 14-day triple therapy in the first line treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 2016;43(4):470–81.
Liou JM, Chen CC, Chang CY, Chen MJ, Chen CC, Fang YJ, et al. Sequential therapy for 10 days versus triple therapy for 14 days in the eradication of Helicobacter pylori in the community and hospital populations: a randomised trial. Gut. 2016;65(11):1784–92.
Villoria A, Garcia P, Calvet X, Gisbert JP, Vergara M. Meta-analysis: high-dose proton pump inhibitors vs. standard dose in triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2008;28(7):868–77.
McNicholl AG, Linares PM, Nyssen OP, Calvet X, Gisbert JP. Meta-analysis: esomeprazole or rabeprazole vs. first-generation pump inhibitors in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 2012;36(5):414–25.
Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65(9):1439–46.
Liou JM, Chen CC, Fang YJ, Chen PY, Chang CY, Chou CK, et al. 14 day sequential therapy versus 10 day bismuth quadruple therapy containing high-dose esomeprazole in the first-line and second-line treatment of Helicobacter pylori: a multicentre, non-inferiority, randomized trial. J Antimicrob Chemother. 2018; https://doi.org/10.1093/jac/dky183. [Epub ahead of print]
Wu DC, Hsu PI, Wu JY, Opekun AR, Kuo CH, Wu IC, et al. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clin Gastroenterol Hepatol. 2010;8(1):36–41 e1.
Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion. 2013;88(1):33–45.
Liou JM, Fang YJ, Chen CC, Bair MJ, Chang CY, Lee YC, et al. Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 2016;388(10058):2355–65.
Hsu PI, Lin PC, Graham DY. Hybrid therapy for helicobacter pylori infection: a systemic review and meta-analysis. World J Gastroenterol. 2015;21(45):12954–62.
Chen MJ CC, Chen YN, Chen CC, Fang YJ, Lin JT, Wu MS, Liou JM. Systematic review with meta-analysis: concomitant therapy versus triple therapy for the first-line treatment of Helicobacter pylori infection. Am J Gastroenterol 2018;(in press).
Molina-Infante J, Lucendo AJ, Angueira T, Rodriguez-Tellez M, Perez-Aisa A, Balboa A, et al. Optimised empiric triple and concomitant therapy for Helicobacter pylori eradication in clinical practice: the OPTRICON study. Aliment Pharmacol Ther. 2015;41(6):581–9.
Losurdo G, Cubisino R, Barone M, Principi M, Leandro G, Ierardi E, et al. Probiotic monotherapy and Helicobacter pylori eradication: a systematic review with pooled-data analysis. World J Gastroenterol. 2018;24(1):139–49.
Dang Y, Reinhardt JD, Zhou X, Zhang G. The effect of probiotics supplementation on Helicobacter pylori eradication rates and side effects during eradication therapy: a meta-analysis. PLoS One. 2014;9(11):e111030.
Lu C, Sang J, He H, Wan X, Lin Y, Li L, et al. Probiotic supplementation does not improve eradication rate of Helicobacter pylori infection compared to placebo based on standard therapy: a meta-analysis. Sci Rep. 2016;6:23522.
McFarland LV, Huang Y, Wang L, Malfertheiner P. Systematic review and meta-analysis: multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United European Gastroenterol J. 2016;4(4):546–61.
Saad RJ, Schoenfeld P, Kim HM, Chey WD. Levofloxacin-based triple therapy versus bismuth-based quadruple therapy for persistent Helicobacter pylori infection: a meta-analysis. Am J Gastroenterol. 2006;101(3):488–96.
Chen PY, Wu MS, Chen CY, Bair MJ, Chou CK, Lin JT, et al. Systematic review with meta-analysis: the efficacy of levofloxacin triple therapy as the first- or second-line treatments of Helicobacter pylori infection. Aliment Pharmacol Ther. 2016;44(5):427–37.
Liou JM, Chen CC, Chen MJ, Chang CY, Fang YJ, Lee JY, et al. Empirical modified sequential therapy containing levofloxacin and high-dose esomeprazole in second-line therapy for Helicobacter pylori infection: a multicentre clinical trial. J Antimicrob Chemother. 2011;66(8):1847–52.
Liou JM, Bair MJ, Chen CC, Lee YC, Chen MJ, Chen CC, et al. Levofloxacin sequential therapy vs levofloxacin triple therapy in the second-line treatment of Helicobacter pylori: a randomized trial. Am J Gastroenterol. 2016;111(3):381–7.
Federico A, Nardone G, Gravina AG, Iovene MR, Miranda A, Compare D, et al. Efficacy of 5-day levofloxacin-containing concomitant therapy in eradication of Helicobacter pylori infection. Gastroenterology. 2012;143(1):55–61 e1. quize e13–4
Hsu PI, Tsai FW, Kao SS, Hsu WH, Cheng JS, Peng NJ, et al. Ten-day quadruple therapy comprising proton pump inhibitor, bismuth, tetracycline, and levofloxacin is more effective than standard levofloxacin triple therapy in the second-line treatment of Helicobacter pylori infection: a randomized controlled trial. Am J Gastroenterol. 2017;112(9):1374–81.
Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence consensus report. Gut. 2012;61(5):646–64.
Liou JM, Chen CC, Chang CY, Chen MJ, Fang YJ, Lee JY, et al. Efficacy of genotypic resistance-guided sequential therapy in the third-line treatment of refractory Helicobacter pylori infection: a multicentre clinical trial. J Antimicrob Chemother. 2013;68(2):450–6.
Liou JM, Chen PY, Luo JC, Lee JY, Chen CC, Fang YJ, et al. Efficacies of genotypic resistance-guided vs empirical therapy for refractory Helicobacter pylori infection. Gastroenterology. 2018;
Acknowledgements
The authors would like to express their special thanks to the staff of the Eighth Core Lab, Department of Medical Research, National Taiwan University Hospital for their technological support.
Taiwan Gastrointestinal Disease and Helicobacter Consortium investigators. Steering committee of The Taiwan Gastrointestinal Disease and Helicobacter Consortium: Jyh-Ming Liou (Taipei), Yi-Chia Lee (Taipei), Mei-Jyh Chen (Taipei), Jaw-Town Lin (Taipei), Chun-Ying Wu (Taipei), Jeng-Yih Wu (Kaohsiung), Ching-Chow Chen (Taipei), Chun-Hung Lin (Taipei), Yu-Ren Fang (Yun-Lin), Ming-Jong Bair (Taitung), Jiing-Chyuan Luo (Taipei), and Ming-Shiang Wu (Taipei). Others investigators of the Taiwan Helicobacter Consortium in this study: Tsu-Yao Cheng (Taipei), Ping-Huei Tseng (Taipei), Han-Mo Chiu (Taipei), Chun-Chao Chang (Taipei), Chien-Chun Yu (Yun-Lin), Min-Chin Chiu (Yun-Lin),Yen-Nien Chen (Hsinchu), Wen-Hao Hu (Hsinchu), Chu-Kuang Chou (Chia-Yi), Chi-Ming Tai (Kaohsiung), Ching-Tai Lee (Kaohsiung), Wen-Lun Wang (Kaohsiung), and Wen-Shiung Chang (Taipei).
Funding
This work was financially supported by the “Center of Precision Medicine” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (Grant Number: NTU- 107 L9014–1), the Ministry of Science and Technology, Executive Yuan, ROC, Taiwan (Grant Number: TCTC-TR2 106–2321-B-002-025 and MOST 107–3017-F-002-002), the Ministry of Health and Welfare of Taiwan (Grant Number: MOHW106-TDU-B-211–113002,MOHW107-TDU-B-211–123002), and National Taiwan University Hospital (Grant Number: NTUH 104-P05, NTUH 106-P06). The funding source had no role in study design, data collection, analysis or interpretation, report writing or the decision to submit this paper for publication.
Author information
Authors and Affiliations
Consortia
Contributions
The study was conceived by JML with input from PYC, YTK, and MSW. JML, PYC, and YTK drafted the article which was critically revised by JML and MSW. All authors commented on drafts and approved the final version. All authors participated in the decision to submit for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Table S1–1. Efficacy of 7-day triple therapy in the first line treatment of the individual studies. Table S1–2. Efficacy of 10-day triple therapy in the first line treatment of the individual studies. Table S1–3. Efficacy of 14-day triple therapy in the first line treatment of the individual studies. Table S2–1. Efficacy of 10-day sequential therapy in the first line treatment of the individual studies. Table S2–2. Efficacy of 14-day sequential therapy in the first line treatment of the individual studies. Table S3–1. Efficacy of 5-day or less concomitant therapy in the first line treatment of the individual studies. Table S3–2. Efficacy of 7-day concomitant therapy in the first line treatment of the individual studies. Table S3–3. Efficacy of 10-day concomitant therapy in the first line treatment of the individual studies. Table S3–4. Efficacy of 14-day concomitant therapy in the first line treatment of the individual studies. Table S4. Efficacy of 10–14 day hybrid therapy in the first line treatment of the individual studies. Table S5–1. Efficacy of 7-day or less bismuth quadruple therapy in the first line treatment of the individual studies. Table S5–2. Efficacy of 10-day bismuth quadruple therapy in the first line treatment of the individual studies. Table S5–3. Efficacy of 14-day bismuth quadruple therapy in the first line treatment of the individual studies. Table S6. Efficacy of Levofloxacin triple therapy in the first line treatment of the individual studies. (DOCX 221 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liou, JM., Chen, PY., Kuo, YT. et al. Toward population specific and personalized treatment of Helicobacter pylori infection. J Biomed Sci 25, 70 (2018). https://doi.org/10.1186/s12929-018-0471-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12929-018-0471-z