Abstract
Background
Malnutrition resulting from protein and calorie deficiency continues to be a major concern worldwide especially in developing countries. Specific deficiencies in the protein intake can adversely influence reproductive performance. The present study aimed to evaluate the effects of curcumin and curcumin nano-emulsion on protein deficient diet (PDD)-induced testicular atrophy, troubled spermatogenesis and decreased reproductive performance in male rats.
Methods
Juvenile rats were fed the protein deficient diet (PDD) for 75 days. Starting from day 60 the rats were divided into 4 groups and given the corresponding treatments for the last 15 days orally and daily as follows: 1st group; curcumin group (C) received 50 mg/kg curcumin p.o. 2ndgroup; curcumin nano-form low dose group (NCL) received 2.5 mg/kg nano-curcumin. 3rd group; curcumin nano-form high dose group (NCH) received 5 mg/kg nano-curcumin. 4th group served as malnutrition group (PDD group) receiving the protein deficient diet daily for 75 days and received distilled water ingestions (5 ml/kg p.o) daily for the last 15 days of the experiment. A normal control group was kept under the same conditions for the whole experiment and received normal diet according to nutrition requirement center daily for 75 days and received distilled water ingestions (5 ml/kg p.o) daily for the last 15 days of the experiment.
Results
PDD induced significant (P < 0.05) reduction in serum testosterone level, sperm motility, testicular GSH, CAT, SOD, testicular cell energy (ATP, ADP and AMP), essential and non-essential amino acids in seminal plasma, an increase in testicular MDA, NOx, GSSG and 8-OHDG. Data was confirmed by histological examination and revealed pathological alteration in the PDD group. Ingestion of curcumin (50 mg/kg) and curcumin nano-emulsion (2.5 and 5 mg/kg) showed significant (P< 0.05) amelioration effects against PDD-induced disrupted reproductive performance as well as biochemical and pathological alterations and the overall results of the nano-emulsion (5 mg/kg) were comparable to curcumin (50 mg/kg).
Conclusions
The present study suggests that administration of curcumin nano-emulsion as a daily supplement would be beneficial in malnutrition- induced troubled male reproductive performance and spermatogenesis cases.
Similar content being viewed by others
Background
Nutrition plays an important role in growth and development of the reproductive system. Evidence that reproductive maturation and function are influenced by malnutrition is now emerging from animal studies and human populations. It is clinically known that among the several forms of under-nutrition, mild-to-moderate protein and/or energy malnutrition is the most common and frequently impairs the growth and development of reproductive system. A huge number of reports have been published relating protein-calorie malnutrition (PCM) in males to several hazardous health complications and it is well established that this problem probably dominates in over populated countries [1,2,3].
Curcumin (1,7-bis (4-hidroxy-3-methoxyphenyl)-1,6-hepadiene-3,5-dione), obtained from Curcuma longa L. (Zengiberaceae family) rhizomes, has been extensively used in ethnic medicine for centuries and has displayed a wide range of physiological and pharmacological activities [4]. It is present in the commonly consumed foodstuff and considered safe and is known to have antioxidant, anti-inflammatory and immunodulatory properties. Numerous researches suggest the protective action of curcumin against oxidative stress-mediated cardiomyopathy, neuropathy, nephropathy, hepatic injury and testicular dysfunction [5,6,7].
From the pharmaceutical point of view, there are many challenges that limit the clinical application of curcumin [8, 9], among which is its poor aqueous solubility, photo-degradation, chemical instability, rapid metabolism and short half-life, leading to poor bioavailability when it is administered as such [10]. Therefore nano-systems for curcumin provide a very efficient solution for the aforementioned problems, leading to enhancement of its in vivo bioavailability and maximization of its therapeutic potential. Nano-emulsions which are nano-carriers composed of oil, surfactant/co-surfactant and an aqueous phase represent a very promising delivery system for curcumin as indicated by previous studies, which reported about 9 folds increase in oral bioavailability and better pharmacokinetic profile for curcumin upon encapsulation in nano-emulsion form [11, 12].
Effect of ingesting such nano-emulsions on male fertility has not been experimentally explored before and hence the present study aimed to evaluate the efficacy of curcumin as contrasted to curcumin nano-emulsion (nano-curcumin) in ameliorating protein deficient diet model-induced testicular atrophy, troubled spermatogenesis and decrease reproductive performance in male rats.
Methods
Animals
Male Wistar albino juvenile rats weighing 60–70 g were obtained from the animal house colony of Faculty of Pharmacy, Ain Shams University (Cairo, Egypt) and were housed in the National Research Centre (Dokki, Giza, Egypt) animal house in standard polypropylene cages and kept under adequate environmental conditions with equal light − dark cycles.
Ethics statement
The protocol for the conducted animal experiments was approved by the Research Ethics Committee of the Faculty of Pharmacy, Ain Shams University which followed the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH publication no. 85–23, revised 1996).
Chemicals and drugs
Curcumin was purchased from Sigma Aldrich, Germany. Tween 80 was purchased from El-Nasr Pharmaceutical Company (Cairo, Egypt). Labrafac PG oil was a kind gift from Gattefosse’ Company, France.
Preparation of curcumin nanoparticles
Curcumin was added to amber glass vials containing appropriate amounts of Labrafac PG oil and Tween 80 at a ratio of 1:1, then placed in a thermostatically controlled shaking water bath at 37 °C for 72 h at 100 rpm (Kottermann GmbH, Uetze/Hanigsen, Germany) to reach equilibrium. Preparation of the nano-emulsion was carried out using the spontaneous emulsification method [13], which was carried out by the portion-wise addition of the vial contents to double distilled water with continuous magnetic stirring for 2 h (Yellow line stirrer, IKA, Germany), for the formation of the o/w nano-emulsion. The nano-emulsion was characterized by measuring its particle size using the Zetasizer device (Nano ZS, Malvern instruments, Worcestershire, UK) after appropriate dilution. Its polydispersity index and surface charge was also measured using the same device [14].
