Abstract
Background
Diabetes mellitus is a chronic disease characterized by hyperglycemia that may occur due to genetic, environmental or lifestyle factors. Natural remedies have been used to treat diabetes since long and many antidiabetic compounds of varied efficacies have been isolated from medicinal plants. Rhazya stricta has been used for decades for the treatment of diabetes mellitus and associated ailments. Considering the folkloric use of R. stricta against diabetes, it was aimed to investigate the effectiveness of its root extracts against diabetes through in vitro assays and in vivo studies using animal model along with phytochemical profiling through GCMS.
Methods
Various fractions of Rhazya stricta obtained through column chromatography were evaluated for a variety of assays including α-glucosidase, Dipeptidyl peptidase-IV (DPP-IV), β-secretase and Glucagon-like peptide-1 (GLP-1) secretion studies. For the in vivo studies the alloxan-induced diabetic mice were treated with root extracts and blood glucose levels, HbA1C, and other biochemical markers along with the histological study of the liver were done. The phytochemical identification was performed using an Agilent 7890B GC coupled to a 7010 Triple Quadrupole (MS/MS) system. GraphPad Prism software version 5.01 was used for statistical analysis.
Results
Majority of the extract fractions showed excellent results against diabetes by inhibiting enzymes DPP-IV (Up to 61%) and β-secretase (Up to 83%) with IC50s 979 μg/ml and 169 μg/ml respectively with increase in the GLP1 secretion. The results of in vivo studies indicated a marked reduction in blood glucose and HbA1c levels along with positive effects on other parameters like lipid profile, liver functions and renal functions of extract-treated mice as compared to control. The histological examination of the liver demonstrated hepatoprotective effects against diabetes led changes and various classes of phytochemicals were also identified through GCMS in different fractions.
Conclusion
The results revealed strong antidiabetic activity of R. stricta root with the potential to protect body organs against diabetic changes. Moreover, a variety of phytochemicals has also been identified through GCMS that might be responsible for the antidiabetic potential of Rhazya stricta root.
Graphical abstract

Similar content being viewed by others
Background
Diabetes is considered among the top five causes of death in most high-income countries and the half of patients died from diabetes mellitus (DM) were under 60 years of age [1]. The rise in the number of patients of type 2 DM is associated with obesity, hypertension, and an increasingly elderly population and one of the main reasons is the unhealthy lifestyle with no adherence to improved diet and poor exercise [2]. Even though a wide range of therapeutics are available to counter diabetes [3], medical science is still facing a challenge of management of diabetes mellitus with fewer side effects [4].
The use of therapeutic agents and changes in lifestyle are the strategies that can prevent or at least delay the development of DM in patients with impaired glucose tolerance [5]. The use of synthetic drugs for diabetes mellitus leads to secondary complications, therefore; alternative medicines system is taken into account by health-care professionals due to their efficacy, fewer side effects and cost-effectiveness [6]. The antihyperglycemic property of the medicinal plants entirely depends on the chemical constituents present inside the plants [7, 8] and many antidiabetic compounds have been isolated from plants like gelagine, pycnogenol and berberine [9].
Rhazya stricta Decne (Apocynaceae) is a folkloric medicinal plant being used in traditional medicine systems against DM [10, 11]. In 1996, Tanira et al. reported an increase in the insulin concentration after administration of R. stricta extracts to animals resulting in glucose levels reduction [12]. In a study by Ahmed et al., a noteworthy decline in blood glucose levels was observed in diabetic mice after oral administration of extracts fractions of Rhazya stricta [13]. In another comparative study, Ahmed et al. [14] also reported Rhazya stricta as the best in lowering blood glucose levels in animals among other medicinal plants used for the management of diabetes.
Along with other reports, a study by Ali BH [15] reported significant decrease in blood glucose in rats after treatment with the R. stricta extracts along with increase in insulin level after 1 h, 2 h and 4 h oral doses of the extracts. Moreover, Baeshen et al. described an improvement in insulin levels in rats along with improved lipid profile and liver function tests by alkaloid rich fraction of R. stricta [16].
