Abstract
Background
Antibiotic resistance is a challenge in the management of infectious diseases and can cause substantial cost. Even without the onset of infection, measures must be taken, as patients colonized with multi-drug resistant (MDR) pathogens may transmit the pathogen. We aim to quantify the cost of community-acquired MDR colonizations using routine data from a German teaching hospital.
Methods
All 2006 cases of documented MDR colonization at hospital admission recorded from 2011 to 2014 are matched to 7917 unexposed controls with the same primary diagnosis. Cases with an onset MDR infection are excluded from the analysis. Routine data on costs per case is analysed for three groups of MDR bacteria: Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococcus (VRE), and multidrug-resistant gram-negative bacteria (MDR-GN). Multivariate analyses are conducted to adjust for potential confounders.
Results
After controlling for main diagnosis group, age, sex, and Charlson Comorbidity Index, MDR colonization is associated with substantial additional costs from the healthcare perspective (€1480.9, 95%CI €1286.4–€1675.5). Heterogeneity between pathogens remains. Colonization with MDR-GN leads to the largest cost increase (€1966.0, 95%CI €1634.6–€2297.4), followed by MRSA with €1651.3 (95%CI €1279.1–€2023.6), and VRE with €879.2 (95%CI €604.1–€1154.2). At the same time, MDR-GN is associated with additional reimbursements of €887.8 (95%CI €722.1–€1053.6), i.e. costs associated with MDR-colonization exceed reimbursement.
Conclusions
Even without the onset of invasive infection, documented MDR-colonization at hospital admission is associated with increased hospital costs, which are not fully covered within the German DRG-based hospital payment system.
Similar content being viewed by others
Background
Antibiotic resistance is a major challenge in the management of infectious diseases [1]. Treatment of healthcare-associated infections (HAIs), a frequent adverse event in health care delivery, is complicated when the causative pathogen is resistant. However, antimicrobial resistance (AMR) also impacts patients who do not become infected. Admission to a hospital while carrying multidrug-resistant (MDR) bacteria can be associated with prolongation of stay or increased medical costs [2,3,4]. MDR bacteria are organisms that are insusceptible to several classes of antibiotics.
Even adjusted for severity of underlying illnesses, length of stay (LOS) is significantly increased if a patient is colonized with a MDR bacteria [5]. Many factors besides morbidity can influence LOS in colonized patients in comparison to non-colonized patients: Patients colonized with an MDR are often put in spatial isolation, which can have negative psychological effects on the patients, affecting clinical outcomes [6].
The way isolation of colonized patients is implemented in the hospitals’ daily routine might also play a role: Anecdotal evidence suggests that hospitals or clinical departments tend to schedule diagnostics for colonized patients at the end of the day. If an emergency leads to an unscheduled patient having to be diagnosed immediately, the MDR patient, as the last patient on the regular schedule, is the patient most likely to be deferred to the next day. Such factors might also contribute to the longer LOS in colonized patients.
Depending on the pathogen, colonization can persist for months or even years, if untreated [7], potentially influencing costs over multiple hospital stays.
Quantifying the costs of colonization without infection therefore is an important piece in the overall picture of cost of MDR, and precise measurement of these costs is vital to efficiently allocate hospital resources to most effectively control them [8]. These costs include expenses for pathogen detection, infection control measures, and loss of reimbursement associated with bed closures due to patient isolation [9]. Costs can differ between MDR organisms such as Methicillin-resistant Staphylococcus aureus (MRSA), Vancomycin-resistant Enterococcus (VRE), or multidrug-resistant gram negative bacteria (MDR-GN), [5, 10]. MDR-GN include multidrug-resistance in K. pneumoniae, A. baumannii, P. aeruginosa, Enterobacter spp., E. coli and other pathogens.
Using a single-centre, matched case-cohort design, we quantify the economic burden of patients admitted to the hospital while colonized with MRSA, VRE, or MDR-GN, focusing on the inclusion of risk adjustment scores as well as accounting for procedure-related fixed costs.
Methods
Data
The data is obtained from the University Medical Centre Freiburg (UMCF), a tertiary care teaching hospital with some 1600 beds. All cases from January 2011 to December 2014 are included. Complete routine data of 185,348 inpatient cases from different wards including intensive care units (ICU) are available. Only patients older than 17 years were selected. The dataset includes detailed information about individual patient characteristics such as an individual unique identifier for each admission, age, sex, main and secondary diagnoses, costs and reimbursement. All patient records were stripped of identifying information prior to release to the researchers in accordance with German data protection law.
In-hospital treatments in Germany are generally reimbursed through predetermined lump-sums based on diagnosis related groups (DRGs). The criteria for grouping DRGs include the patients’ diagnoses, sex and age, treatment procedures, and comorbidities, among others. Hospitals receive additional reimbursement for every day that a patient stays above the upper length of the stay threshold to compensate for unusually long stays [11]. These daily surcharges are, however, much lower than the mean reimbursement per day below this threshold, and designed to not entirely cover additional variable costs to create incentives to reduce length of stay. Additional reimbursement is made for cases with very severe illnesses necessitating complex intensive care treatments [12].
About 250 hospitals are tasked with generating detailed real-life cost figures by recording the individual services delivered to each patient. Until 2016, the sample was made up entirely of hospitals that had volunteered for this task. Since then the sample has been expanded with hospitals randomly selected from those hospitals whose patient and procedure profiles had previously been underrepresented to improve the representativeness of the sample. The cost numbers conform to a standardised costing system developed by the Institute for the Payment system in Hospitals (InEK), the authority responsible for reimbursement rates [11]. Reimbursement of inpatient cases is therefore informed by real cost data. Direct costs, which are mandatory for implants, blood products or drugs etc., are based upon documented utilization. Overhead costs and costs on primary cost units on the other hand are based upon key cost drivers, i.e. time on ward or in the ICU or operating room. Indirect cost units such as on demand medications or dressings are allocated to primary cost units and excluded unless they are relevant for the corresponding DRG [13,14,15].
Main and secondary diagnoses are coded with the International Classification of Diseases 10th revision, German Modification (ICD-10-GM).
Microbiological data was obtained from the Institute for Infection Prevention and Hospital Epidemiology on the three most relevant groups of MDR bacteria MRSA, VRE and MDR-GN.
Community-onset cases
Community-onset cases of colonization are identified using the timestamps from the microbiological data. The threshold is a pathogen detection < 48 h after admission. A detection more than 48 h after admission leads to exclusion from further analyses, as these are considered hospital-acquired [16]. In a second step all onset infections associated with a resistant pathogen are excluded. Although this step leads to a study population containing on average more healthy individuals, it is necessary to isolate the costs of colonization, as infection in itself leads to substantial increases in costs and reimbursements. Moreover, transplantations are excluded, since transplantations involve two patients, but are only reimbursed by the health insurance of the recipient such that costs are assigned to both donor and recipient, while reimbursements are only attributed to the recipient. Inpatient cases with documented MDR-colonisation at hospital admission are compared to controls never infected or colonized with a MDR organism.
Matching
Each of the positive cases (n = 2006) is randomly matched with up to four controls (n = 7917) within the same primary diagnosis (4 digit, ICD-10). Eligible controls are required to not have a positive resistant pathogen status during their stay. For a few cases there were less than four controls satisfying the matching criteria available. Again, patients colonized > 48 h after admission, patients with onset infections and transplant patients were excluded from the pool of potential controls. The primary diagnosis matching is used since in the G-DRG payment system, costs and reimbursements are highly clustered within main diagnosis groups, as most costs are disease- or procedure-related. This within-main-diagnosis approach prevents the comparison of controls with cases with different fixed costs unrelated to the colonization with resistant pathogens. Multivariate analyses are conducted to include additional potential confounders such as age, sex and comorbidities.
Risk adjustment
For risk adjustment, the Charlson comorbidity index (CCI) is applied [17]. The CCI is a weighted index consisting of 19 comorbid conditions. The score was adjusted as described by Quan et al. [18] to comply with the ICD-10 systematic and is a widely used tool to control for underlying differences in comorbidities when evaluating attributable mortality, length of stay or costs in patients.
Regression model
For the multivariate analyses, a generalized linear model (GLM) is chosen, to account for the right skewed distribution of health care cost data and reduce sensitivity to outliers [19, 20]. For the outcome (InEK-)costs and reimbursements, a log link and a gamma distribution were chosen, based on the results of Modified Park Tests. The models include the main diagnosis groups as fixed effect as well as age, age2, sex, and the CCI as continuous and categorical covariates, respectively. Two different models are estimated for each outcome, first using the aggregated binary variable, indicating a positive pathogen status, and second the three pathogens separately. All models use robust standard errors. As log links are used, exponentiated coefficients represent the multiplicative increase in the costs (or reimbursements) from a one-unit increase in the respective independent variable. Because GLMs focus inference on the overall marginal mean, predictions of mean costs (or reimbursements) are estimated from the model [21]. Computation of standard errors or confidence intervals for the additive difference in means is obtained using the “margins” command in Stata [22], which computes standard errors using the delta method.
For the statistical analysis Stata Version 14.1 (Stata Corp, College Station, Texas, USA) is used.
Results
Patient population
Table 1 presents the descriptive statistics for cases and unexposed controls. Due to the within-main-diagnosis matching process, 7917 controls are elected for the multivariate analysis from the pool of 183,378 possible controls. As can be seen, the selected actual controls are much more similar to the cases in terms of relevant characteristics such as cost, illness severity (CCI) and age as a result of the matching process.
Descriptive statistics regarding the differences between the three resistant pathogens MRSA, VRE and MDR-GN are shown in Additional file 1. Co-colonization with more than one pathogen is possible. Heterogeneity between the pathogens is visible, with VRE being associated with the largest costs and reimbursements. Since the results are unadjusted for possible confounding factors, the effects are most likely driven by comorbidities and/or advanced age.
Adjusted statistical analysis
Table 2 shows the results of the regression for the outcomes cost and reimbursement, at first for all community-acquired cases combined, then separately for each of the three pathogens. In order to interpret the estimation results, the coefficients were transformed to present the percentage increase of the variable of interest [23]. Below the estimates, marginal effects are calculated. Being colonized with a resistant pathogen increases costs per case by 26% or 1500€ compared to controls. The cost effects differ between the pathogens. While MDR-GN leads to the largest cost increase, of nearly 2000€ per case, MRSA is associated with a cost increase of over 1600€ per case, followed by VRE with nearly 900€ all other things equal.
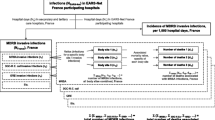
Results for the outcome reimbursement show a similar pattern. Patients colonized with a resistant pathogen accrue additional reimbursements of around 887€ per case or 16% more than controls. Cases with the highest cost estimates have larger reimbursements, as visualized in Fig. 1, although the difference is negative for all three pathogens. We find cost increases for MDR-GN cases of about 1300€ compared to unexposed controls, 1200€ for MRSA cases, and about 300€ for VRE cases.
Additional costs of MDR-colonised patients in comparison to non-colonised controls. Notes: Risk adjusted estimates with 95% confidence intervals as calculated in Table 2
As shown in Fig. 1, additional costs associated with colonizations are higher than the compensation payment.
Discussion
We find that cases colonized with MDR pathogens are associated with additional costs even without the onset of an infection. Even though these cases are asymptomatic carriers, they incur a considerable financial burden. Additionally, our study focused on community-onset cases of colonization, estimating the financial burden of hospitals due to the influx of MDR cases from the community. Even excluding transmissions occurring in the hospital, costs due to colonization are considerable and exceed reimbursement, in effect penalizing hospitals for events outside their control. These extra costs may be due to measures to prevent the spread of pathogens such as single room isolation, but can also indicate higher treatment cost [24].
Second, the results show that it is important to account for possible heterogeneity between different pathogens, as the large differences between our findings for MRSA, VRE and MDR-GN attest. This may be explained by the observation that some pathogens are more commonly found in specific tissues than others, so that the pathogens differ regarding their detectability via screening and testing [25]. It is, for example, easier to screen for skin colonizing MDR organisms than those colonizing the gut. Additionally, decolonisation measures are sometimes used for some pathogens, such as MRSA [26]. Decolonization measures would incur cost through the expenses for the measures themselves, but, if successful, reduce costs for the remainder of that patient’s hospital stay, since the decolonized patient would no longer have to be isolated [27]. This may explain some of the differences in the cost of MRSA compared to MDR-GN or VRE, for which no decolonisation regimens yet exist.
Finally, it is sometimes difficult to assess the risk of subsequent infections by the colonising MDR. For example, in case of asymptomatic colonisation of the respiratory tract by a MDR-GN, physicians still might choose to treat the patient with antibiotics to prevent subsequent pneumonia. Treatment costs would increase, and possibly even LOS if patients are kept longer in the hospital for observation.
Limitations of this study include the definition of the variable of interest, community-onset resistant colonization. It is not possible to distinguish between genuine community-acquired and previous healthcare-associated colonization from previous visits to health care facilities or nursing homes. Previous studies found a correlation between colonization and previous hospitalization [28, 29]. Unfortunately, our routine data does not provide information about previous visits to such facilities. It is possible that the variable is driven by unobserved comorbidities, as it can be hypothesized that a patient being transferred from a health care facility is on average not as healthy as a patient transferred from home, keeping age and sex constant. Following this assumption, the variable identified in this study would thus not directly measure the economic burden of colonization cases, but rather work as an additional indicator for unobserved comorbidities.
However, our matching of controls and MDR carriers included the CCI as a comparable and standardised proxy indicator for comorbidity. With the exception of VRE, CCI was lower in patients than controls, suggesting that the number of previous stays in other health care facilities - based on the assumption that higher morbidity increases the chance of such stays - is not different between controls and colonized patients. VRE on the other hand are usually not as pathogenic as MRSA or MDR-GN, more often causing colonizations rather than infections [30], i.e. instead of killing multimorbid patients, VRE remain colonizing bystanders and are therefore often found in highly morbid patients with increased likelihood of previous hospital stays [31]. These colonizations might thus be a predictor of previous stays in health care facilities.
Only considering cases and controls that never developed an infection can be considered conditioning on the future, as this information is not known at baseline. To circumvent this bias, exposure density sampling is suggested [32]. However, as the infections are very rare within eligible controls, the bias is likely negligible.
Since routine data is used, coding errors may be present. However, according to the department collecting the data, these errors are likely to be random rather than systematic for purposes of our analysis.
Finally, generalizability is another possible limitation, since our data is for one German hospital only. Despite a similar regulatory framework these findings may be different in other German settings and hospitals elsewhere.
While interpreting the results, the definition of the case group as well as the control has to be kept in mind. All patients with an onset infection are excluded. As infections tend to be cost intensive, the selection leads to an observation group which is on average healthier and less expensive. This step is nonetheless necessary in order to isolate the economic burden of MDR colonization, and results may be biased downward, so the conclusion still remains. However, estimates for costs and reimbursements in the literature vary, which is due to heterogeneities in the methodologies and datasets used.
Conclusions
Taking all strengths and limitations into account, this study demonstrates the importance of accounting for the cost of cases of colonization without infection when analysing the economic burden of antibiotic resistance. The results suggest that MDR bacteria present at hospital admission can add a serious financial burden during a patient’s hospital stay. Since this penalizes the hospitals for events outside their control, a case could be made to classify pre-existing colonization as a type of co-morbidity justifying higher reimbursement.
Abbreviations
- AMR:
-
Antimicrobial resistance
- CCI:
-
Charlson comorbidity index
- DRG:
-
Diagnosis related groups
- GLM:
-
Generalized linear model
- HAI:
-
Healthcare-associated infection
- ICD-10-GM:
-
International Classification of Diseases 10th revision, German Modification
- InEK:
-
Institute for the Payment system in Hospitals
- LOS:
-
Length of stay
- MDR:
-
Multi-drug resistant
- MDR-GN:
-
Multi-resistant gram-negative bacteria
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- UMCF:
-
University Medical Centre Freiburg
- VRE:
-
Vancomycin-resistant enterococcus
References
World Health Organization. Worldwide country situation analysis: response to antimicrobial resistance. [Internet]. 2015 [cited 2018 Jun 11]. Available from: http://apps.who.int/iris/bitstream/10665/163468/1/9789241564946_eng.pdf?ua=1&ua=1
Kim T, Oh PI, Simor AE. The economic impact of methicillin-resistant Staphylococcus aureus in Canadian hospitals. Infect Control Hosp Epidemiol. 2001 Feb;22(02):99–104.
Neidell MJ, Cohen B, Furuya Y, Hill J, Jeon CY, Glied S, et al. Costs of healthcare- and community-associated infections with antimicrobial-resistant versus antimicrobial-susceptible organisms. Clin Infect Dis. 2012;55(6):807–15.
Resch A, Wilke M, Fink C. The cost of resistance: incremental cost of methicillin-resistant Staphylococcus aureus (MRSA) in German hospitals. Eur J Health Econ. 2009;10(3):287–97.
Mutters NT, Günther F, Sander A, Mischnik A, Frank U. Influx of multidrug-resistant organisms by country-to-country transfer of patients. BMC Infect Dis. 2015 [cited 2018 Jun 11];15(1). Available from: http://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-015-1173-8
Ibert F, Eckstein M, Günther F, Mutters NT. The relationship between subjective perception and the psychological effects of patients in spatial isolation. GMS Hyg Infect Control. 2017;12:Doc11.
Cluzet VC, Gerber JS, Nachamkin I, Metlay JP, Zaoutis TE, Davis MF, et al. Duration of colonization and determinants of earlier clearance of colonization with methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2015;60(10):1489–96.
Graves N, Harbarth S, Beyersmann J, Barnett A, Halton K, Cooper B. Estimating the cost of health care–associated infections: mind your p’s and q’s. Clin Infect Dis. 2010;50(7):1017–21.
Hübner C, Ried W, Flessa S. Assessing the opportunity costs of patients with multidrug-resistant organisms in hospitals. Eur J Health Econ. 2017 [cited 2018 Jun 11]; Available from: http://link.springer.com/10.1007/s10198-017-0949-8
Mutters NT, Günther F, Frank U, Mischnik A. Costs and possible benefits of a two-tier infection control management strategy consisting of active screening for multidrug-resistant organisms and tailored control measures. J Hosp Infect. 2016;93(2):191–6.
Quentin W, Geissler A, Scheller-Kreinsen D, Busse R. DRG-type hospital payment in Germany: the G-DRG system. Euro Obs. 2010;12(3):4–6.
Braun J-P, Bause H, Bloos F, Geldner G, Kastrup M, Kuhlen R, et al. Peer reviewing critical care: a pragmatic approach to quality management. GMS Ger Med Sci. 2010;8:Doc23 ISSN 1612–3174.
Gutmann A, Kaier K, Sorg S, von zur Mühlen C, Siepe M, Moser M, et al. Analysis of the additional costs of clinical complications in patients undergoing transcatheter aortic valve replacement in the German health care system. Int J Cardiol. 2015;179:231–7.
Reinöhl J, Gutmann A, Kollum M, von zur Mühlen C, Baumbach H, Avlar M, et al. Transfemoral aortic valve implantation: bleeding events, related costs and outcomes. J Thromb Thrombolysis. 2013;35(4):469–75.
Vogl M. Assessing DRG cost accounting with respect to resource allocation and tariff calculation: the case of Germany. Health Econ Rev. 2012;2(1):15.
Zarb P, Coignard B, Griskeviciene J, Muller A, Vankerckhoven V, Weist K, et al. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2012;17(46).
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J-C, et al. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med Care. 2005;43(11):1130–9.
Barber J, Thompson S. Multiple regression of cost data: use of generalised linear models. J Health Serv Res Policy. 2004;9(4):197–204.
Griswold M. Analyzing health care costs: a comparison of statistical methods motivated by Medicare colorectal Cancer charges. Biostatistics. 2004;1(1):1–23.
Smith VA, Maciejewski ML, Olsen MK. Modeling Semicontinuous Longitudinal Expenditures: A Practical Guide. Health Serv Res. 2018 [cited 2018 Jun 11]; Available from: http://doi.wiley.com/10.1111/1475-6773.12815
Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J. 2012;12(2):308.
Lloyd-Smith P, Younger J, Lloyd-Smith E, Green H, Leung V, Romney MG. Economic analysis of vancomycin-resistant enterococci at a Canadian hospital: assessing attributable cost and length of stay. J Hosp Infect. 2013;85(1):54–9.
Tran K, Bell C, Stall N, Tomlinson G, McGeer A, Morris A, et al. The effect of hospital isolation precautions on patient outcomes and cost of care: a multi-site, retrospective, propensity score-matched cohort study. J Gen Intern Med. 2017;32(3):262–8.
Robinson J. Colonization and infection of the respiratory tract: what do we know? Paediatr Child Health. 2004;9(1):21–4.
Huang SS, Septimus E, Kleinman K, Moody J, Hickok J, Avery TR, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368(24):2255–65.
Nelson RE, Samore MH, Smith KJ, Harbarth S, Rubin MA. Cost-effectiveness of adding decolonization to a surveillance strategy of screening and isolation for methicillin-resistant Staphylococcus aureus carriers. Clin Microbiol Infect. 2010;16(12):1740–6.
Jung E, Byun S, Lee H, Moon SY, Lee H. Vancomycin-resistant enterococcus colonization in the intensive care unit: clinical outcomes and attributable costs of hospitalization. Am J Infect Control. 2014;42(10):1062–6.
Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis. 2003;36(2):131–9.
Mutters NT, Frank U. Sources of systematic errors in the epidemiology of vancomycin-resistant enterococci. Infection. 2013;41(2):305–10.
Mutters NT, Brooke RJ, Frank U, Heeg K. Low risk of apparent transmission of vancomycin-resistant enterococci from bacteraemic patients to hospitalized contacts. Am J Infect Control. 2013;41(9):778–81.
Wolkewitz M, Beyersmann J, Gastmeier P, Schumacher M. Efficient risk set sampling when a time-dependent exposure is present. Methods Inf Med. 2009;48(5):438–43.
Funding
This work was supported by the German Research Foundation [grant no. KA 4199/1–1 to TH and SEH]. KK has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115737–2 (Combatting bacterial resistance in Europe - molecules against gram negative infections [COMBACTE-MAGNET]). The funders had no role in data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
Due to German data protection laws and the European General Data Protection Regulation, we are unable to provide the data to the public: The UMCF data protection guidelines restrict the processing of the data the study is based on to the hospital’s internal IT network.
Author information
Authors and Affiliations
Contributions
SEH, TH and KK developed the research question and designed the methodology. NM provided the medical knowledge of hospital infection control practice informing the study design. NM and JW collected the data. SEH developed and implemented the formal analysis and statistical programming, with TH and KK contributing. SHE, NM and JW interpreted and contextualized the results. SEH wrote the initial draft, with all other authors contributing. All authors participated in the critical revision of the article, provided final approval of the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors meet the criteria for authorship as outlined in the BMC Health Services Research editorial policies.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Patients provided informed written consent on admission to the use of their pseudonymized data for scientific purposes, in accordance with German data protection law. The study only included pseudonymized routine data and the researchers were unable to connect data to individual patients. The study was conducted as part of the research project “Towards a full picture of the costs of antimicrobial resistance” funded by the German Research Foundation (DFG KA 4199/1–1). The project and its individual work packages were approved by the Ethics Committee of the University of Freiburg (Application 305/14) covering the present study. The data used were obtained from and used with permission of the Institute for Infection Prevention and Hospital Epidemiology and the Hospital Administration Department.
Consent for publication
Not applicable: no individual patient data.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Descriptive statistics separated by pathogens. Shows cost, reimbursement, length of stay, age, Charlson comorbidity index, in-hospital mortality and sex for controls as well as for cases separated by pathogen. (DOCX 15 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Engler-Hüsch, S., Heister, T., Mutters, N.T. et al. In-hospital costs of community-acquired colonization with multidrug-resistant organisms at a German teaching hospital. BMC Health Serv Res 18, 737 (2018). https://doi.org/10.1186/s12913-018-3549-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-018-3549-0