Experimental design
Rats were divided into five groups (n = 8), they were fed protein deficient diet (PDD) consisting of pellets of shelled corn grains for 75 days [15, 16]. Drug treatments started from day 60 and for 15 days; rats were divided as follows: (1). PDD control: given daily 5 ml/kg distilled water ingestions. (2). Curcumin group (C): administered curcumin (50 mg/kg, orally) [17]. (3, 4). Nano-curcumin groups (NCL, NCH): respectively administered nano-curcumin (2.5 and 5 mg/kg body weight, orally). A group of 8 rats was kept in a separate cage in the same room, under the same conditions and fed normal standard recommended rats’ pellet diet for 75 days to serve as Normal control. Starting from day 60 this group received daily 5 ml/kg distilled water ingestions for 15 days. On day 76; 24 hs. after the last drugs ingestions; blood samples were collected and then rats were sacrificed by decapitation, testes and epididymides were collected. Tissues were either used for biochemical analyses or fixed in 10% formalin for histochemical studies.
Sample preparation
The testes and epididymides were gently excised. Each testis was weighted and homogenized in saline (10% w/v); samples were centrifuged at (4500 rpm) for 15 min, the supernatant was isolated and stored at −20 °C until assay. Each epididymal caudal was minced by using sharp scissors to release sperm in 1 ml of PBS (pH = 7.4). The liquefied semen samples were centrifuged at 600 xg for 20 min at 4 °C. The supernatant seminal plasma were separated and stored at −20 °C until assay.
Mass motility study
A drop of freshly collected semen was placed on a slide kept near body temperature (37–38 °C) and was examined under low magnification (X120), motility of semen samples were rated according to the vigor of the motility of sperms as follows: (0) Seminal samples showing no movement. (1) Seminal samples showing very slow movement. (2) Seminal samples showing slow movement. (3) Seminal samples showing moderate movement. (4) Seminal samples showing vigorous movement.
Percentage of progressive motility of spermatozoa
Immediately after each collection, it was assessed by microscopic examination by placing a small drop of fresh semen on a clean warm glass slide (37 °C), diluted with two drops of warm 0.9% NaCl and covered with a cover slip. Examination is made under the high power (X400).
Percentage of normal spermatozoa
To determine sperm vitality, 40 μl of freshly liquefied semen was thoroughly mixed with 10 μl of eosin Y (1℅), and 1 drop of this mixture was transferred to a clean slide. At least 200 sperms were counted. Sperms that were stained pink or red were considered dead, and the unstained sperms were considered viable. The percentage of normal sperm was calculated.
Sperm cell concentration
500 μl of the sperm suspension was diluted with formaldehyde fixative (10% formalin in PBS). Approximately 10 μl from the diluted solution was transferred into a haemocytometer and let to stand for 7 min. Then the settled sperms were counted and evaluated per 250 small squares of a haemocytometer [18,19,20,21].
Biochemical analysis
Determination of seminal plasma essential and non-essential amino acids levels
Amino acids of seminal plasma were determined using HPLC according to the method of Saunders et al. (1988) with some modifications where; the samples were clarified by centrifugation, 400 μl of seminal plasma supernatant were evaporated under reduced pressure and resuspended in 100 μl of coupling buffer composed of acetonitrile: ethanol: tri-ethylamine: water (10: 5: 2: 3) and then re-evaporated under reduced pressure. Derivatization by Phenyl isothiocyanate (PITC) was accomplished by resuspending the samples in 90 μl coupling buffer plus 10 μl of PITC and incubated for 5 min at room temperature, after that the samples were evaporated, and then resuspended in 240 μl of 50 mM ammonium acetate buffer + 10 μl methanol, pH (6.5). A 20 -μ1, sample was used for HPLC analysis with an Agilent HP 1200 series HPLC apparatus (USA) as described above. Separation of amino acids was conducted using an ultrasphere C18 reversed phase column at wave length 254 nm with UV detector. The mobile phase was a seven step gradient of increasing concentrations of solvent B from 5 to 70%. Solvent A was composed of 50 mM ammonium acetate (pH 6.5) and solvent B was 100 mM ammonium acetate (pH 6.5): acetonitrile (1:1). Flaw rate was 2 ml/min at temperature 500 C [22].
Determination of serum testosterone level (ng/ml)
Serum testosterone level was determined by ELISA (Enzyme Linked Immunosorbant Assay) kit; obtained from Fortrees Diagnostic Limited, United Kingdom and north Ireland.
Determination of the testicular tissue GSH and GSSG levels (μmol/g tissue) by HPLC
The thiols compounds of oxidized and reduced glutathione were detected by HPLC system of Agilent HP 1200 series (USA) that consisted of quaternary pump, a column oven, Rheodine injector and 20 μl loop, UV variable wavelength detector. The report and chromatogram taken from Chemstation program purchased from Agilent. 30 cm × 3.9 mm C18 μBondapak column was used. The flow rate was 1 ml/min and UV detection at wavelength 190 nm was applied. 0.0025 M sodium phosphate buffer, pH 3.5, containing 0.005 M tetrabutylammonium phosphate and 13% methanol was used as mobile phase. Samples were compared to glutathione (oxidized and reduced) reference standard purchased from Sigma Chemical Co. The results were expressed as μmol/g tissue [23, 24].
Determination of the testicular tissue MDA level (nmol/g tissue) by HPLC
For determination of malondialdehyde (MDA) levels; the samples were analyzed on an Agilent HP 1200 series HPLC apparatus (USA) as described above. The analytical column was Supelcosil C18 (5 μm particle and 80 Ao pore size) (250 × 4.6 ID). The mobile phase was 82.5:17.5 (v/v) 30 mM monobasic potassium phosphate (pH 3.6)–methanol and the flow rate was 1.2 ml/min, wavelength 250 nm was applied for detection. MDA standard was prepared by dissolving 25 μl 1,1,3,3 tetraethoxypropane (TEP) in 100 ml of water to give a 1 mM stock solution. Working standard was prepared by hydrolysis of 1 ml TEP stock solution in 50 ml 1% sulfuric acid and incubation for 2 h at room temperature. The resulting MDA standard of 20 nmol/ml was further diluted with 1% sulfuric acid to yield the final concentration of 1.25 nmol/ml to get the standard for the estimation of total MDA [25,26,27].