For DM therapy, the advancement of medical care uses a different array of lifestyle and pharmaceutical interventions [17]. The use of alpha-glucosidase inhibitors like Acarbose, Miglitol, and Voglibose can hinder the release of glucose from complex carbohydrates in the small intestine, consequently suppressing postprandial hyperglycemia. Along with beneficial effects, the use of alpha-glucosidase inhibitors is associated with gastro-intestinal disorders [18]. Likewise, glucagon-like peptide-1 (GLP-1) is an incretin hormone that plays different roles in blood glucose regulation in the gut [19]. This hormone is rapidly inactivated by an enzyme dipeptidyl peptidase-IV (DPP-IV) also present in the gut within less than a minute after being secreted [20]. In the treatment of DM, the inhibition of DPP-IV enzyme is a novel strategy that could potentially affect glucose regulation through multiple effects [21,22,23]. In addition, the beta-amyloid (APP) cleaving enzyme (BACE1) levels may also play an important role in glucose and lipid homeostasis in conditions of chronic nutrient excess [24] and central BACE-1 level was reported to be higher after insulin deficiency produced through streptozotocin injection [25]. BACE1 is also found to be involved in the regulation of the insulin receptor in the liver [26]. Hence, considering BACE1 as a therapeutic target, efforts to discover and develop BACE1 inhibitors have been pursued in the last few years [27].
To address many biological and biomedical questions and to increase human knowledge, the role of animal models in experimental research cannot be denied. Many of the animal species are being used in the field of biology due to their phylogenetic proximity to humans [28]. Animal models have a major contribution in the field of diabetic research and we can get valuable information about pathways that contribute to the induction of diabetes mellitus in humans [29]. Moreover, animal models are considered as an essential tool to understand the molecular basis, pathogenesis of diabetes and to evaluate the different therapeutic agents [30].
In the present study, keeping in view the folkloric use of R. stricta for the treatment of DM, roots of R. stricta were evaluated for anti-diabetic effects through various assays including animal study. Alloxan induced diabetic mice were treated with the extracts and effects of root extracts on blood glucose and other biochemical parameters were evaluated. Liver histology of mice was also done to determine the hepatoprotective/ hepatotoxic effects of the extracts on the liver as it is the vital organ involves in the detoxification of metabolites, synthesis of various proteins and other biochemicals necessary for digestion.
Methods
Plant collection and extract preparation
Roots of R. stricta plant were collected in the month of April from District Karak, KPK, Pakistan, after approval of the study from Bioethics Committee of Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad, Pakistan. Roots were identified by Prof. Dr. Rizwana Aleem Qureshi, Taxonomist, department of plant sciences (Voucher Specimen No. 130290). Roots were carefully inspected and all deteriorated parts or foreign material was separated and thoroughly washed with tap water for the removal of sand or dust particles. After drying, roots were cut into small pieces followed by shade drying for about 2–3 weeks in a well-ventilated area. The dried material was then ground using a laboratory grinder to ease the extraction process.
The crude extracts of dried roots was prepared through maceration (ammonical-chloroform: methanol = 1:1) at room temperature and fractionated through solvent-solvent extraction which resulted in four fractions termed as First Aqueous Layer (FAL), Second Chloroform Layer (SCL), Third Aqueous Layer (TAL) and Forth Chloroform Layer (FCL). In continuation of our previous work [31] in which the Second Chloroform Layer exhibited the most promising results against diabetes; it was decided to further fractionate the SCL fraction through normal phase column chromatography and evaluate for antidiabetic potential.
Further fractionation of Second Chloroform Layer (37.3 g) was carried out by normal phase column chromatography using a thick glass column with silica (260–280 mesh) and eluted with chloroform, ethyl acetate, and methanol by gradient change in mobile phase with increasing polarity (starting from 10% ethyl acetate in chloroform to 100% ethyl acetate and then 5% methanol in ethyl acetate to 100% methanol). Similar fractions were combined on the basis of analytical normal phase TLC. Resulting 23 combinations were termed as A-W master fractions and E-W fractions (755-2876 mg) were further purified through flash column chromatography (FCC). The complete fractionation scheme is shown below diagrammatically (Fig. 1).
Fraction S4 was the only fraction obtained as a whitish powdered form containing some noticeable compounds based on TLC which were expected to be isolated easily and weight of fraction was higher than all fractions so it was further processed through column chromatography and eluted with same mobile phases mentioned above and four sub-fractions (S4a, S4b, S4c and S4d) were obtained. Fractions A-D were not further processed due to a minute quantity of waxy material (un-dissolved in the solvent of choice) and in total, 95 fractions (A to W5) were obtained through fractionation of SCL (Table 1). It is important to mention here, that all obtained fractions were tested for the evaluation of antidiabetic potential through in vitro enzyme inhibition assays and on the basis of these results, fractions with good activity were selected for further evaluation as described in the results section of each assay.
