Abstract
Background
Hydrolates, complex mixtures containing traces of essential oils (EOs), are inexpensive, easy to make and less toxic than their corresponding EOs. The antibacterial and antifungal activity of the hydrolate of Coridothymus capitatus (L.) Reichenb. fil. (Lamiaceae) alone and in combination with antimicrobial drugs, such as tetracycline and itraconazole, were evaluated.
Methods
The chemical composition was analysed by gas-chromatography-mass spectrometry (GC-MS). Standard methods were performed to evaluate the susceptibility of some Gram-positive and Gram-negative bacteria, and Candida spp. to the hydrolate, in comparison with its EO. The hydrolate mechanism of action was assayed by propidium iodide and MitoTracker staining. Checkerboard tests were carried out for combinations studies.
Results
GC-MS identified 0.14% (v/v) of total EO content into hydrolate and carvacrol as a dominant component. The hydrolate showed a good antimicrobial activity against bacteria and yeasts. It exhibited a synergistic effect with itraconazole against Candida krusei, and an additive effect with tetracycline against methicillin-resistant Staphylococcus aureus strains. Hydrolate changed the membranes permeability of bacteria and yeasts and altered mitochondrial function of yeasts.
Conclusions
Our study extends the knowledge by exploiting non-conventional antimicrobial agents to fight the emergence of antibiotic resistance.
Similar content being viewed by others
Background
Essential oils (EOs) and aromatic oily compounds extracted from plant material, have been suggested as potential sources of new antimicrobial and therapeutic products [1,2,3,4,5]. In nature, EOs play a role in plant defence against microorganisms and insects [6]. In addition, they are valuable natural compounds used in many fields, i.e. pharmaceutical and food and cosmetic industries [7]. EOs composition is influenced by many factors such as species, cultivar, geographic and climatic conditions, cultivation practices, storage conditions of raw materials. Thus, wild plants of the same species due to different backgrounds can express many characters and chemical composition [8].
The genus Thymus L. (Lamiaceae) includes several species with biological properties, based on a number of active components such as carvacrol, thymol, p-cymene and γ-terpinene, known to exhibit different antimicrobial activity [9]. Medicinal plants of the genus Thymus are traditionally administered for whooping coughs, upper respiratory congestions, acute and chronic bronchitis, and gastrointestinal disorders [10].
Coridothymus capitatus (L.) Reichenb. fil. [syn. Thymus capitatus (L.) Hoffmanns. & Link], also known as Spanish origanum, is a Mediterranean aromatic shrub, extensively found all over Italy [11]. The EO, obtained from the flowers by hydrodistillation, is important for pharmaceutical and food and cosmetic industries. During distillation, parts of the EO components remain dissolved in the distillation water and the “product” is called hydrolate, which is also known as the aromatic water, floral water or hydrosol [12]. Hydrolates are complex mixtures containing traces of related EOs, and many water-soluble components. They are easy and inexpensive to produce and less toxic than their corresponding EOs [13, 14]. Hydrolates are used in the aqueous phase in the manufacture of lotions, and creams and soaps, or independently as tonics and air fresheners, as well as in the food industries. Since some previous evidence showed that hydrolates could have antimicrobial properties [15], in the present research we evaluated the antibacterial and antifungal activity of the hydrolate obtained from C. capitatus (L.) Reichenb. fil. grown wild on the sunny slopes and rocky crags of Ragusa, arid area of Sicily (Italy). Moreover, we studied the effect of hydrolate alone and in association with antimicrobial agents, such as tetracycline (TC) and itraconazole (ITC). TC is an inexpensive broad-spectrum antibiotic extensively used in the prophylaxis and therapy of bacterial diseases. However, the widespread of bacterial resistance caused to efflux and ribosomal protection mechanisms limited tetracycline’s effectiveness [16]. ITC is a lipophilic antifungal drug with lower toxicity, and may be used in association with EOs that are lipophilic compounds [17].
Methods
Plant material and extraction procedure
The hydrolate and EO of C. capitatus (L.) Reichenb. fil. (Batch: BI25A10102. Exp. 10/2017) were supplied by Exentiae S.R.L. (Catania, Sicily, Italy). They were obtained from plants grown in a classified area “Lauretum-Rosmarinetum”, of the Hyblaean Mountains, near Ragusa, Sicily. A voucher specimen was deposited in the Herbarium Mediterraneum Panormitanum housed in the Botanical Garden of Palermo, Italy (id number 71381). The hydrolate and EO were obtained by steam distillation in a Clevenger-type apparatus from dried flowers collected at the beginning of the flowering stage.
Gas chromatography/mass spectrometry (GC-MS) analysis
The C. capitatus hydrolate and EO were analysed using a Bruker Scion SQ gas chromatograph (Bruker Daltonics, Macerata, Italy), coupled with a single quadrupole (SQ) detector. A Zebron ZB -5HT Inferno capillary column (VF-5 ms, 30 m × 0.25 mm i.d., film thickness 0.25 μm) was used for separation. GC ramp: 60 °C (hold time 3 min), 60 to 150 °C (3.0 °C/min, hold 1 min), 150–380 °C (10 °C/min, hold 3 min). Injector temperature: 250 °C, hold 20 min. Helium 5.5 was used as carrier gas and the column gas flow settled to 1.00 mL/min.; ionization energy − 70 eV. Split/splitless ratio 1:30 after 45 s. Peaks identification was performed by retention indices, evaluated using the homologue n-alkane scale and by comparison of the experimental mass spectra fragments with those of the NIST mass spectral database (vers. 2.0, 2011), as well as with those of commercial standards. The relative percentage of the components was obtained by normalization of the peaks area.
EO (batch: BI25A10102) was diluted 1:1000 (v/v) in Ethylacetate, and 1 μL injected in GC-MS. The hydrolate was diluted 1:5 (v/v) in EtOH, and 1 μL injected in GC-MS.
The EO percentage (m/m) content in the hydrolate was evaluated by extracting 3 mL of hydrolate with 9.0 mL of CH2Cl2 (n = 3) [18]. After separation, the organic phases were pooled and the solvent evaporated under reduced pressure (IKA HB 10 basic, 280 rpm, T = 40 °C), until constant weight. The carvacrol (Aldrich-Fluka-Sigma S.r.l., Milan, Italy) percentage was evaluated by comparison of the GC-MS peak area with a calibration curve (y = 118415306.12x - 1083867346.94, r2 = 0.99946, n = 5 points). Results were obtained from three independent experiments performed in triplicate.
Antimicrobial agents
TC hydrochloride (superior quality) was purchased from Ningxia Qiyuan Pharmaceutical Co. LTD (No. I Qiyuan Street, Wangyyuan Industrial Area, Yinchuan, Ningxia, China) and ITC was obtained from Sigma-Aldrich (purity determined by HPLC = ≥98 percentage; n° I6657). TC stock solution was dissolved in phosphate buffered solution, pH 7 (PBS; Sigma-Aldrich), whereas ITC stock solutions in dimethylsulfoxide (100%; DMSO; Sigma-Aldrich), and then stored at − 20 °C.
Bacteria
Bacteria included in this study were Gram-positive and Gram-negative strains obtained from the in-house culture collection of the Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina (Italy). The Gram-positive bacteria included reference strains: Staphylococcus aureus ATCC 6538, S. aureus ATCC 43300, S. epidermidis ATCC 35984; Listeria monocytogenes ATCC 13932; Bacillus subtilis ATCC 6633 and some clinical isolates: S. aureus 7786, S. aureus 815 (methicillin resistant S. aureus-MRSA) and S. aureus 74CCH-MRSA. The Gram-negative strain was Pseudomonas aeruginosa ATCC 9027 [19]. The identification of all isolates was conducted using API systems (bioMérieux, Firenze, Italy). Isolates were stored at − 70 °C in Microbanks™ vials (DID, Pro-Lab Diagnostics, Ontario, Canada), until use.
Yeasts
The following yeast strains, obtained from the MiBat-TUCC collection of the Public Health and Pediatrics Department, Microbiology Section, Bacteriology and Mycology Laboratory, University of Turin (Italy), were tested: Candida albicans ATCC 90028, C. albicans ATCC 10231, C. glabrata ATCC 90030; clinical isolates of C. albicans 183, C. krusei 398, C. glabrata 32–09, C. norvegensis 112, C. lusitaniae 103, C. valida 287, C. guilliermondii 209, C. parapsilosis 198, and C. tropicalis 16–09. The clinical yeasts were collected from hospitalized patient specimens in Torino, identified by API systems (API ID32C panel), and stored at − 80 °C in Microbanks™ vials (DID), until use.
Antibacterial susceptibility testing
Bacterial cultures for antibacterial activity assays were grown in Mueller-Hinton Broth (MHB, Oxoid, Basingstoke, United Kingdom) for 24 h at 37 °C. Working cultures of bacteria were adjusted to the required inoculum of 105 CFU/mL. The Minimum Inhibitory Concentration (MIC) and the Minimum Bactericidal Concentration (MBC) of drugs and natural compounds were established by broth microdilution method, according to Clinical and Laboratory Standards Institute (CLSI, document M07-Ed11, 2018), with some modifications for hydrolate, and EO [20]. The hydrolate was used as such. The EO was dissolved to 5% using DMSO and further diluted using MHB to 2–0.016%. DMSO maximum concentration was 1% (v/v). Serial doubling dilutions of the hydrolate (100% product) and EO were prepared in a 96-well microtiter plates over the range of 50–0.1% (v/v) and 2–0.016% (v/v) in MHB, respectively. Growth controls (medium with inocula but without hydrolate or EO) were included. TC was tested against all strains at concentrations ranging from 32 to 0.016 μg/ml. Plates were incubated at 37 °C for 24 h. The MIC was considered as the lowest concentration of the hydrolate or the EO, at which there was no microbial growth. To determine MBC, bacterial aliquots (10 μL) were taken from each well and inoculated in Mueller-Hinton Agar (MHA, Oxoid). Cultures were incubated for 24 h at 37 °C. Results were obtained from three independent experiments performed in triplicate.
Antifungal susceptibility testing
Yeast cultures for antifungal activity tests were grown at 30 °C (24 h) in RPMI-1640 (0.2% glucose) supplemented with L-glutamine (Sigma-Aldrich), and 0.165 M 3-(N-Morpholino) propanesulfonic acid (MOPS) (pH 7) (Sigma-Aldrich), without sodium bicarbonate. Working cultures of yeasts were adjusted to the required concentration of 103 CFU/mL. The MIC and the Minimum Fungicidal Concentration (MFC) of drugs and natural compounds were detected by broth microdilution method, according to CLSI document M27-A3, 2008, with some modifications for hydrolate, and EO [21].
The hydrolate was used as such. The EO was dissolved to 5% using DMSO, and then diluted using RPMI-1640 plus MOPS to 2–0.016% (v/v). DMSO maximum concentration was 1% (v/v). Serial doubling dilutions of the hydrolate (100% product) and EO were prepared in a 96-well microtiter plates over the range of 50–0.1% (v/v) and 2–0.016% (v/v) in RPMI-1640 plus MOPS, respectively. Cultures were incubated for 24 h at 35 °C. Growth controls (medium with inocula but without hydrolate or EO) were included. MIC was considered the lowest concentration of hydrolate or the EO at which no microorganism growth was detected. MIC of ITC was defined as the lowest drug concentration that inhibited ≥50% growth inhibition in comparison with the control. To determine the MFC, fungal aliquots (10 μL) were taken from each well and spreaded onto Sabouraud Dextrose Agar (SDA). Cultures were incubated at 35 °C for 48 h [22, 23]. To define yeasts resistance to ITC published epidemiological cut-off values (ECVs) were used (ECV, 1 μg/mL) [24]. Results were obtained from three independent experiments performed in triplicate.
Checkerboard test
Based on the antibacterial and antifungal susceptibility testing results, MRSA strains (S. aureus ATCC 43300, S. aureus 815, S. aureus 74CCH), and some yeasts strains (C. albicans ATCC 90028, C. albicans ATCC 10231, C. albicans 183, C. glabrata 32–09, and C. krusei 398) were used to evaluate the efficacy of hydrolate in combination with TC or ITC, respectively. The checkerboard assay was used to assess drug synergism [25]. The ranges of TC/hydrolate were based on the MIC of the two compounds. Bacterial suspensions were prepared in MHB to yield an inoculum of 5 × 105 CFU/mL. The ranges of ITC/hydrolate were based on the MIC of the two compounds. Yeast suspensions were prepared in RPMI plus MOPS to yield an inoculum of 1.5 × 103 CFU/mL. Microplates were read after 24-48 h at 37 °C (bacteria and yeasts). Data were interpreted by the fractional inhibitory concentration index (FICI). A FICI value ≤0.5 was referred to synergy, whereas values between 0.5 and 1.0 were considered as additive. FICI values > 4.0 were interpreted as antagonism and FICI values between 1.0 and 4.0 were considered as indifferent [25]. Results were obtained from three independent experiments performed in triplicate.
Propidium iodide staining
To analyse the membrane integrity, fraction of surviving cells of S. aureus ATCC 6538 and C. albicans ATCC 10231 exposed to hydrolate were stained with propidium iodide (PI) solution (Sigma-Aldrich) [26]. The control sample of both strains was performed for comparison. Briefly, treated cells (0.5 MIC) were washed and suspended in PBS. Then, PI solution (final concentration of 1.25 μg/mL) was added to these cell suspensions and incubated for 10 min at 25 °C. After that, cells were washed twice with PBS to eliminate the excess of the stain and immediately examined under the inverted microscope Axio Observer.Z1 with ApoTome.2 (Zeiss, Milan, Italy). PI has a maximum emission peak at 606 nm. Results were obtained from three independent experiments performed in triplicate.
MitoTracker staining
To detect permeability changes of mitochondrial membrane, fraction of surviving cells of C. albicans ATCC 10231 exposed to hydrolate was stained with mitochondrion-specific dye MitoTracker®RedCMXRos (MTR) according to manufacturer’s instructions (Invitrogen, Fisher Scientific Italia, Rodano-Milan, Italy). The control sample of the strain was performed for comparison. The treated cells (0.5 MIC) were washed with PBS by centrifugation, and stained with 50 nM MTR for 15 min at 35 °C [26,27,28]. Stained cells were rinsed twice with PBS and examined by the above-mentioned fluorescence microscope. MTR has a peak of excitation at 579 nm and a peak of emission at 599 nm. Results were obtained from three independent experiments performed in triplicate.
Results
Chemical analysis of C. capitatus essential oil and hydrolate
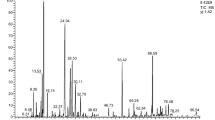
As reported in Table 1, the most abundant components identified in the C. capitatus EO were oxygenated structures (70.76%). The largest percentage was represented by phenols, carvacrol (67.58%) and thymol (0.16%), followed by alcoholic monoterpenes, such as β-linalool (0.97%), L-terpinen-4-ol (0.93%), borneol (0.48%) and α-terpineol (0.09%) (Fig. 1). Among the minority oxygenated compounds, a small percentage was represented by alcohols (1-octen-3-ol, 0.21%; 3-octanol, 0.02%), ketones (carvone, 0.04%; camphor, 0.02%; thujone, 0.01%), ethers (eucalyptol, 0.07%), and esters (carvacrol acetate, 0.03%; bornyl acetate 0.01%). Among the not oxygenated compounds (total amount 28.34%), monoterpenes amounted to 19.82% (mainly constituted by γ-terpinene: 6.80%; p-cymene: 6.44%; α-terpinene: 1.88%; β-myrcene: 1.53%; α-pinene: 1.47%; limonene: 0.57%) and sesquiterpenes to 8.50% (β-caryophyllene 7.74%; β-bisabolene: 0.32%; α-bisabolene 0.27%; humulene, 0.14%).
A. Effect of hydrolate on S. aureus (a1) and C. albicans (a2) by propidium iodide (PI) staining. The images showed PI positive staining of both strains due to altered membrane permeability. B. Effect of hydrolate on C. albicans mitocondria by Mitotracker staining. The images showed the mitochondria concentrated in compact masses at one side of the nucleus, none hyphal formation and extracellular material probably released by altered membrane (b1). Mitochondrial morphology of yeast: the images showed punctiform mitochondrial staining in untreated spores and hyphae (b2)
The EO content of the hydrolate was gravimetrically evaluated to be 0.1403% (v/v) (Table 1). The main constituents of the hydrolate identified in this study are carvacrol (93.11%) and thymol (6.34%). Terpineols (such as endo-borneol (0.18%), terpinen-4-ol (0.15%), and α-terpineol (0.08%)) and camphor (0.04%) were also present.
The quantitative analysis of carvacrol achieved by interpolation of the GC-MS peak area with the corresponding pure standard calibration curve (y = 119740852.99 x - 1005897752.26, r2= 0.99946, range of linearity 20–1000 ng) was of 76.31 mg/L (0.0952% v/v), in good accordance with the TGSC information system established for carvacrol water solubility (1250 mg/L) [29].
Antibacterial activity
Table 2 showed MIC and MBC data for hydrolate and EO against all tested strains. The order of susceptibility to hydrolate (MIC) was B. subtilis = S. aureus ATCC > S. aureus clinical isolates (including MRSA strains) = S. epidermidis = L. monocytogenes > P. aeruginosa strains, with MIC ranged from 12.5 to 50% of product (corresponding to 0.0175–0.07% (v/v) of EO in it). The order of susceptibility to EO (MIC) was B. subtilis > L. monocytogenes > S. aureus ATCC > S. aureus clinical isolates (including MRSA strains) = P. aeruginosa with MIC values ranged from 0.031 to 0.25% (v/v). The MBC values of both hydrolate and EO were generally equivalent or one more concentration above the MIC ones, except for P. aeruginosa. The MIC values of TC were in the range from < 0.125 μg/mL to > 4 μg/mL. The order of susceptibility was staphylococci ATCC strains (0.125 μg/mL) > MRSA strains (0.25 μg/mL) = B. subtilis ATCC 6633 > L. monocytogenes ATCC 13932 (1 μg/mL) > P. aeruginosa ATCC 9027 (4 μg/mL) (data not shown).
Antifungal activity
Table 3 showed MIC and MFC data for hydrolate and EO against all tested yeasts. The order of susceptibility to hydrolate (MIC) was C. glabrata > C. albicans = C. guilliermondii = C. parapsilosis > C. krusei = C. tropicalis > C. norvegensis = C. lusitaniae = C. valida strains, with values ranged from 6.25 to 50% of product, corresponding to 0.009–0.07% (v/v) of EO in it. The order of susceptibility to EO (MIC) was C. albicans ATCC = C. glabrata = C. lusitaniae = C. parapsilosis = C. tropicalis > C. krusei = C. albicans clinical isolate = C. norvegensis = C. valida = C. guilliermondii strains with MICs from 0.125 to 0.25% (v/v). The MFC values of both hydrolate and EO were generally equivalent or one more concentration above the MIC ones, indicating a fungicidal effect of the two samples. The MIC values of ITC were aligned from 0.5 μg/mL to 8 μg/mL. The order of susceptibility was C. albicans ATCC 90028 and clinical strains (0.5 μg/mL) > C. albicans ATCC10231 = C. glabrata (1 μg/mL) > C. parapsilosis = C. krusei = C. lusitaniae = C. valida = C. norvegensis = C. tropicalis (2 μg/mL) > C. guilliermondii (8 μg/mL) (data not shown).
Checkerboard test
The FICIs of hydrolate in association with TC were calculated to ascertain their possible interactions towards all MRSA strains. The combinations of hydrolate with TC showed additive interactions, with FICI values ranged from 0.75 to 1 (Table 4).
The FICI of hydrolate in combination with ITC was evaluated to determine the possible interactions against C. albicans, C. glabrata and C. krusei clinical strains. The data (Table 5), for hydrolate in combination with ITC, indicated synergistic interaction against C. krusei strains (FICI = 0.375), an additive interaction against C. albicans strains (FICI = 0.62) and an indifferent interaction against C. glabrata strains (FICI = 2). Values of the combination against C. albicans and C. glabrata indicated indifferent interactions; however, they were considerably lower than the antagonistic value of > 4.
Propidium iodide staining
Propidium iodide, a red-fluorescent nuclear stain, is a membrane impermeant dye that is generally excluded from viable cells. Microscopic examination demonstrated that S. aureus ATCC 6538 and C. albicans ATCC 10231 cells, treated with hydrolate (0.5 MIC), were stained red (about 84.2–78%, respectively), probably because they lose cell membrane integrity (Fig. 1-Aa1). Moreover, hydrolate clearly inhibited yeast-form growth (Fig. 1-Aa2). The untreated bacterial and yeast cells, about 26.8–21.3% respectively, lost their membrane permeability. Positively stained cells (PI+) were observed under inverted fluorescence microscope.
MitoTracker staining
MitoTracker is a specific mitochondrial stain in live cells and its accumulation depends on the membrane potential. However, once incorporated in the mitochondria, it can chemically link to thiol groups and will not leave the mitochondria when the membrane potential decreases as a result of fixation and/or cell death. Fluorescence microscope images of the treated C. albicans ATCC 10231 cells (about 70%) with hydrolate (0.5 MIC) highlighted absence of punctiform mithocondrial staining and showed only diffuse cytoplasmic staining, indicating that mitochondrial function was reduced. Moreover, the staining highlighted morphological changes of treated cells, none hyphal formation and extracellular material between cells caused probably, by the action of the hydrolate on the membrane integrity (Fig. 1-Bb1). The images of healthy non-treated C. albicans cells showed punctiform mitochondrial staining (Fig. 1-Bb2).
Discussion
Antibiotic resistance is a growing public health problem and the discovery and development of new antimicrobial drugs is becoming an important priority [30,31,32]. In recent years, researchers have been evaluating the antimicrobial activity of many Thymus EOs and their components. These compounds are of particular interest as it has never been reported any kind of resistance nor any form of bacterial adaptation to them [33,34,35].
In this research, we investigated the antimicrobial activity of the hydrolate from C. capitatus grown in a very dry territory of southeastern part of the Sicily. This hydrolate contained 0.14% (v/v) of total EO and the carvacrol was the dominant component in it. It exhibited higher antimicrobial activity towards Gram-positive bacteria (including MRSA strains) and yeasts such as C. glabrata and C. krusei, species that at the present time are often resistant to currently conventional drugs such as azoles (i.e fluconazole) and/or echinocandins (i.e caspofungin) [36]. C. krusei is intrinsically resistant to fluconazole and in both these two species the resistance to voriconazole is increasing mainly following exposure to fluconazole [37]. C. glabrata is the second most prevalent cause of candidiasis in USA, Australia, and Northern European countries [38]. This yeast has a reduced susceptibility to fluconazole and recently an increase in acquired echinocandin resistance has also been reported [36, 38].
The antimicrobial activity of hydrolate and EO could be related to carvacrol, the main compound contained in both products. Carvacrol (5-isopropyl-2-methylphenol) is a volatile phenolic monoterpene with antimicrobial properties [39, 40]. Terpenes have a great potential to traverse cell walls of bacteria and yeasts due to their large lipophilicity [41, 42]. Their antimicrobial action is due to the hydroxyl group and a delocalized electron system which cause destabilization of the membrane integrity of different microorganisms [1, 43,44,45,46]. Hydroxyl groups are highly reactive and form hydrogen bonds with active sites of target enzymes inactivating them and consequently a dysfunction or rupture of the cell membrane [39, 41, 44, 47]. In fact, our findings showed that bacteria and yeasts membrane and mitochondria were affected by hydrolate (Fig. 1 A-B). In addition, carvacrol acts as a proton exchanger, thereby reducing the pH gradient across the cytoplasmic membrane and shows ATPase-inhibiting activity that lead to reduction in other energy-dependent cell processes including synthesis of enzymes and toxins [48, 49]. Furthermore, it inhibits the synthesis of ergosterol in fungi [41].
Other components of this hydrolate, such as thymol and terpinen-4-ol have also demonstrated antimicrobial effects [1, 50]. The synergistic action between carvacrol and these components can occur despite their concentrations were lower than that of carvacrol, suggesting that the hydrolate overall high efficiency is also probably due to its high-water solubility. It has also been found that terpenoids exhibit antiseptic potential according to their solubility in water [51]. Active components of the hydrolate can better diffuse in the aqueous medium around microorganisms and their activity is increased compared to the EO that needs initial solubilisation in an organic solvent (DMSO) before introduction into the aqueous medium [34].
Moreover, the hydrolate in combination with ITC displayed a synergistic effect against C. krusei and an additive effect against C. albicans. This is a very positive result if it is taken into account the fact that C. krusei is intrinsically resistant to fluconazole and can cause breakthrough candidemia in immunocompromised patients receiving long-term prophylactic treatment with this azole. The mechanism of resistance to fluconazole and itraconazole could be due to low sensitivity of the drug target Erg11p to azole antifungals and of the constitutive expression of the multidrug efflux pumps [52].
Whereas in combination with TC, the hydrolate demonstrated an additive effect against MRSA strains. Resistant multi-drug strains, such as MRSA, are becoming a growing worldwide concern and a pressing need to improve MRSA infection therapies has been considered. In fact, S. aureus is a major pathogen both in hospitals and in the community [53]. In S. aureus, efflux mechanisms are able to confer resistance to various antimicrobial agents including tetracyclines [54].
The hydrolate is able to inhibit microbial growth and to reduce the required active amount of antibiotics when it is in combination. The synergic or additive effects of hydrolate against resistant bacteria and yeasts showed the promising tendency to apply it as an antibiotic adjuvant in combination with drugs. Probably, the main constituent carvacrol after its passage across the cell wall damaged the lipid bilayer of bacterial cell membrane increasing its permeability and enhancing TC effect. The same membrane damage on yeasts enhanced penetration of ITC into the cytoplasm and therefore increased its activity.
Conclusions
This study reported the characterization of the hydrolate of C. capitatus for the first time. It contained traces of total EO and the carvacrol was the main component in it. The antimicrobial activity of hydrolate was mostly direct against resistant bacteria (MRSA strains) and yeasts (C. glabrata and C. krusei). The mode of action could be due to increase of permeabilization of cell membrane of bacteria and yeasts and mitochondrial dysfunction in yeasts. In addition, the combination of C. capitatus hydrolate with antimicrobial agents reduced antibiotic minimum effective dose. Combination therapy between natural compounds and drugs may be able to recover the loss of function for existing antimicrobial agents improving the action and reducing side-effects.
In conclusion, these findings lay the ground for further more extensive investigations in order to identify new natural, cheap, safe and readily available antimicrobial agents with applicability for pharmaceutical topical formulations.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CFU:
-
Colony forming unit
- DMSO:
-
Dimethyl sulfoxide
- ECVs:
-
Epidemiological cut-off values
- GC-FID:
-
Gas-chromatography-flame ionization detector
- GC-MS:
-
Gas-chromatography-mass spectrometry
- ITC:
-
Itraconazole
- MIC:
-
Minimum Inhibitory Concentration
- MBC:
-
Minimum Bactericidal Concentration
- MFC:
-
Minimum Fungicidal Concentration
- MHB/MHA:
-
Mueller-Hinton Broth/Agar
- MiBat-TUCC:
-
Mycetes and Bacteria/Turin University Culture Collections
- PBS:
-
Phosphate buffered solution
- TC:
-
Tetracycline
References
Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94:223–53.
Solórzano-Santos F, Miranda-Novales MG. Essential oils from aromatic herbs as antimicrobial agents. Curr Opin Biotechnol. 2012;23:136–41.
Chouhan S, Sharmaand K, Guleria S. Antimicrobial activity of some essential oils—present status and future perspectives. Medicines. 2017;4:58.
Zhang N, Lan W, Wang Q, Sun X, Xie J. Antibacterial mechanism of Ginkgo biloba leaf extract when applied to Shewanella putrefaciens and Saprophytic staphylococcus. Aquacult Fisheries. 2018;3:163–9.
Geetha V, Chakravarthula SN. Chemical composition and anti-inflammatory activity of Boswellia ovalifoliolata essential oils from leaf and bark. J For Res. 2018;29:373–81.
Sainz P, Andrés MF, Martínez-Díaz RA, Bailén M, Navarro-Rocha J, Díaz CE, González-Coloma A. Chemical Composition and Biological Activities of Artemisia pedemontana subsp. assoana essential oils and hydrolate. Biomolecules. 2019;9. https://doi.org/10.3390/biom9100558.
Zuzarte M, Salgueiro L. Essential oils chemistry. In: de Sousa DP, editor. Bioactive essential oils and cancer. Switzerland: Springer International Publishing; 2015. p. 19–28.
Russo MT, Serra D, Suraci F, Postorino S. Effectiveness of electronic nose system to detect bergamot (Citrus bergamia Risso et Poiteau) essential oil quality and genuineness. J Essent Oil Res. 2012;24:137–51.
Nabavi SM, Marchese A, Izadi M, Curti V, Daglia M, Nabavi SF. Plants belonging to the genus Thymus as antibacterial agents: from farm to pharmacy. Food Chem. 2015;173:339–47.
Basch E, Ulbricht C, Hammerness P, Bevins A, Sollars D. Thyme (Thymus vulgaris L.), thymol. J Herb Pharmacother. 2004;4:49–67.
Pignatti S. Flora d’Italia. Bologna: Edagricole New-Business Media; 1982.
Wajs-Bonikowska A, Sienkiewicz M, Stobiecka A, Maciąg A, Szoka Ł, Karna E. Chemical composition and biological activity of Abies alba and A. koreana seed and cone essential oils and characterization of their seed hydrolates. Chem Biodivers. 2015;12:407–18.
Prusinowska R, Śmigielski K, Stobiecka A, Kunicka-Styczyńska A. Hydrolates from lavender (Lavandula angustifolia)-their chemical composition as well as aromatic, antimicrobial and antioxidant properties. Nat Prod Res. 2016;30:386–93.
Tornuk F, Cankurt H, Ozturk I, Sagdic O, Bayram O, Yetim H. Efficacy of various plant hydrosols as natural food sanitizers in reducing Escherichia coli O157:H7 and Salmonella typhimurium on fresh cut carrots and apples. Int J Food Microbiol. 2011;148:30–5.
Sagdic O, Ozcan M. Antibacterial activity of Turkish spice hydrosols. Food Control. 2003;14:141–3.
Chopra I. New developments in tetracycline antibiotics: glycylcyclines and tetracycline efflux pump inhibitors. Drug Resist Updat. 2002;5:119–25.
Scalas D, Mandras N, Roana J, Tardugno R, Cuffini AM, Ghisetti V, Benvenuti S, Tullio V. Use of Pinus sylvestris L. (Pinaceae), Origanum vulgare L. (Lamiaceae), and Thymus vulgaris L. (Lamiaceae) essential oils and their main components to enhance itraconazole activity against azole susceptible/not-susceptible Cryptococcus neoformans strains. BMC Complement Altern Med. 2018;18:143.
Monsef-Esfahani HR, Amanzade Y, Alhani Z, Hajimehdipour H, Faramarzi MA. GC/MS analysis of Citrus aurantium L. hydrolate and its comparison with the commercial samples. Iranian J Pharm Res. 2004;3:177–9.
Marino A, Blanco AR, Ginestra G, Nostro A, Bisignano G. Ex vivo efficacy of gemifloxacin in experimental keratitis induced by methicillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 2016;48:395–400.
Clinical and Laboratory Standards Institute-document M07-Ed11. Wayne, CLSI; 2018.
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; Approved Standard M27-A3. Wayne: CLSI; 2008.
Mandras N, Nostro A, Roana J, Scalas D, Banche G, Ghisetti V, Del Re S, Fucale G, Cuffini AM, Tullio V. Liquid and vapour-phase antifungal activities of essential oils against Candida albicans and non-albicans Candida. BMC Complement Altern Med. 2016;16:330.
Tullio V, Nostro A, Mandras N, Dugo P, Banche G, Cannatelli MA, Cuffini AM, Alonzo V, Carlone NA. Antifungal activity of essential oils against filamentous fungi determined by broth microdilution and vapour contact methods. J Appl Microbiol. 2007;102:1544–50.
Pfaller MA, Messer SA, Woosley LN, Jones RN, Castanheira M. Echinocandin and triazole antifungal susceptibility profiles of opportunistic yeast and mould clinical isolates (2010–2011): application of new CLSI clinical breakpoints and epidemiological cutoff values to characterize geographic and temporal trends of antifungal resistance. J Clin Microbiol. 2013;51:2571–81.
Van Vuuren S, Viljoen A. Plant-based antimicrobial studies–methods and approaches to study the interaction between natural products. Planta Med. 2011;77:1168–82.
Blanco AR, Nostro A, D’Angelo V, D’Arrigo M, Mazzone MG, Marino A. Efficacy of a fixed combination of tetracycline, chloramphenicol, and colistimethate sodium for treatment of Candida albicans keratitis. Invest Ophthalmol Vis Sci. 2017;58:4292–8.
Oliver BG, Silver PM, Marie C, Hoot SJ, Leyde SE, White TC. Tetracycline alters drug susceptibility in Candida albicans and other pathogenic fungi. Microbiology. 2008;154:960–70.
Shibata T, Takahashi T, Yamada E, Kimura A, Nishikawa H, Hayakawa H, Nomura N, Mitsuyama J. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob Agents Chemother. 2012;56:5892–7.
Carvacrol, 499–75-2 - The Good Scents Company. TGCS information system. (http://www.thegoodscentscompany.com/data/rw1027311.html).
Högberg LD, Heddini A, Cars O. The global need for effective antibiotics: challenges and recent advances. Trends Pharmacol Sci. 2010;31:509–15.
Owen L, Laird K, Wilson PB. Structure-activity modelling of essential oils, their components, and key molecular parameters and descriptors. Mol Cell Probes. 2017. https://doi.org/10.1016/j.mcp.2017.12.004.
Marino A, Zengin G, Nostro A, Ginestra G, Dugo P, Cacciola F, Miceli N, Taviano MF, Filocamo A, Bisignano G, Aktumsek A. Antimicrobial activities, toxicity and phenolic composition of Asphodeline anatolica E. Tuzlaci leaf extracts from Turkey. Nat Prod Res. 2016;30:2620–3.
Chorianopoulos NG, Giaouris ED, Skandamis PN, Haroutounian SA, Nychas GJ. Disinfectant test against monoculture and mixed-culture biofilms composed of technological, spoilage and pathogenic bacteria: bactericidal effect of essential oil and hydrosol of Satureja thymbra and comparison with standard acid–base sanitizers. J Appl Microbiol. 2008;104:1586–96.
Karampoula F, Giaouris E, Deschamps J, Doulgeraki AI, Nychas GJ, Dubois-Brissonnet F. Hydrosol of Thymbra capitata is a highly efficient biocide against Salmonella enterica Serovar Typhimurium biofilms. Appl Environ Microbiol. 2016;82:5309–19.
Yap PSX, Lim SHE, Hu CP, Yiap BC. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine. 2013;20:710–3.
Arendrup MC, Patterson TF. Multidrug-resistant Candida: epidemiology, molecular mechanisms, and treatment. J Infect Dis. 2017;216supp:S445–51. https://doi.org/10.1093/infdis/jix131.
Schell WA, Jones AM, Garvey EP, Hoekstra WJ, Schotzinger RJ, Alexander BD. Fungal CYP51 inhibitors VT-1161 and VT-1129 exhibit strong in vitro activity against Candida glabrata and C. krusei isolates clinically resistant to azole and echinocandin antifungal compounds. Antimicrob Agents Chemother. 2017;61(3):e01817–6. https://doi.org/10.1128/AAC.01817-16.
Colombo AL, Júnior JNA, Guinea J. Emerging multidrug-resistant Candida species. Curr Opin Infect Dis. 2017;30:528–38.
De Martino L, De Feo V, Formisano C, Mignola E, Senatore F. Chemical composition and antimicrobial activity of the essential oils from three chemotypes of Origanum vulgare L. ssp. hirtum (link) letswaart growing wild in Campania (southern Italy). Molecules. 2009;14:2735–46.
Magi G, Marini E, Facinelli B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant group a streptococci. Front Microbiol. 2015;6:165. https://doi.org/10.3389/fmicb.2015.00165.
Ahmad A, Khan A, Akhtar F, Yousuf S, Xess I, Khan LA, Manzoor N. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur J Clin Microbiol Infect Dis. 2011;30:41–50.
Zacchino SA, Butassi E, Cordisco E, Svetaz LA. Hybrid combinations containing natural products and antimicrobial drugs that interfere with bacterial and fungal biofilms. Phytomedicine. 2017;37:14–26.
Altintas A, Tabanca N, Tyihák E, Ott PG, Móricz AM, Mincsovics E, Wedge DE. Characterization of volatile constituents from Origanum onites and their antifungal and antibacterial activity. J AOAC Int. 2013;96:1200–8.
de Sousa JP, Torres Rde A, de Azerêdo GA, Figueiredo RC, Vasconcelos MA, de Souza EL. Carvacrol and 1,8-cineole alone or in combination at sublethal concentrations induce changes in the cell morphology and membrane permeability of Pseudomonas fluorescens in a vegetable-based broth. Int J Food Microbiol. 2012;158:9–13.
Mancini E, Camele I, Elshafie HS, De Martino L, Pellegrino C, Grulova D, De Feo V. Chemical composition and biological activity of the essential oil of Origanum vulgare ssp. hirtum from different areas in the southern Apennines (Italy). Chem Biodivers. 2014;11:639–51.
Nostro A, Marino A, Blanco AR, Cellini L, Di Giulio M, Pizzimenti F, Sudano Roccaro A, Bisignano G. In vitro activity of carvacrol against staphylococcal preformed biofilm by liquid and vapour contact. J Med Microbiol. 2009;58:791–7.
Guimarães AC, Meireles LM, Lemos MF, Guimarães MCC, Endringer DC, Fronza M, Scherer R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules. 2019;24:2471. https://doi.org/10.3390/molecules24132471.
Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013;6:1451–74.
Nostro A, Papalia T. Antimicrobial activity of carvacrol: current progress and future prospectives. Recent Pat Antiinfect Drug Discov. 2012;7:28–35.
Burt SA, van der Zee R, Koets AP, de Graaff AM, van Knapen F, Gaastra W, Haagsman HP, Veldhuizen EJ. Carvacrol induces heat shock protein 60 and inhibits synthesis of flagellin in Escherichia coli O157:H7. Appl Environ Microbiol. 2007;73:4484–90.
Mahizan NA, Yang SK, Moo CL, Song AAL, Chong CM, Chong CW, Abushelaibi A, Lim SHE, Lai KS. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules. 2019;24:2631. https://doi.org/10.3390/molecules24142631.
Lamping E, Ranchod A, Nakamura K, Tyndall JDA, Niimi K, Holmes AR, Niimi M, Cannon RD. Abc1p is a multidrug efflux transporter that tips the balance in favor of innate azole resistance in Candida krusei. Antimicrob Agents Chemother. 2009;53:354–69.
Edelsberg J, Weycker D, Barron R, Li X, Wu H, Oster G, Badre S, Langeberg WJ, Weber DJ. Prevalence of antibiotic resistance in US hospitals. Diagn Microbiol Infect Dis. 2014;78:255–62.
Costa SS, Junqueira E, Palma C, Viveiros M, Melo-Cristino J, Amaral L, Couto I. Resistance to antimicrobials mediated by efflux pumps in Staphylococcus aureus. Antibiotics. 2013;2:83–99.
Acknowledgements
The authors thank Vincenza Isabella for the expert English language assistance.
Funding
The authors received no specific grant from any funding agency.
Author information
Authors and Affiliations
Contributions
AM and VT formulated and realized the study; NM, JR, GG collected and identified yeast strains; AM, AN, NM, JR, NM, MFT, FG, GB made the experiments and interpreted the data; AM and VT wrote the manuscript. AM and VT revised the manuscript. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Natalizia Miceli is a member of the editorial board (Associate Editor) of this journal. The other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Marino, A., Nostro, A., Mandras, N. et al. Evaluation of antimicrobial activity of the hydrolate of Coridothymus capitatus (L.) Reichenb. fil. (Lamiaceae) alone and in combination with antimicrobial agents. BMC Complement Med Ther 20, 89 (2020). https://doi.org/10.1186/s12906-020-2877-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-020-2877-x