Determination of the testicular tissue NOx level (μmol/g tissue) by HPLC
Nitrates + nitrites (NOx) level was determined using Agilent HP 1200 series HPLC apparatus (USA) as described above. The analytical column was anion exchange PRP-X100 Hamilton, 150 × 4.1 mm, 10 μm. The mobile phase was a mixture of 0.1 M NaCl - methanol, at a volume ratio 45:55.The flow rate of 2 ml/min, wavelength adjusted to 230 nm. The resulting chromatogram identified the concentration from the sample as compared to that of the standard purchased from Sigma Aldrich [28].
Determination of the testicular tissue CAT activity (U/mg protein) by spectrophotometer
Catalase activity was measured by spectrophotometric method based on the decomposition of H2O2 [29].
Determination of the testicular tissue SOD activity (U/mg protein) by spectrophotometer
SOD activity was assayed for 2 min interval. Activity was expressed as the amount of enzyme that inhibits the auto oxidation of pyrogallol and was expressed as U/mg protein. [30]
Determination of the testicular tissue 8-OHDG content (pg/g tissue) by HPLC
The separation of 8-hydroxy-2-deoxyguanosine (8-OHDG) was performed with an Agilent HP 1200 series HPLC apparatus (USA) as described above. The analytical column was Supelcosil C18 (5 μm particle and 80 Ao pore size) (250 × 4.6 ID). The eluting solution was H2O/methanol at a ratio (85: 15) with 50 mM KH2PO4, pH 5.5 at a flow rate of 0.68 ml/min. The UV detector was set at 245 nm. The resulting chromatogram identified the concentration from the sample as compared to that of the standard purchased from Sigma Aldrich [31].
Determination of testicular tissue ATP, ADP and AMP contents (μg/g tissue) by HPLC
The separation of tissue adenosine tri, di and mono phosphate (ATP, ADP and AMP) was performed with an Agilent HP 1200 series HPLC apparatus (USA) as described above. The analytical column was Ultrasphere ODS EC 250 × 4.6 mm column. Mobile phase A consisted of 0.06 mol/l K2HPO4 and 0.04 mol/l KH2PO4 dissolved in deionized water and adjusted to pH 7.0 with 0.1 mol/l KOH, while mobile phase B consisted of 100% acetonitrile. Flow rate of the mobile phase was 1.2 ml/min. ATP, ADP and AMP in the samples were identified by comparison with standards purchased from Sigma Aldrich. The report and chromatograms were taken from chemstation program at wave length 254 nm [32, 33].
Total adenylate energy charge (AEC) was calculated according to the equation [34]:
AEC = (ATP + 0.5ADP)/(ATP + ADP + AMP)
Histopathological examination
For a variety of reasons, the testis presents a problem for good fixation. All of the regulatory guidelines relating to reproductive studies recommend that the testes should be fixed in Bouin’s or a comparable fixative for observing cellular details as meiosis, mitosis or even apoptosis. Since our study is concerned with normal spermatogenesis not fertility leveling; our samples were fixed in formalin, because formalin fixation of rodent testes results in better preservation of structure than with Bouin’s [35]. Specimens were taken from all groups subjected to our study, which were sliced and fixed in 10% buffered formalin. Paraffin blocks were prepared from those samples after a serial of dehydration, clearing and embedding. The paraffin-embedded material was prepared in 5-μm-thick slices, which were mounted on microscope slides and stained with hematoxylin and eosin and examined by optical microscopy to evaluate the morphologic aspects.
N.B. Minimal artifacts were produced due to formalin fixation.
Image Morphometry
Morphometric studies on H&E stained slides were performed using the Leica Qwin 500 Image Analyzer (LEICA Imaging Systems Ltd., Cambridge, England,) which consists of Leica DM-LB microscope with JVC color video camera attached to a computer system Leica Q 500IW.
Morphometric measurements
Detection of circumferences (μm)
Means of seminiferous tubules circumferences of the tested groups were determined by lining of transitionally cut seminiferous tubules (n = 8) selected on each field at a magnification of 50X (longitudinal cuts were excluded).
Total sperm cells maturation count
Counting of spermatogenic cells were performed including spermatogonia, primary & secondary spermatocytes, spermatids and spermatozoa.
Modified Johnsen spermatogenesis scoring
(1) No seminiferous epithelium, (2) No germinal cells, Sertoli cells only, (3) Spermatogonia only, (4) No spermatozoa or spermatids, few spermatocytes, (5) No spermatozoa or spermatids, many spermatocytes, (6) No spermatozoa, no late spermatids, few early spermatids, (7) No spermatozoa, no late spermatids, many early spermatids, (8) Less than five spermatozoa per tubule, few late spermatids, (9) Slightly impaired spermatogenesis, many late spermatids, disorganized epithelium, (10) Full spermatogenesis [36].
Statistical analyses
Statistical analyses for Total sperm cells maturation count and Modified Johnsen spermatogenesis scoring were carried out using Kruskal-Wallis test followed by Dunn’s multiple comparisons test. All other parameters measured were carried out using one way ANOVA followed by Tukey’s multiple comparisons test using Graph prism software (version 6); where P < 0.05 was accepted as being significant in statistical tests. Values were expressed as means ± S.E. Pearson’s correlations studies were conducted using SPSS software (version 17) where P < 0.05, P < 0.01 and P < 0.001 were used to designate the degree of correlation between parameters.
Results
Nano-emulsion specifications
The curcumin nano-emulsion was successfully prepared using the spontaneous emulsification method stated above. The prepared nano-emulsion showed a particle size of 141 ± 5.3 nm, a surface charge of −5.15 ± 0.98, and a polydispersity index of 0.369 ± 0.01, indicating its uniform particle size distribution. (Fig. 1a). The nanometer size was further confirmed by the transmission electron microscopy. (Fig. 1b).
Effects of curcumin and curcumin nano-emulsion on body weight, testis weight, relative testis weight and sperm cell concentration
As shown in present data PDD resulted in a significant decrease in the rate of normal body weight increase, testis weight, relative testis weight as well as sperm cell concentration, % of normal sperm cell, mass motility and progressive motility%. Furthermore, the % of abnormal sperm cell was increased. On the other hand curcumin and curcumin nano-emulsion at high dose showed significant increase of final body weight, testis weight, relative testis weight in addition to sperm cell concentration, % of normal sperm cell, mass motility and progressive motility%. Besides, the % of abnormal sperm cell was reduced. Their results were comparable to each other. (Table 1).
Effect of curcumin and curcumin nano-emulsion on essential and non-essential amino acids levels
PDD significantly reduced all essential and non-essential amino acids levels as compared to normal control. Curcumin significantly elevated all essential amino acids levels including L-arginine as compared to PDD. Moreover; curcumin normalized most non-essential amino acids levels including taurine. Nano curcumin at the lower dose level significantly elevated most essential amino acids including L-arginine as compared to PDD control, the high dose significantly elevated all essential amino acids levels as compared to PDD control. Furthermore; nano curcumin at the lower dose level significantly elevated all non-essential amino acids levels including taurine as compared to PDD control and at the high dose normalized their levels. Comparing the results of curcumin and nano-curcumin at the high dose; it was easily demonstrated that nano-curcumin at the high dose showed superior results especially regarding both L-arginine and taurine (Table 2).
Effect of curcumin and curcumin nano-emulsion on serum testosterone level
PDD resulted in significant reduction in serum testosterone level (0.92 ± 0.04 vs. 3.43 ± 0.22 ng/ml) as compared to the normal control. Ingestion of the conventional dose of curcumin as well as nano-curcumin at both dose levels resulted in a significant elevation of the testosterone level (2.39 ± 0.1, 1.89 ± 0.08 and 2.61 ± 0.06 vs. 0.92 ± 0.04 ng/ml) respectively as compared to the PDD control; where the nano-curcumin at the higher dose level resulted in superior results over the conventional curcumin formula. (Fig. 2).
Effect of curcumin and curcumin nano-emulsion on testicular tissue oxidative and nitrosative stresses parameters
PDD resulted in significant elevation of the MDA and NOx levels and GSSG/GSH ratio as well as significant reduction in the GSH level, CAT and SOD activities as compared to the normal control indicating extensive oxidative and nitrosative stresses. Ingestion of the conventional dose of curcumin (50 mg/kg) resulted in a significant decrease in the MDA and NOx levels and GSSG/GSH ratio in addition to a significant increase in the GSH content, CAT and SOD activities as compared to the PDD control. Administration of nano-curcumin at both dose levels also resulted in a pronounced anti-oxidant effect where the results of the higher dose were in most; superior over the conventional curcumin formula (Table 3).
Effect of curcumin and curcumin nano-emulsion on testicular tissue 8- OHDG level
PDD resulted in significant elevation in testicular tissue 8-hydroxy-2-deoxyguanosine (8-OHDG) level (352.6 ± 6.30 vs. 119.3 ± 3.64 pg/g) as compared to the normal control. Ingestion of the conventional dose of curcumin as well as nano-curcumin at both dose levels resulted in a significant reduction in the testicular tissue 8-OHDG level (218.9 ± 2.42, 263.1 ± 3.97 and 231.4 ± 1.21 vs. 352.6 ± 6.30 pg/g) respectively as compared to the PDD control indicating DNA preservation (Fig. 3).
Effect of curcumin and curcumin nano-emulsion on testicular tissue cell energy performance
PDD resulted in significant reduction in cell energy represented by decreased adenylate energy charge (AEC) and increased AMP/ATP ratio as compared to normal control. Ingesting the conventional dose of curcumin as well as nano-curcumin at both dose levels reversed that disruption in cell energy; normalizing the AMP/ATP ratio and the AEC, where the higher dose on nano-curcumin showed superior results over the conventional curcumin formula (Table 4).
Correlation studies
Our results revealed positive correlation between the increased essential & non-essential amino acids contents in the seminal plasma and the increased seminal plasma sperm cell concentration & progressive sperm motility also there was a positive correlation between the levels of L- arginine & taurine and the increased seminal plasma sperm cell concentration & progressive sperm motility. Moreover the incidence of testicular tissue oxidative stress, DNA damage and disrupted cell energy turned to be negatively correlated to the essential and non-essential amino acids contents in the seminal plasma (Table 5, Fig. 4).
Histopathological examination
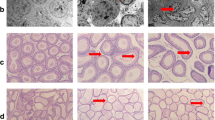
H& E staining for the group ingesting protein deficient diet (PDD diet) for 75 days revealed tubular atrophy with extensive degeneration in testicular tissue. The atrophic irregular seminiferous tubules X40 were characterized by a depletion of germ cells, exhibiting sertoli cell-only type and a few spermatogonia. Reduced seminiferous epithelial layers were found in numerous tubules, and irregular and diminished tubules containing a few germ cells were also seen X100. Ingesting curcumin at the conventional dose level resulted in normal spermatogenic cell layers at different stages of development with normal seminiferous tubules and interstium, all levels of sperm maturation were present. Examining sections from the group ingesting nano-curcumin at the low dose level revealed remarkable regenerative features in all the tubules reaching the spermatid level but lipid deposition in the basal layer could be observed at some tubules figure (a) and fragmentation in some tubules (b) H&E X100. Normal gaps between seminiferous tubules is preserved with no atrophy or interstitial edema. On the other hand; ingesting nano-curcumin at the high dose level resulted in sections with almost reduced seminiferous epithelial layers in numerous tubules with maturation to sperms H&E X100. Magnified photography of the section revealing highly remarkable regenerative features with cellular ballooning in few seminiferous tubules with diminished normal intratubular gaps relatively higher than group of low dose level. H&E X100. (Fig. 5). Furthermore; morphometric measures revealed that PDD significantly reduced the mean seminiferous tubules circumferences and total sperm cells maturation count as compared to the normal control. On the other hand; ingesting curcumin and curcumin nano-emulsion at the two dose levels significantly increased both the mean tubules circumferences and total sperm cells maturation count indicating enhancement in the overall spermatogenesis process. According to Modified Johnsen spermatogenesis scoring; taking in consideration that Johnsen scoring is covering only the spermatogenic leveling in testicular biopsy away from other pathological features that could be detected; it was clearly demonstrated that the normal control group showed a score of 10 while the PDD control scored 2. Ingesting curcumin in its conventional form resulted in retrieving the score of 10. On the other hand; administration of nano-curcumin in the lower dose level showed mixed scores of 8 & 9 according to captures from different sections. Moreover; ingesting curcumin nano-emulsion at the higher dose level displayed score of 8 & 9 at some fields and 10 at other. This result further supports our findings and prove the effectiveness of curcumin nano-emulsion (Table 6).