Enzyme inhibition assays
Dipeptidyl peptidase-IV (DPP-IV) inhibition assay
All obtained fractions were investigated for their inhibitory potential against the dipeptidyl peptidase-IV enzyme as done earlier by Fujiwara & Tsuru [32]. The assessment of DPP-IV inhibition was done fluorometrically using Gly-Pro-aminomethyl coumarin substrate, purified porcine DPP-IV enzyme (1 U/ml) and Berberine (Flouorochem, UK) as a positive control. All samples were run in triplicate using 96 well microtiter plate. Briefly, 20 μl sample, 30 μl AMC substrate and 20 μl DPP-IV enzyme (1 U/ml) were added in each of the respective well and reaction mixture was incubated at 37 °C with gentle agitation for one hour. After incubation, 100 μl Acetic acid (3 mM) was added in each well to stop the reaction. The amount of free AMC after liberation from the substrate is monitored using Excitation and Emission wavelength at 351 nm and 430 nm respectively [33] with the help of the Tecan Safire fluorometer (Reading, England). Extracts were tested at different concentrations like 1.0, 0.5 and 0.25 mg/ml. HEPES buffer was used as negative control and percentage inhibition was calculated by the given formula. Finally, IC50 values were calculated which represents the 50% inhibition of DPP-IV activity by each fraction.
Where “Fc” is the fluorescence of negative control and “Fs” is of the sample.
α-Glucosidase inhibition assay
Fractions obtained through fractionation of SCL fraction were investigated for α-glucosidase inhibition activity according to the method described earlier [34] with the help of enzymatic assay kit and PGM7 Micro-Stat Analyzer (Analox Instruments Ltd.; London, UK). Briefly, the crude enzyme was prepared from rat intestinal acetone powder (Sigma, UK) with a 50 mM citrate buffer (pH 6.0) in a 1:9 (w/v) ratio and the supernatant obtained was used as the crude enzyme solution for assays. Extract samples were dissolved in PBS buffer pH 7.4 which was prepared by mixing KCl (0.2 g), NaCl (8.0 g), Na2HPO4 (1.44 g) and KHPO4 in 1000 ml of distilled water. Experimentally, the reaction mixture contained substrate (Maltose) 300 μl, rat intestinal acetone extract 10 μl and plant extract 150 μl. Acarbose (Sigma-Aldrich, UK) was used as positive control and PBS buffer as a negative control. The enzyme activity or its inhibition was monitored by the formation of glucose from carbohydrate during the reaction at specific time intervals include 0, 30 and 60 min (the whole intervals) during incubation of reaction mixture at 37 °C using PGM7 analyzer.
β-Secretase inhibition assay
β-Secretase or BACE1 activity was done as described earlier by Cox et al., [35] using a kit of Sigma (Sigma-Aldrich, Inc.) with slight modification. The kit was provided with BACE-1 enzyme, substrate, standard, buffer and stop solution. The same buffer was used for the preparation of enzyme and substrate at required concentration 0.3 units/μl and 50 μM respectively according to the manufacturer’s instruction. β-secretase inhibitor (KF-14, Mol. Formula C73H118N16O27, Mol.wt. 1651.85) was also procured from GL Biochem (Shanghai) Ltd. China. Assay components (test samples/inhibitor/buffer, enzyme, substrate) were mixed to a final volume of 100 μL in each well of a 96-well microtiter plate. The fluorescence level was measured at zero and 2 h after incubation at 37 °C using Excitation and Emission wavelength at 320 nm and 405 nm respectively with the help of Tecan Safire fluorometer (Reading, England, UK). Stop solution was added in all wells to stabilize the signal for up to 24 h.
Secretion of glucagon-like peptide-1 (GLP-1)
Cell culturing (Secondary cell culture/ Cell lines) and GLP-1 secretion studies were done according to the procedure of Gillespie et al. [36] and measurement of the GLP-1 level using GLP-1 ELISA kit of EMD-Millipore Corporation USA. Dulbecco’s Modified Eagle Medium (DMEM) was used for cell culturing which comprised 4.5 g/l D-glucose with L-glutamine, without sodium pyruvate (Gibco, Paisley, UK) and supplemented with 17.5% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. Culturing was done by seeding about two million cells per well into 12-well plates in 1 ml culture medium with 18 h incubation at 37 °C in a 5% CO2 humidified atmosphere.
Washing with buffer (10 mM Glucose, 20 mM HEPES, 4.5 mM KCl, 140 mM NaCl, 1.2 mM MgCl2, 1.2 mM CaCl2) was done after removing cells from medium and incubated further for 1 h with the same buffer. The buffer aspired off and cells were incubated for 3 h with 400 μl extract samples. The same volume (400 μl) of HEPES buffer was added in respective well as vehicle (negative) control. In the final step before ELISA, centrifugation of vehicle control and test agents was done to get rid of cellular debris and stored at − 80 °C.
GLP-1 measurement
Measurement of GLP-1 was done in duplicate by following the procedure of GLP-1 ELISA assay kit of EMD-Millipore, USA. Anti-GLP-1 monoclonal antibodies were already coated in the 96-well microtiter plate. The standards, QC 1, QC 2 and samples were added after assay buffer in respective wells, mixed gently and the plate was incubated at 4 °C for 20–24 h. On next day detection conjugate was added, incubated for 2 h and after washing three times with wash buffer, the substrate was added in each well. After 20 min incubation stop solution was added to stop the reaction and incubated again for a further 5 min at room temperature in the dark to arrest phosphatase activity. The fluorescence was measured using Excitation and Emission wavelength at 355 and 460 nm respectively with the help of the Tecan Safire2 fluorometer (Reading, England, UK). The amount of GLP-1 was determined in the sample from the standard curve constructed with standards provided along with the kit. For GLP-1 secretion studies, some selected fractions with good enzyme inhibitory action were evaluated at a single concentration of 1 mg/ml.
In-vivo studies
On the basis of results of in vitro assays, two fractions S4c and V4 were selected for in vivo studies along with SCL fraction as these fractions showed good inhibitory activity against DPP-IV and BACE1 enzymes along with some GLP-1 secretory potential. Although almost similar results were observed with some other fractions but only S4c and V4 were obtained with reasonable weight, as for animal study a good weight of testing material is required for a group of animals and this was another reason of selection of these fractions. Both these fractions were semi-purified fractions containing off-white powder obtained from fractionation of the second chloroform layer.
Approval of the study protocol
Study protocols were approved by the Institutional Animal Use and Care Committee (IAUCC) of the National Institute of Health, Islamabad, Pakistan and permission for collection of animal samples was also sought prior to the study. All institutional procedures and protocols regarding animal handling and care (NIH guidelines) were followed and adherence to the ARRIVE guidelines [37] was also taken into account.
Selection and housing of animals
Briefly, 2 m (8–10 weeks) old healthy male BALB/c albino mice (weight 28.2 ± 1.5 g) were procured from the animal house of the National Institute of Health (NIH), Islamabad, Pakistan. The complete in vivo study was done in the animal house of NIH because of established standardized conditions like 12/12 light-dark cycle with temperature 25 ± 5 °C and relative humidity 35–60%. Initially, all animals were housed in cages to acclimatize for 1 week and fed with standard pellet diet and tap water ad libitum. The cages were solid-bottomed provided with softwood bedding and enough space for movement. Frequent cage cleaning and change of bedding were ensured to maintain hygienic conditions.
Experimental procedure
Induction of diabetes was done intraperitoneally by injecting freshly prepared 150 mg/kg of alloxan monohydrate (Sigma-Aldrich Co., USA) in normal saline and 5% glucose solution was also placed in bottles to avoid hypoglycemic shock for 24 h. The blood glucose level was checked after 2 weeks of alloxan injection with Glucometer (ACCU-CHEK® Performa, Roche) by tail puncture and mice with fasting glucose level > 200 mg/dl were selected for the study. Fifty-four mice were randomly grouped into nine groups each containing six mice as described below and every group was housed in a separate cage with free access to pellet diet and water.
-
Group I- Normal Control (NC)
-
Group II- Alloxan-induced Diabetic Untreated Control (DC)
-
Group III- Alloxan-induced Diabetic Treated Control received Glibenclamide 5 mg/kg b.wt/day (TC)
-
Group IV- Alloxan-induced Diabetic received SCL extract 10 mg/kg b.wt /day (SCL-I)
-
Group V- Alloxan-induced Diabetic received SCL extract 20 mg/kg b.wt /day (SCL-II)
-
Group VI- Alloxan-induced Diabetic received S4c fraction 10 mg/kg b.wt /day (S4c-I)
-
Group VII- Alloxan-induced Diabetic received S4c fraction 20 mg/kg b.wt /day (S4c-II)
-
Group VIII- Alloxan-induced Diabetic received V4 fraction 10 mg/kg b.wt /day (V4-I)
-
Group X- Alloxan-induced Diabetic received V4 fraction 20 mg/kg b.wt /day (V4-II)
All samples were given through oral intubation in 5% DMSO in physiological saline [38] and to harmonize the experiment, 5% DMSO in saline was also given to normal and diabetic untreated control groups throughout the study.
Blood collection and samples processing
On 28th day at 9:00 am, all animals were anesthetized {Forane® (Isoflurane) Batch No. 6048245 of Abbott Laboratories (Pakistan) Ltd.}without fasting. Anesthesia was given by expert veterinarian using cotton soaked with 3% Isoflurane individually by drop jar method aiming to immediate effects and less distress. Blood was withdrawn through heart puncture (diaphragmatic approach) and placed in proper blood collection tubes. Euthanasia was carried out immediately after each blood collection by higher doses of anesthetic. Animals were given few more minutes in the chamber and death was confirmed by examining absence of respiratory activities, no heartbeat and no response to toe pinch. After that, livers were dissected and preserved in 10% formalin for histopathology.
After complete tissue processing of liver [39], microscopic examination was done using 40x objectives (Nikon). All biochemical parameters like blood glucose level, glycosylated hemoglobin (HbA1C), alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase, urea, creatinine, uric acid, cholesterol, triglyceride, total protein, and albumin were measured with the help of fully automated Spectrophotometer “Vitalab Selectra E” of Vital Scientific N.V. /Clinical Data Inc. USA, using reagents of “DiaSys” Diagnostic Systems, GmbH Germany.
GC-MS analysis
The GC-MS analysis was performed using an Agilent 7890B GC with the split-splitless inlet, coupled to a 7010 Triple Quadrupole (MS/MS) system from Agilent, equipped with EI ion source. The system was operated by MassHunter Software with MSD ChemStation. The separation was carried out on an Agilent J&W DB-5 ms capillary column (30 m × 0.25 mm i.d., 0.25 mm film thickness). Helium (purity 99.999%) was employed as carrier gas at a constant column flow of 1.0 mL/min. The GC oven temperature was programmed from 50 °C (held 1 min) to 270 °C at 10 °C/min (held 5 min). The temperature of the transfer line, the quadrupole and the ion source were set at 280, 150 and 230 °C respectively. The injector temperature was set at 250 °C. The injector was operating in the split-less mode and programmed to return to the split mode after 2 min from the beginning of a run. The MSD was operated in full scan acquisition mode. Mass spectra deconvolution of chromatographic signals and tentative identification of unknown bioactive compounds was carried out using the Agilent MassHunter Unknown Analysis tool and the NIST Mass Spectral Library (NIST MS Search 2.0).
Statistical analysis
Statistical analysis was performed using GraphPad Prism software version 5.01. All values are expressed as mean ± SEM using one-way ANOVA with Tukey’s posttest for comparison. The p-value ≤0.05 was considered significant.
Results
All of the samples in the vehicle were given to mice for 4 weeks until sacrifice and no restlessness, any irritation or any adverse event was noted during the study.
DPP-IV inhibition
The fractions obtained from the second chloroform layer have displayed significant activity against diabetes by inhibiting DPP-IV enzyme (p < 0.001). Initially, all fractions obtained from the second chloroform layer (SCL) were analyzed at a single concentration (1 mg/ml) and then some fractions were chosen on the basis of the highest DPP-IV inhibition and processed further. The majority of fractions showed a good dose-dependent inhibitory response to DPP-IV but some of them like H4, N6, N7, Q1, S3, S4c, U2, U5, and V4, have displayed marked inhibition of DPP-IV (Fig. 2a). IC50 values are therefore calculated using a linear regressing curve with interpolation from the standard curve and given in Table 2.
α-Glucosidase inhibition assay
Alpha-glucosidase inhibition assay was performed for all fractions of the second chloroform layer (SCL) initially at 1 mg/ml concentration to pick fractions with the highest inhibition. Most of the fractions were able to inhibit the enzyme activity but fractions P1, Q4, W4, and W5 exhibited maximum (> 25%) inhibition activity like 36.7, 25.5, 31.8 and 39.2% respectively (Fig. 2b). Three fractions with the highest activity were further processed for the calculation of IC50 value which caused enzyme inhibition in a concentration-dependent manner. IC50 values are shown in Table 3.
β-Secretase inhibition
The second chloroform layer and its fractions represented promising inhibitory effects against the BACE-1 enzyme. Initially, all fractions were analyzed at 400 μg/ml concentrations and based on these results, only fractions with > 70% BACE-1 inhibitory activity (Fig. 2c) were re-examined at a concentration ranging from 50 μg/ml to 400 μg/ml to determine the IC50 values. The IC50 values for the highly active fractions are afforded in Table 4. All fractions showed inhibition in a dose-dependent manner.
Secretion of GLP-1
GLP-1 secretory studies are made to examine the secretory response of some of the selected fractions in cultured cells at a single concentration (1 mg/ml) and measurement was done with ELISA based kit. The fraction P2 showed a significant increase in acute GLP-1 secretion by 116.6 pM/106 cells (p < 0.001) while fraction Q1 also increased secretion by 79.0 pM/106 cells (p < 0.005). All other tested fractions were unable to stimulate a significant response (p > 0.05). Overall, the fraction P2 was able to increase GLP-1 secretion by 3.3 fold (p < 0.001) while fraction Q1 exhibited a good response by 2.2 fold increase (p < 0.05) as compared to vehicle control (Fig. 2d).
In vivo studies
Reduction of blood glucose and glycosylated hemoglobin levels
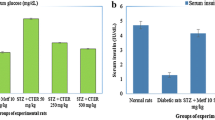
A significant increase in glucose concentration was observed in mice of the untreated diabetic control group (DC) as compared to the normal control group (NC). After 28 days of oral administration of the extract fractions, an observable reduction was noted in the glucose level of almost all groups in comparison with the untreated control group (Fig. 3). Similar results were observed in glycosylated hemoglobin (HbA1C) level and the noticeable decrease was shown by all treated groups of mice as compared to the untreated diabetic control group (DC). Overall, maximum activity has been shown by the SCL-II and its glucose-lowering effects are comparable with the results of the group treated with standard drug glibenclamide (TC).
Anti-hyperlipidemic potential of R. stricta root
The mice of untreated diabetic control groups (DC) have shown an increase in cholesterol and triglycerides level as compared to the normal control group (NC). On the other hand, a noteworthy decrease in these parameters was observed in the mice of the extract-treated group including the diabetic treated control group (TC) as compared to the untreated diabetic control group (DC). However, the best results have been observed in mice of the group treated with fraction SCL-II (Fig. 4).
Effects of root extract on kidney functions tests
It has been observed that the root extract fractions showed a positive effect on kidney functions of the mice of extract-treated groups. All parameters (urea, creatinine, uric acid) were observed to be high in the diabetic untreated control group and treatment of the mice with the extract fractions decreased blood urea, creatinine and uric acid levels in all groups on comparison with the diabetic untreated control group (Fig. 5).
Liver profile and the histological study
The liver profile done during this study has shown strong hepatoprotective effects of the root extract of the R. stricta against diabetic changes. Liver enzymes of mice of the diabetic untreated diabetic control group (DC) were increased while a marked decrease was observed in the total protein and albumin contents of mice of this untreated control group as compared to the normal control group (NC). The root extract and its fractions exhibited strong hepatoprotective effects as a significant decrease (p < 0.001) was observed in the liver enzymes like AST, ALT and ALP along with improvement in total protein and serum albumin levels in the extract-treated groups as compared to the diabetic untreated control group (DC) (Fig. 6).
Histological examinations of liver sections of the normal control group showed normal hepatocytes and veins with uniformity in cells but on the comparison, the untreated diabetic control group (DC) was observed to have hepatic alterations like vascular dilation, congestion and increased number of inflammatory cells. The extract fractions presented their hepatoprotective effects and preserved the normal texture of liver cells and no major cellular alterations were noted in the treated mice groups (Fig. 7).
Representative microscopic photographs of liver sections of mice of all groups (H&E Staining): Liver sections of normal mice showing normal hepatic cells a, untreated diabetic mice showing vascular dilation, congestion and infiltration of inflammatory cells b, treated mice with standard drug c, treated with fraction SCL-I d, treated with fraction SCL-II e, treated with fraction S4c-I f, treated with fraction S4c-II g, treated with fraction V4-I h and liver section of diabetic mice treated with fraction V4-II i
Identification analysis by GC-MS
The results obtained from the GC-MS analysis led to the tentative identification of a good number of compounds, based on the positive match of experimental mass spectra with NIST MS database, and data reported in the literature. The fractions showed the presence of many classes of compounds like diterpenes, sesquiterpenes, phenolics, quinolines, quinoxaline, isoxazoles, phenyl indoles, carbonyls, beta-carboline, and other indole group-containing compounds. Many phytochemicals were identified in the SCL fraction including alkaloids such as aspidospermidine, eburnamenine, quebrachamine, aspidofractinine, eburenine (1,2-didehydro-aspidospermidine), β-hydroxyquebrachamine, secoaspidospermidine as well as several fatty acid methyl esters like heptadecanoic acid methyl ester, 9-octadecenoic acid (Z)- methyl ester and eicosanoic acid methyl ester along with identification of several other heterocyclic compounds such as 2,4-dimethyl-quinoline, hexahydro-1H-Isoindole-1,3(2H)-dione and 4-(3-methyl-2-butenyl)-1H-Indole. Moreover, some Vinca alkaloids have also been identified for the first time in Rhazya stricta root like Vinburnine, Vincamine and Apovincamine.
Many of the compounds present in the SCL fraction were also identified in other fractions like aspidospermidine, 1,2-didehydroaspidospermidine, quebrachamine and some fatty acid methyl esters. The other analyzed fractions exhibited many compounds similar to the main fraction SCL and various other compounds which have not been identified in the main fraction SCL like N-methyl-Aspidodasycarpine, Talbotine, Astaxanthin, p-Cymene, Nerinine, 2-Anthracenamine, alfa-Copaene, Deoxyspergualin, D-Limonene and dozens of other compounds with pharmacological potential. A complete list of phytochemicals identified in fraction SCL with their presence in all other fractions is given in Table 5.
Discussion
Rhazya stricta is claimed to contain various medicinal properties including antihyperglycemic effects like some other members of Apocynaceae family e.g., Telosma procumbens Merr., Vinca rosea L., Picralima nitida [47]. Root extracts of the plant inhibited α-glucosidase enzyme that can lead to better glycemic control through a reduction in disaccharide hydrolysis [48]. The inhibition of the DPP-IV enzyme is another strategy to keep incretin hormones available in the blood for insulin-dependent disposal of glucose from the blood. Our study revealed that R. stricta root has dual activity to cope with DM as it inhibited the DPP-IV enzyme as well as stimulated GLP1 secretion. Despite the availability of many synthetic DPP-IV inhibitors, various studies have been conducted on medicinal plants and several plants are found to be active against DPP-IV including Mangifera indica, Berberis aristata (turmeric), Inonotus obliquus (a mushroom), Ocimum sanctum and Momordica charantia [49].
Rhazya stricta root extracts remarkably inhibition β-Secretase in a dose-dependent manner that is supposed to be involved in diet-induced obesity and related complications including diabetes. Recently, a study by Meakin et al., (2018) reported that BACE1 is a regulator of insulin signaling and the amount of insulin receptor in the liver and during diabetes, the amount of insulin receptor is found to be reduced due to the degradation by the BACE1 enzyme. In this connection, they suggested that insulin signaling can be improved with the use of BACE1 inhibitors during diabetes [50].
Continuous monitoring of the blood glucose levels of diabetic patients and HBA1C levels are considered important factors in the treatment of DM. The root extracts of R. stricta considerably reduced the blood glucose level and HbA1c in accordance with previous reports where aerial parts of the plant were seen reducing blood glucose levels and HbA1c accordingly [13, 14]. Another study reported that long-term oral administration of alkaloids rich fractions of R. stricta significantly improved the insulin level and insulin resistance which showed insulin secretagogue activity of the plant for the disposal of blood glucose [16].
An array of secondary metabolites is well-known for the treatment of diabetes reported from plant species. Initial in vitro, in vivo and enzyme based assays were considered strong tools to assess the plants’ antidiabetic potentials. After the initial screening, we have found a variety of the compounds of different phytochemical classes through GCMS. Interestingly, among the identified compounds in the SCL fraction, five have already been documented as antihyperglycemic compounds namely; Hexadecane [40], n-Hexadecanoic acid [41], Vinburnine, Vincamine [44] and Squalene [45]. Some other classes of compounds have also been identified and derivatives of which are reported of antidiabetic potential like Benzofuran [51], Quinoxaline [52] Quinoline [53, 54], Pyridine [55], Sydnones [56] Thioureas [57] and Isoquinolines [58]. Moreover, Berberine, that is an Isoquinoline alkaloid isolated from Rhizoma coptidis has shown hypoglycemic and anti-dyslipidemic activities [58].
Several studies reported the link of reactive oxygen species with diabetes and oxidative stress is considered as one of the main factors that have an important role in beta-cells dysfunction, resulting in diabetes [59]. The role of oxidative stress has also been determined in the pathogenesis and complication of diabetes [60, 61]. In this regard, various reports suggested the use of antioxidants in the aetiology of diabetes and its associated complications [62, 63]. Up-till now, many plants have been investigated extensively to search natural antioxidants to prevent/minimize the destructive process caused by the oxidative stress [64]. Several natural products are reported here which have proven antioxidant attributes including thioureas derivatives [57] trans-13-Octadecenoic acid, methyl ester, 9-Octadecenoic acid (Z)-, methyl ester [42], uleine [43], acetamide derivatives [65] and squalene [46]. Thus, the Rhazya stricta root extracts displayed its potential for DM by lowering blood glucose level through different mechanisms that might be a synergistic effect of many of the phytoconstituents present in the extracts.
In this study, positive effects of the extracts on other biochemical parameters like lipid profile, renal function tests, liver function tests, total protein and serum albumin of mice have also been observed. The outcome of these biochemical parameters pointed out lipid-lowering potential and beneficial effects of the root extract on kidneys and liver functions of the mice. The extract of the leaves of R. stricta was reported in lowering the levels of cholesterol and triglycerides in animals [13, 14, 16, 66]. The histological examination of the mice liver has shown that the administration of the root extract fractions has not posed any substantial alterations of liver cells and any kind of known hepatic variations like dilation, congestion, necrosis or hypertrophy were not seen in all treated groups. These results suggested that the root extracts of R. stricta has potential to preserve the hepatic cells against diabetes led changes and its associated complaints.
Furthermore, the acceptance of findings of plant based natural products lead animal research for human is debatable but keeping in view a wide range of commonalities in both human and mice, the results of basic research are required to be tested on human considering that these secondary metabolites are safe. We suggest root extract fractions of R. stricta as a strong candidate possessing antidiabetic activity and these results shows that our methodology achieved the main objectives of the study. The main limitation of using mice in this kind of research is the “blood volume” as effects of plant extracts on many other blood parameters could be done. However, number of animals can be reduced for this kind of basic research which may have no or limited impacts on outcome.
Conclusion
The root part of R. stricta is a potential source for the treatment of DM as it has demonstrated its effectiveness by inhibiting various key enzymes involved in the hyperglycemia. All the conducted assays clearly indicated the role of R. stricta root in lowering of blood glucose levels with a positive impact on the associated biochemical parameters and liver cells. Moreover, a variety of natural products including alkaloids, heterocyclic compounds etc. are identified in the root extract of R. stricta. We suggest a possible synergistic involvement of these identified phytochemicals in the antidiabetic activity of the extract.
Availability of data and materials
All data/material is available on request from the corresponding author.
Change history
08 December 2020
Open Access Funding note was added to backmatter text of the article. This article has been updated to correct this.
Abbreviations
- ALT:
-
Alanine Transaminase
- AST:
-
Aspartate Transaminase
- ALP:
-
Alkaline Phosphatase
- BACE-1:
-
Beta-amyloid (APP) cleaving enzyme
- DM:
-
Diabetes Mellitus
- DPP-IV:
-
Dipeptidyl peptidase-IV
- DMEM:
-
Dulbecco’s Modified Eagle Medium
- FAL:
-
First Aqueous Layer
- FCL:
-
Forth Chloroform Layer
- FCC:
-
Flash column chromatography
- GLP-1:
-
Glucagon-like peptide-1
- HbA1c:
-
Glycosylated hemoglobin
- GC-MS:
-
Gas Chromatography-Mass Spectrometry
- SCL:
-
Second Chloroform Layer
- TAL:
-
Third Aqueous Layer
- TLC:
-
Thin Layer Chromatography
References
Diabetes and impaired glucose tolerance. IDF Diabetes Atlas, 5th ed. Brussels, Belgium: International Diabetes Federation. 2012.
King DE, Iii AGM, Carnemolla M, Everett CJ. Adherence to Healthy Lifestyle Habits in US Adults, 1988–2006. AJM. 2009;122(6):528–34.
Bailey C, Kodack M. Patient adherence to medication requirements for therapy of type 2 diabetes. Int J Clin Pract. 2011;65(3):314.
Ortiz-Andrade R, Rodríguez-López V, Garduño-Ramírez M, Castillo-España P, Estrada-Soto S. Anti-diabetic effect on alloxanized and normoglycemic rats and some pharmacological evaluations of Tournefortia hartwegiana. J Ethnopharmacol. 2005; 101(1–3):37–42. Available from: https://www.sciencedirect.com/ science/ article/ pii/ S0378874105002618?via%3Dihub.
Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hamäläinen H, Ianne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–50.
Samyal ML, Ahuja A, Ahmed Z. Evaluation of Antidiabetic activity of isolated compound from Ougeinia oojeinensis bark extract in diabetic rats. UK J Pharm Biosci. 2014;2(5):27–33.
Liu C, Song J, Teng M, Zheng X, Li X, Tian Y, et al. Antidiabetic and Antinephritic Activities of Aqueous Extract of Cordyceps militaris Fruit Body in Diet-Streptozotocin-Induced Diabetic Sprague Dawley Rats. Vol. 2016, Oxidative Med Cell Longev 2016.
Bowman WCRM. Textbook of pharmacology. Oxford: Blackwell Scientific Publication; 1980. p. 121–36.
Ríos JL, Francini F, Schinella GR. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015;81(12–13):975–94.
Baeshen NA, Elkady AI, Abuzinadah OA, Mutwakil MH. Potential anticancer activity of the medicinal herb, Rhazya stricta, against human breast cancer. Afr J Biotechnol. 2012;11(37):8960–72.
Gilani SA, Kikuchi A, Shinwari ZK, Khattak ZI, Watanabe KN. Phytochemical, pharmacological and ethnobotanical studies of Rhazya stricta Decne. Phyther Res. 2007;21(4):301–7.
Tanira MO, Ali BH, Bashir AK, Chandranath I. Some pharmacologic and toxicologic studies on Rhazya stricta decne in rats, mice and rabbits. Gen Pharmacol. 1996;27(7):1261–7.
Ahmed A, Asad MJ, Ahmad MS, Rehmatullah Q, Shah SI, Hina G, et al. Antidiabetic and hypolipidemic potential of Rhazya stricta Decne extract and its fractions. Int Curr Pharm J. 2015;4(January):353–61.
Ahmed A, Gulfraz M, Asad MJ, Qureshi R, