Abstract
Background
Vitamin D-dependent rickets is rare in animals and humans. Several types of this condition are associated with genetic variants related to vitamin D metabolism. This is the first report of type 1B vitamin D-dependent rickets in a cat.
Case presentation
Here, we describe the case of a 3-month-old female domestic short-haired cat previously fed on commercial kitten food that presented at our clinic with seizures, lethargy, and generalized pain. Serum and ionized calcium concentrations and 1,25-dihydroxycholecalciferol in this cat were low, and radiographs showed skeletal demineralization and abnormally wide growth plates on the long bones. Initially, simple vitamin D deficiency was suspected; however, the cat’s profile, which included fed a well-balanced commercial diet, together with the findings of additional laboratory tests and the cat’s unresponsiveness to various treatments, raised the suspicion of vitamin D-dependent rickets. Examination of the DNA sequences of CYP2R1 and CYP27B1 genes, which are genes linked with vitamin D metabolism, showed a CYP2R1 frameshift mutation in exon 5 (where T is deleted at position c.1386). This mutation alters the amino acid sequence from position 462, while the stop codon introduced at position 481 prematurely truncates the 501 amino acid full-length protein. With this knowledge, a new treatment regime based on a standard dose of calcitriol was started and this markedly improved the cat’s condition.
Conclusions
To the best of our knowledge, the present case is the first description of type 1B vitamin D-dependent rickets linked with a genetic variant of CYP2R1 in a cat.
Similar content being viewed by others
Background
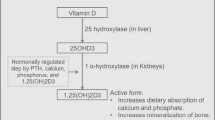
Hypocalcemia and skeletal abnormalities can occur in cats as a result of primary hypoparathyroidism, nutritional secondary hyperparathyroidism, renal secondary hyperparathyroidism, intestinal malabsorption, or vitamin D-dependent rickets (VDDR), but the latter disease is rare in cats. In domestic cats, vitamin D3 is only acquired from dietary sources, and a two-step enzymatic pathway is required for its conversion to the active form by the same process that occurs with human vitamin D3 (calcitriol) (Fig. 1) [1]. In the liver, vitamin D 25-hydroxylase (CYP2R1) catalyzes the initial hydroxylation of vitamin D (cholecalciferol) to 25-hydroxycholecalciferol (calcidiol). In the kidneys, 1-alpha-hydroxylase (CYP27B1) then catalyzes the hydroxylation and metabolic activation of calcidiol to hormonally active 1,25-hydroxycholecalciferol (calcitriol). Calcitriol binds to and activates the nuclear vitamin D receptor (VDR), which regulates calcium homeostasis. Mutations in the CYP27B1, CYP2R1, and VDR genes result in types 1A, 1B, and 2 VDDR, respectively, in humans [2]. Feline cases of VDDR have been characterized, and the treatment strategies for it documented, but the causal mutations have rarely been determined [3,4,5,6,7,8,9]. In veterinary medicine, only two cats were previously identified with a genetic defect resulting in feline type 1A VDDR [4, 5], and type 1B VDDR has not been reported previously in a cat.
Case presentation
A 3-month-old 1.1 kg female domestic short-haired cat presented with a 3-week history of seizure and generalized pain. The cat had been fed on a commercial kitten food. The cat was examined by the referring veterinarian because of its seizures and reluctance to move for 2 weeks before its initial presentation. The cat’s serum biochemistry profile revealed hypocalcemia (total calcium 1.27 mmol/L; reference range 1.97–2.82 mmol/L) and high alkaline phosphatase (835 U/L; reference range 14–192 U/l), aspartate transaminase (70 U/L; reference range 0–32 U/L), total bilirubin (3.4 mg/dL; reference range 0–0.9 mg/dL), and creatine kinase (3470 U/L; reference range 0–394 U/L). Urea and creatinine were within the reference range (Table 1). The complete blood count revealed no abnormalities, and the tests results for feline leukemia virus antigen and antibodies against feline immunodeficiency virus were negative. Based on these results, the referring veterinarian suspected that the cause of the seizures was related to hypocalcemia. Therefore, the cat was treated with levetiracetam (20 mg/kg, IV, BID) and calcium gluconate 8.5% (0.5 ml/kg, PO, BID) for 2 weeks, but its clinical condition did not improve.
On initial examination, the cat was lethargic and reluctant to move. Her appetite was good, but defecation appeared to be painful. Her family history, including whether the parents and littermates were alive, was unknown. Serum biochemistry revealed that both the total and ionized calcium levels were low (total calcium 1.55 mmol/L; reference range 2.05–3.02 mmol/L, ionized calcium 0.74 mmol/L; reference range 1.20–1.35 mmol/L). Radiographs showed skeletal demineralization and the presence of abnormally wide growth plates on the long bones (Fig. 2). The differential diagnosis for hypocalcemia with skeletal abnormalities includes primary hypoparathyroidism, nutritional secondary hyperparathyroidism, renal secondary hyperparathyroidism, intestinal malabsorption, and VDDR. However, the cat had previously eaten a well-balanced commercial kitten food (Science Diet kitten, Hill’s Colgate Japan) before becoming obviously ill, so nutritional deficiency seemed highly unlikely. The levels of parathyroid hormone (Immulyze intact PTH III, Simens) and 1,25-dihydroxycholecalciferol (1,25(OH)2D RIA Kit FR, Immunodiagnostic Systems) were determined as elements of the differential diagnosis of hypocalcemia. The parathyroid hormone level was high (99.7 pg/ml; reference range 8.0–25.0 pg/ml), but 1,25-dihydroxycholecalciferol (23.1 pg/ml) was low compared with the levels of healthy cats (Table 2). The urinalysis revealed a specific gravity of 1.032 and traces of protein. No bacterial growth was detected in the urine culture. Consequently, VDDR was suspected to be the cause of hypocalcemia and skeletal abnormalities in this cat.
On the day after the initial examination, the cat experienced seizures suspected of being hypocalcemic in origin. Slow administration of calcium gluconate 8.5% IV (1 ml/kg) was commenced, and the cat was continuously monitored by ECG, but no arrhythmia was detected. Several calcium gluconate 8.5% (1 ml/kg, IV) treatment stopped the seizures, but neither her total calcium nor her ionized calcium level increased sufficiently. Therefore, the cat was administered 8.5% calcium gluconate (starting dose, 1.0 ml/kg/h) in 0.9% saline solution by continuous rate infusion (CRI) (Table 3).
Starting on day 2, the cat received alfacalcidol (Onealfa Tablets) by CRI of 8.5% calcium gluconate. However, she did not respond well to treatment with the standard dose (0.01–0.03 μg/kg, PO, SID) of alfacalcidol so the CRI of 8.5% calcium gluconate was stopped. The final dose of alfacalcidol was very large, and 1 μg/kg, PO, TID was required to maintain the cat’s general condition in the absence of CRI of 8.5% calcium gluconate (Table 3). On day 16, the cat was discharged from hospital. Radiographs were taken again 2.5 months after the initial treatment was started. The cat’s bone structure was still abnormal, but showed marked improvement (Fig. 3).
The hypocalcemia, low 1,25-dihydroxycholecalciferol, radiographic changes, and poor response to alphacalcidol led us to suspect a genetic abnormality of vitamin D metabolism in this cat. In humans and cats, type 2 VDDR is characterized by high 1,25-dihydroxycholecalciferol levels. In the cat examined in the present study, 1,25-dihydroxycholecalciferol was lower than in healthy cats, and a huge dose of alfacalcidol was required to maintain her serum calcium concentration. Hence, we decided to analyze the gene sequences of CYP2R1 (ENSEMBL Gene ID ENSFCAG00000011383) and CYP27B1 (ENSEMBL Gene ID ENSFCAG00000014701) in healthy control cats (2 intact males and a spayed female, all domestic short-haired cats owned by our veterinary teaching hospital staff) and the affected this cat. The feline CYP2R gene contains five exons encoding an enzyme of 501 amino acids, and the CYP27B1 gene contains nine exons encoding an enzyme of 508 amino acids. We designed primer pairs for polymerase chain reaction (PCR) amplification of the individual exons and the immediate flanking regions of CYP2R1 and CYP27B1. Genomic DNA was extracted from the blood samples with the DNeasy Blood & Tissue Kit (Qiagen). PCR amplification of the fragments was performed with 20 ng of genomic DNA in GoTaq Green Master Mix (Promega), which also contained the primer pair (10 μM each) and water, with 35 cycles of 30 s at 95 °C, 30 s at 55 °C, and 1 min at 72 °C (Table 4). The PCR product sizes were verified as correct by agarose gel electrophoresis separation, the products were prepared for sequencing using the FastGene Gel/PCR Extraction Kit (Nippon Genetics), and then sequenced by a commercial DNA sequencing service (Fasmac). The sequences from the healthy cats and the affected cat were compared, and although no abnormalities in the CYP27B1 gene were present in the affected cat, we did identify the CYP2R1 variant in exon 5; c.1380G > G/A and c.1386del in this cat (Fig. 4). The variant, where T is deleted at position c.1386, causes a frameshift mutation in the gene, resulting in chain termination at the position encoding amino acid 481 in the full-length 501 amino acid protein (p.Phe462Leufs*20). The partial sequence of CYP2R1 exon 5 in this cat has been available in the DDBJ/EMBL/GenBank databases under the accession number LC424161. We next searched for the structural information for proteins affected by this nonsynonymous mutation using the protein database HOMOCOS (http://homcos.pdbj.org/). The amino acid sequences between human and feline CYP2R1 were almost identical (96.7% identity) and all contact sites with vitamin D3 were conserved (Additional file 1). Therefore, we referred an already-known crystal structure of human CYP2R1-vitamin D3 complex (pdb ID 3c6g) [10]. Two contact sites (amino acids 487 and 488) in the CYP2R1-vitamin D3 complex were missing in the sequence from the affected cat (Additional file 2), suggesting that the T deletion in exon 5 of CYP2R1 linked with a remarkable loss of CYP2R1 protein function in this cat.
a A portion of the DNA sequence chromatogram CYP2R1 exon 5 (shown 3′ → 5′ sequence), which shows the T deletion at nucleotide position c.1386 (arrow) and the heterozygous variant at position c.1380 (arrow). b Protein sequence alignment. The normal protein has 501 amino acids, but the sequence from the affected cat is altered from position 462 on, and a stop codon at position 481 truncates the protein
After the CYP2R1 variant was identified, the affected cat was treated with a standard dose of calcitriol (Caldemin; 2.5–3.5 ng/kg, PO, SID), which restored the total and ionized calcium levels to within the reference range. The level of 25-hydroxycholecalciferol at − 80 °C stored serum sample of initial presentation was additionally measured using a commercial ELISA kit (HVD3 ELISA Kit, MyBioSource). The low level of 25-hydroxycholecalciferol (12 nmol/L) compared with the levels of healthy cats (Table 2) is likely to type 1B VDDR in humans [11,12,13].
Discussion and conclusions
To the best of our knowledge, there have been no reports of the CYP2R1 variant seen in this study in cats or in veterinary medicine generally. In humans, a genetic defect in CYP2R1 was first reported as a cause of rickets in 1994, in two brothers of Nigerian descent [14]. Both had rickets associated with low baseline 25-hydroxyvitamin D3 levels. Their 25-hydroxyvitamin D3 concentrations were restored to the normal range with a relatively high dose of vitamin D. The older sibling was homozygous for the transition variant c.296 T > C in exon 2 of the CYP2R1 gene, which changes a leucine at position 99 in the encoded protein to a proline (p.Leu99Pro) [11]. A different study of familial rickets in Nigerians identified both homozygous and heterozygous c.296 T > C variants (which results in p.Leu99Pro), while the heterozygous c.726A > C nucleotide variant these researchers identified was found to result in p.Lys242Asn. Type 1B VDDR in the above-mentioned siblings resulted from a variant of CYP2R1 [12]. Type 1B VDDR characteristically manifests as rickets in patients who fail to respond to a standard dose of vitamin D, as shown by an appropriately increased 25-hydroxyvitamin D3 levels [2]. A more recent study on type 1B VDDR in human patients described the genetic variants in CYP2R1 as homozygous loss-of-function mutation, and found that calcifediol treatment brought about a substantive improvement in these patients [15].
After we identified the genetic mutation in CYP2R1, the affected cat was successfully treated with a standard dose of calcitriol. Because alfacalcidol is a prodrug, it must undergo hepatic 25-hydroxylation by CYP2R1 to become the active form, calcitriol. Hence, the total serum calcium level in the affected cat was maintained within the reference range through a standard dose of calcitriol rather than an overdose of alfacalcidol. From our examination of the crystallographic structure of human CYP2R1 complexed with vitamin D3, it is clear that the frameshift mutation in this gene will lead to an abnormal CYP2R1 protein structure in the affected cat, and this will affect the contact sites for vitamin D3. Therefore, the binding ability between CYP2R1 and vitamin D3 would be lost or at least very much reduced, thereby impairing 25-hydroxylation activity in the cat.
In conclusion, VDDR is a rare vitamin D-related deficiency disease, and type 1B VDDR has never been reported in a cat before now. The affected cat from this study will need to be treated with vitamin D3 supplementation throughout its lifetime and its serum calcium concentration will require close monitoring. It is recommended that vitamin D-dependent rickets is suspected when a poor serum calcium concentration response is seen despite administering alfacalcidol at high dosage.
Abbreviations
- CRI:
-
Continuous rate infusion
- CYP27B1:
-
1-alpha-hydroxylase
- CYP2R1:
-
vitamin D 25-hydroxylase
- VDR:
-
Vitamin D receptor, VDDR: vitamin D-dependent rickets
References
How KL, Hazewinkel HA, Mol JA. Dietary vitamin D dependence of cat and dog due to inadequate cutaneous synthesis of vitamin D. Gen Comp Endocrinol. 1994;96:12–8.
Thacher TD, Levine MA. CYP2R1 mutations causing vitamin D-deficiency rickets. J Steroid Biochem Mol Biol. 2017;173:333–6.
Grahn RA, Ellis MR, Grahn JC, Lyons LA. A novel CYP27B1 mutation causes a feline vitamin D-dependent rickets type IA. J Feline Med Surg. 2012;14:587–90.
Geisen V, Weber K, Hartmann K. Vitamin D-dependent hereditary rickets type I in a cat. J Vet Intern Med. 2009;23:196–9.
Phillips AM, Fawcett AC, Allan GS, Wilkinson M, Fraser DR, Malik R. Vitamin D-dependent non-type 1, non-type 2 rickets in a 3-month-old Cornish rex kitten. J Feline Med Surg. 2011;13:526–31.
MacKenzie JM, Crawford J, Ghantous S. Successful therapy of vitamin D-dependant rickets in a kitten. J Am Anim Hosp Assoc. 2011;47:290–3.
Tanner E, Langley-Hobbs SJ. Vitamin D-dependent rickets type 2 with characteristic radiographic changes in a 4-month-old kitten. J Feline Med Surg. 2005;7:307–11.
Godfrey DR, Anderson RM, Barber PJ, Hewison M. Vitamin D-dependent rickets type II in a cat. J Small Anim Pract. 2005;46:440–4.
Schreiner CA, Nagode LA. Vitamin D-dependent rickets type 2 in a four-month-old cat. J Am Vet Med Assoc. 2003;222:337–9.
Strushkevich N, Usanov SA, Plotnikov AN, Jones G, Park HW. Structural analysis of CYP2R1 in complex with vitamin D3. J Mol Biol. 2008;380:95–106.
Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci U S A. 2004;101:7711–5.
Al Mutair AN, Nasrat GH, Russell DW. Mutation of the CYP2R1 vitamin D 25-hydroxylase in a Saudi Arabian family with severe vitamin D deficiency. J Clin Endocrinol Metab. 2012;97:E2022–5.
Thacher TD, Fischer PR, Singh RJ, Roizen J, Levine MA. CYP2R1 mutations impair generation of 25-hydroxyvitamin D and cause an atypical form of vitamin D deficiency. J Clin Endocrinol Metab. 2015;100:1005–13.
Casella SJ, Reiner BJ, Chen TC, Holick MF, Harrison HE. A possible genetic defect in 25-hydroxylation as a cause of rickets. J Pediatr. 1994;124:929–32.
Molin A, Wiedemann A, Demers N, Kaufmann M, Do Cao J, Mainard L, et al. Vitamin D-dependent rickets type 1B (25-hydroxylase deficiency): a rare condition or a misdiagnosed condition? J Bone Miner Res. 2017;32:1893–9.
Acknowledgements
We thank the staff of Veterinary Medical Teaching Hospital of Nippon Veterinary and Life Science University for their support in caring for the affected cat. We thank Sandra Cheesman, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Contributions
Writing and revising the manuscript: TT, SK, TS, HM, HK; clinical evaluation and treatment: TT, HM, AN, DA, NK; DNA sequence analysis: TT, SK; protein crystallography analysis: TT, TS; radiographic examination: TT, AN, AD, NK. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent for publication of the present case was obtained from the cat’s owner. Verbal informed consent for publication of the control cases was obtained from the cat’s owner.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Amino acid sequence alignment of human and feline CYP2R1 proteins. The amino acid sequence identity score is 96.7% (JPG 279 kb)
Additional file 2:
(A) DNA sequence alignment of exon 5 in CYP2R1 from a control cat and the affected cat. (B) Amino acid sequence alignment of CYP2R1 from a control cat and the affected cat. The CYP2R1 protein from the affected cat is mutated from amino acid position 462 to 501. (C) Protein crystallography of CYP2R1 based on the structure of human CYP2R1 complexed with vitamin D3. Two contact sites for vitamin D3 in the protein (positions 487 and 488) are deleted. (JPG 200 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Teshima, T., Kurita, S., Sasaki, T. et al. A genetic variant of CYP2R1 identified in a cat with type 1B vitamin D-dependent rickets: a case report. BMC Vet Res 15, 62 (2019). https://doi.org/10.1186/s12917-019-1784-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-019-1784-1