Discussion
Curcumin, a component of the spice turmeric (Curcuma longa), is widely used as a traditional herbal medicine in the treatment of various diseases. This compound can inhibit oxidative stress and could ameliorate tissue atrophy [37]. Previous investigators reported the beneficial effects of ingesting curcumin on testicular tissue; both in acute and chronic models of testicular tissue injury; which could be clearly confirmed on both histological level and biochemical parameters estimated; suggesting the possibility of using curcumin as a potential therapeutic in the treatment of stress-mediated testicular dysfunction. Treatment with curcumin markedly decreased apoptosis in rat testes, increased the mean seminiferous tubule diameter and mean testicular biopsy score values. A significant reduction in the testicular tissue oxidative stress and decrease in sperm abnormalities as well as increased total sperm count and elevation in testosterone level were demonstrated [6, 38,39,40].
In the present investigation protein deficient diet (PDD) based on ingesting pellets of corn grains was used to create a state of malnutrition in rats. This diet was used according to previous researches indicating that corn grains contained very low protein content as compared to the recommended protein contents in standard rat diet [15, 41, 42]. Former investigators reported that sperm maturation needs about 70 days in rats [16]; therefore, rats were given the diet for 75 days and the drug treatments were administered daily during the last 15 days of the diet regimen.
The relationship between reproduction and malnutrition has been studied in laboratory animals by employing diets deficient in or totally devoid of protein. It has been demonstrated that under-nutrition during the fetal and/or pre-pubertal period is accompanied by changes in testicular structure with a consequent decrease in daily sperm production [1,2,3]. Besides; supplementation of diet with amino acids improved sperm quality, and subsequently increased fertilization capacity [43]. Furthermore; adequate dietary intake of protein in general is considered essential for maintenance of adequate tissue adenylate energy charge (AEC) and increased ATP production in all body organs [44].
With respect to redox signaling, it has recently been demonstrated that reactive oxygen and nitrogen species (ROS and RNS) and the resultant oxidative and nitrosative stresses are implicated in male infertility. When the oxidative and nitrosative stresses exceed the antioxidant capacity of any body tissue an extensive amount of malondialdehyde (MDA) is generated, intense cellular damage and consequential accelerated apoptosis follows. The overall status affects tissue DNA and membrane phospholipids integrity; resulting in severe tissue injury and/or cell death [45,46,47]. 8-hydroxylated guanine species such as 8-oxoguanine and 8-hydroxy-2-deoxyguanosine (8-OHDG) are repair products of oxidized guanine lesions. 8-OHDG content is considered a sensitive biomarker of the oxidative DNA damage and repair [48]. Besides the superoxide anion is the main undesired by-product of mitochondrial oxidative phosphorylation. Superoxide dismutase (SOD) converts superoxide anion to hydrogen peroxide, which can be then converted by catalase (CAT) to harmless H2O [49].
During malnutrition and protein deficiency, there is excess production ROS and RNS. Furthermore; it was demonstrated that diets deficient in methionine are usually accompanied by progressive increase in MDA and NOx levels as well as pronounced reduction in GSH level along with marked decrease in CAT and SOD activities. Taking into consideration that necessity of GSH, CAT and SOD for body tissues as defense strategy against free radical damage; preserving cellular content of those antioxidant moieties could be critical for maintaining optimum health and wellbeing [46, 50,51,52].
Semen consists of spermatozoa suspended in a fluid medium called seminal plasma; which is a complex fluid portion and mediates the chemical function of the ejaculate. Biochemical components of seminal plasma are synthesized and secreted by the rete testis, epididymis, and accessory sex glands of the male reproductive tract. The conventional role for seminal plasma is acting as a survival medium that facilitates transport of spermatozoa. Generally; seminal plasma plays important roles in increasing the overall sperm quality parameters, activation and augmentation of the motility of spermatozoa as well as buffering to provide the optimal osmotic and nutrient medium. Moreover; it has also been observed that the use of preserved semen for artificial insemination in livestock species, which often involves extensive dilution or removal of seminal plasma, results in lower fertility rates than with natural mating. These evidences suggest that seminal plasma components participate in key events related to sperm function, fertilization, and embryo development in the female reproductive tract. Therefore; the role of seminal plasma constituents in regulating sperm functions must be highlighted in reproductive investigations. Seminal plasma amino acids in particular; serve as a readily oxidizable substrate for energy-yielding reactions in semen to fuel the progressive and hyperactive motility of sperms; necessary for capacitation and fertilization. One of the most important essential amino acids involved in the energy production process would be L-Arginine which acts as a source of energy for normal sperm motility in the form of arginine phosphoric acid. In addition; taurine has been identified as one of the major free amino acids of the seminal fluid. Several physiological functions of taurine have been demonstrated, such as membrane stabilization, sperm motility factor, energy storage and acting as an anti-oxidant [53, 54].
That is why our research was based on examining the effect of PDD on the testicular tissue as well as the spermatogenesis process, sperm motility and seminal plasma essential and non-essential amino acids content with special focus on the levels of both L-arginine and taurine.
The results of the current study clearly showed that PPD resulted in severe reduction in the rate of total body weight increase as well as marked decrease in testes weight, relative testes weight, total sperm count, mass and progressive motilities. On biochemical level; testicular tissue adenylate energy charge (AEC) was lowered, pronounced increase in 8-OHDG level and marked oxidative and nitrosative stresses in the testicular tissue were demonstrated. Serum testosterone level as well as several seminal plasma essential and non-essential amino acids levels were reduced and generally troubled spermatogenesis was definite. Histopathological examination revealed the existence of testicular tissue atrophy and degeneration as well as alterations in the whole spermatogenesis process.
Furthermore, our results also revealed the existence of a negative correlation between testicular tissue elevated level of 8-OHDG, increased oxidative stress as well as alteration in cell energy and the levels of both essential and non-essential amino acids in the seminal plasma as well as sperm cell concentration and progressive sperm motility. It was also demonstrated that the levels of essential and non-essential amino acids and in particular L-arginine and taurine were positively correlated to the sperm cell concentration and progressive sperm motility.
Our results revealed that ingesting curcumin and nano-curcumin protected against the adverse effects of PDD. Curcumin and nano-curcumin resulted in elevated rate in total body weight increase as well as marked increase in testes weight, relative testes weight, testicular tissue cell energy in addition to pronounced decrease in testicular tissue 8-OHDG level as well as decreased oxidative and nitrosative stresses. Furthermore; there was a pronounced elevation in serum testosterone level, as well as seminal plasma essential and non-essential amino acids content specially L-arginine and taurine. Regarding spermatogenesis process there was a marked increase in the total sperm cell concentration, mass and progressive sperm motility and sperm viability and normality. Histopathological examination demonstrated normal spermatogenesis with remarkable regenerative features in seminiferous tubules with no atrophy or interstitial edema. The steroidogenic effect of curcumin may be due to the indirect increase of testosterone level through blocking the metabolism of testosterone. The mechanism by which curcumin has been considered to be a testosterone-increasing agent is by acting as an aromatase inhibitor; an enzyme that converts testosterone to estrogen [55].
The superiority of curcumin nano-emulsion compared to mere curcumin; as manifested by high therapeutic efficacy at a much lower dose could be attributed to the reported internalization of nano-emulsion droplets into the enterocytes by clathrin-mediated endocytosis pathway, which would allow the curcumin loaded nano-emulsion to be transported into the systemic circulation by the portal vein and lymphatic pathway [56]. Furthermore, the co-ingestion of a lipid source; in our case the oil of the nano-emulsion, was reported to enhance the bioaccessibility of lipophilic compounds by increasing their solubility in gastrointestinal tract (GIT) fluids, as the lipids stimulate the production of digesting enzymes as well as bile salts. Moreover, the nano size of the emulsion is postulated to have induced higher lipid hydrolysis rate leading to higher bioaccessibility of curcumin, thus consequently leading to bioavailability enhancement [57].
Conclusion
As can be inferred from the previous results that both curcumin and curcumin in nano-emulsion form managed to exhibit favorable outcomes regarding the reproductive profile in rats indicated by testicular tissue cell energy preservation, assistance in DNA repair as well as improvement of seminal plasma quality as confirmed by the amino acids profile and improvement in the overall spermatogenesis process. Curcumin in nano-emulsion proved to be more superior in several aspects, owing to nanometer size, solubilizing potential for curcumin, and lipophilicity leading to enhanced bioavailability. Futuristic studies should focus on more possible beneficial effects from using curcumin in nano-emulsion on other male fertility parameters and the advantageous consequences from using such nano-emulsion form of curcumin on sperm-egg interaction and fertilization.
Abbreviations
- 8-OHDG:
-
8-hydroxy-2-deoxyguanosine
- ADP:
-
Adenosine diphosphate
- AEC:
-
Adenylate energy charge
- Ala:
-
Alanine
- AMP:
-
Adenosine monophosphate
- Arg:
-
L-arginine
- Asp:
-
Aspartate
- ATP:
-
Adenosine triphosphate
- C:
-
Curcumin group
- CAT:
-
Catalase
- Glu:
-
Glutamate
- Gly:
-
Glycine
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- H&E:
-
Hematoxylin and Eosin
- His:
-
Histidine
- Isoleu:
-
Isoleucine
- Leu:
-
Leucine
- Lys:
-
Lysine
- MDA:
-
Malondialdehyde
- Met:
-
Methionine
- NCH:
-
Curcumin nano-form high dose group
- NCL:
-
Curcumin nano-form low dose group
- NOx:
-
Nitric oxide nitrate and nitrite forms
- PDD:
-
Protein deficient diet
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen
- Ser:
-
Serine
- SOD:
-
Superoxide dismutase
- Tau:
-
Taurine
- Tyr:
-
Tyrosine
- Val:
-
Valine
References
Herbert DC. Growth patterns and hormonal profile of male rats with protein-calorie malnutrition. Anat Rec. 1980;197(3):339–54.
Muzi-Filho H, Bezerra CG, Souza AM, Boldrini LC, Takiya CM, Oliveira FL, et al. Undernutrition affects cell survival, oxidative stress, Ca2+ handling and signaling pathways in vas deferens, crippling reproductive capacity. PLoS One. 2013;8(7):e69682.
Carvalho M, Mateus L, Afonso F, Van Harten S, Cardoso LA, Redmer DA, et al. Testicular angiogenic activity in response to food restriction in rabbits. Reproduction. 2009;137(3):509–15.
Reyes-Gordillo, K, J Segovia, M Shibayama, P Vergara, MG Moreno, P Muriel. Curcumin protects against acute liver damage in the rat by inhibiting NF-kappaB, proinflammatory cytokines production and oxidative stress. Biochim Biophys Acta 2007;1770(6):989-996.
Sanmukhani J, Anovadiya A, Tripathi CB. Evaluation of antidepressant like activity of curcumin and its combination with fluoxetine and imipramine: an acute and chronic study. Acta Pol Pharm. 2011;68(5):769–75.
Rashid K, Sil PC. Curcumin ameliorates testicular damage in diabetic rats by suppressing cellular stress-mediated mitochondria and endoplasmic reticulum-dependent apoptotic death. Biochim Biophys Acta. 2015;1852(1):70–82.
Yan X, Pan B, Lv T, Liu L, Zhu J, Shen W, et al. Inhibition of histone acetylation by curcumin reduces alcohol-induced fetal cardiac apoptosis. J Biomed Sci. 2017;24(1):1.
Lu WP, Mei XT, Wang Y, Zheng YP, Xue YF, Xu DH. Zn(II)-curcumin protects against oxidative stress, deleterious changes in sperm parameters and histological alterations in a male mouse model of cyclophosphamide-induced reproductive damage. Environ Toxicol Pharmacol. 2015;39(2):515–24.
Huang H, Chen X, Li D, He Y, Li Y, Du Z, et al. Combination of alpha-Tomatine and Curcumin inhibits growth and induces apoptosis in human prostate cancer cells. PLoS One. 2015;10(12):e0144293.
Mehanny M, Hathout RM, Geneidi AS, Mansour S. Exploring the use of nanocarrier systems to deliver the magical molecule; Curcumin and its derivatives. J Control Release. 2016;225:1–30.
Onoue S, Takahashi H, Kawabata Y, Seto Y, Hatanaka J, Timmermann B, et al. Formulation design and photochemical studies on nanocrystal solid dispersion of curcumin with improved oral bioavailability. J Pharm Sci. 2010;99(4):1871–81.
Lin W, Hong JL, Shen G, Wu RT, Wang Y, Huang MT, et al. Pharmacokinetics of dietary cancer chemopreventive compound dibenzoylmethane in rats and the impact of nanoemulsion and genetic knockout of Nrf2 on its disposition. Biopharm Drug Dispos. 2011;32(2):65–75.
Nasr M. Development of an optimized hyaluronic acid-based lipidic nanoemulsion co-encapsulating two polyphenols for nose to brain delivery. Drug Deliv. 2016;23(4):1444–52.
Nasr M, Nawaz S, Elhissi A. Amphotericin B lipid nanoemulsion aerosols for targeting peripheral respiratory airways via nebulization. Int J Pharm. 2012;436(1–2):611–6.
Li X, Rezaei R, Li P, Wu G. Composition of amino acids in feed ingredients for animal diets. Amino Acids. 2011;40(4):1159–68.
Guo QY, He LX, Zhu H, Shang JL, Zhu LY, Wang JB, et al. Effects of 90-day feeding of transgenic maize BT799 on the reproductive system in male Wistar rats. Int J Environ Res Public Health. 2015;12(12):15309–20.
Kim, SK, H Seok, HJ Park, HS Jeon, SW Kang, BC Lee, et al. Inhibitory effect of curcumin on testosterone induced benign prostatic hyperplasia rat model. BMC Complement Altern Med. 2015;15:380.
WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization; 2010.
Mohammadi Roushandeh A, Salehi I, Mortazavi M. Protective effects of restricted diet and antioxidants on testis tissue in rats fed with high-fat diet. Iran Biomed J. 2015;19(2):96–101.
Heidari R, Alizadeh R, Abbasi N, Pasbakhsh P, Hedayatpour A, Farajpour M, et al. Do Pilea Microphylla improve sperm DNA fragmentation and sperm parameters in Varicocelized rats? Acta Med Iran. 2015;53(9):547–54.
Seed J, Chapin RE, Clegg ED, Dostal LA, Foote RH, Hurtt ME, et al. Methods for assessing sperm motility, morphology, and counts in the rat, rabbit, and dog: a consensus report. ILSI risk science institute expert working group on sperm evaluation. Reprod Toxicol. 1996;10(3):237–44.
Saunders JA, Saunders JM, Morris S, Wynne SA II. Amino acid analysis of subcellular fractions by pitc and opa. Chromatogram. 1988;9(1):2–4.
Yoshida T. Determination of reduced and oxidized glutathione in erythrocytes by high-performance liquid chromatography with ultraviolet absorbance detection. J Chromatogr B Biomed Appl. 1996;678(2):157–64.
Jayatilleke E, Shaw S. A high-performance liquid chromatographic assay for reduced and oxidized glutathione in biological samples. Anal Biochem. 1993;214(2):452–7.
Karatas F, Karatepe M, Baysar A. Determination of free malondialdehyde in human serum by high-performance liquid chromatography. Anal Biochem. 2002;311(1):76–9.
Lazzarino G, Di Pierro D, Tavazzi B, Cerroni L, Giardina B. Simultaneous separation of malondialdehyde, ascorbic acid, and adenine nucleotide derivatives from biological samples by ion-pairing high-performance liquid chromatography. Anal Biochem. 1991;197(1):191–6.
Karatepe M. Simultaneous determination of ascorbic acid and free malondialdehyde in human serum by HPLC–UV. LCGC ASIA PACIFIC. 2004;7(2):36–8.
Papadoyannis IN, Samanidou VF, Nitsos CC. Simultaneous determination of nitrite and nitrate in drinking water and human serum by high performance anion-exchange chromatography and uv detection. J Liq Chromatogr Relat Technol. 1999;22(13):2023–41.
Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6.
Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47(3):469–74.
Lodovici M, Casalini C, Briani C, Dolara P. Oxidative liver DNA damage in rats treated with pesticide mixtures. Toxicology. 1997;117(1):55–60.
Teerlink T, Hennekes M, Bussemaker J, Groeneveld J. Simultaneous determination of creatine compounds and adenine nucleotides in myocardial tissue by high-performance liquid chromatography. Anal Biochem. 1993;214(1):278–83.
Liu, H, Y Jiang, Y Luo, W Jiang. A Simple and Rapid Determination of ATP, ADP and AMP Concentrations in Pericarp Tissue of Litchi Fruit by High Performance Liquid Chromatography. Food Technol Biotechnol 2006;44(4):531-534.
Atkinson DE, Walton GM. Adenosine triphosphate conservation in metabolic regulation. Rat liver citrate cleavage enzyme. J Biol Chem. 1967;242(13):3239–41.
Tu, L, L Yu, H Zhang. Morphology of rat testis preserved in three different fixatives. J Huazhong Univ Sci Technolog Med Sci 2011;31(2):178-180.
Mushtaq H, Alam S, Khan M. Histopathological patterns of testicular biopsies in male infertility. J Islamabad Med Dent Coll (JIMDC). 2013;2(4):81–6.
Ono T, Takada S, Kinugawa S, Tsutsui H. Curcumin ameliorates skeletal muscle atrophy in type 1 diabetic mice by inhibiting protein ubiquitination. Exp Physiol. 2015;100(9):1052–63.
Takhtfooladi MA, Asghari A, Takhtfooladi HA, Shabani S. The protective role of curcumin on testicular tissue after hindlimb ischemia reperfusion in rats. Int Urol Nephrol. 2015;47(10):1605–10.
Lonare M, Kumar M, Raut S, More A, Doltade S, Badgujar P, et al. Evaluation of ameliorative effect of curcumin on imidacloprid-induced male reproductive toxicity in wistar rats. Environ Toxicol. 2016;31(10):1250–63.
Aktas C, Kanter M, Erboga M, Ozturk S. Anti-apoptotic effects of curcumin on cadmium-induced apoptosis in rat testes. Toxicol Ind Health. 2012;28(2):122–30.
Nutrient Requirements of Laboratory Animals: Fourth Revised Edition, 1995. Washington (DC).
Clarke, HE, ME Coates, JK Eva, DJ Ford, CK Milner, PN O'Donoghue, et al. Dietary standards for laboratory animals: report of the laboratory animals Centre diets advisory committee. Lab Anim 1977;11(1):1-28.
Dong HJ, Wu D, Xu SY, Li Q, Fang ZF, Che LQ, et al. Effect of dietary supplementation with amino acids on boar sperm quality and fertility. Anim Reprod Sci. 2016;172:182–9.
van Zutphen T, Ciapaite J, Bloks VW, Ackereley C, Gerding A, Jurdzinski A, et al. Malnutrition-associated liver steatosis and ATP depletion is caused by peroxisomal and mitochondrial dysfunction. J Hepatol. 2016;65(6):1198–208.
Ziolkowski, N,AK Grover. Functional linkage as a direction for studies in oxidative stress: alpha-adrenergic receptors. Can J Physiol Pharmacol 2010;88(3):220-232.
Ghone RA, Suryakar AN, Kulhalli PM, Bhagat SS, Padalkar RK, Karnik AC, et al. A study of oxidative stress biomarkers and effect of oral antioxidant supplementation in severe acute malnutrition. J Clin Diagn Res. 2013;7(10):2146–8.
Freitas I, Boncompagni E, Tarantola E, Gruppi C, Bertone V, Ferrigno A, et al. In situ evaluation of oxidative stress in rat fatty liver induced by a Methionine- and Choline-deficient diet. Oxidative Med Cell Longev. 2016;2016:9307064.
Ahmed-Farid, O, R Ahmed, D Saleh. Combination of resveratrol and fluoxetine in an acute model of depression in mice: Prevention of oxidative DNA fragmentation and monoamines degradation. J Appl Pharm Sci. 2016:001–7.
Suski JM, Lebiedzinska M, Bonora M, Pinton P, Duszynski J, Wieckowski MR. Relation between mitochondrial membrane potential and ROS formation. Methods Mol Biol. 2012;810:183–205.
Jorgacevic B, Mladenovic D, Ninkovic M, Prokic V, Stankovic MN, Aleksic V, et al. Dynamics of oxidative/nitrosative stress in mice with methionine-choline-deficient diet-induced nonalcoholic fatty liver disease. Hum Exp Toxicol. 2014;33(7):701–9.
Lee SJ, Kang JH, Iqbal W, Kwon OS. Proteomic analysis of mice fed methionine and choline deficient diet reveals marker proteins associated with steatohepatitis. PLoS One. 2015;10(4):e0120577.
Stankovic MN, Mladenovic D, Ninkovic M, Ethuricic I, Sobajic S, Jorgacevic B, et al. The effects of alpha-lipoic acid on liver oxidative stress and free fatty acid composition in methionine-choline deficient diet-induced NAFLD. J Med Food. 2014;17(2):254–61.
Juyena NS, Stelletta C. Seminal plasma: an essential attribute to spermatozoa. J Androl. 2012;33(4):536–51.
Yang J, Wu G, Feng Y, Lv Q, Lin S, Hu J. Effects of taurine on male reproduction in rats of different ages. J Biomed Sci. 2010;17(Suppl 1):S9.
Dhawan K, Kumar S, Sharma A. Beneficial effects of chrysin and benzoflavone on virility in 2-year-old male rats. J Med Food. 2002;5(1):43–8.
Gao F, Zhang Z, Bu H, Huang Y, Gao Z, Shen J, et al. Nanoemulsion improves the oral absorption of candesartan cilexetil in rats: performance and mechanism. J Control Release. 2011;149(2):168–74.
Salvia-Trujillo L, Martin-Belloso O, McClements DJ. Excipient Nanoemulsions for improving oral bioavailability of bioactives. Nanomaterials (Basel). 2016;6(1):17.
Acknowledgments
Not applicable.
Consent to participate
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
All authors contributed equally in the study design, interpretation of the data and writing of the final manuscript. Regarding the practical work part; MN was responsible for the preparation of nano-emulsions and conducting the pharmaceutics studies, OAH and RF conducted the pharmacological and biochemical studies, RM was responsible for the histopathological examinations.
Corresponding author
Ethics declarations
Ethics approval
The protocol for the conducted animal experiments was approved by the Research Ethics Committee of the Faculty of Pharmacy, Ain Shams University which followed the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH publication no. 85–23, revised 1996).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ahmed-Farid, O.A., Nasr, M., Ahmed, R.F. et al. Beneficial effects of curcumin nano-emulsion on spermatogenesis and reproductive performance in male rats under protein deficient diet model: enhancement of sperm motility, conservancy of testicular tissue integrity, cell energy and seminal plasma amino acids content. J Biomed Sci 24, 66 (2017). https://doi.org/10.1186/s12929-017-0373-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12929-017-0373-5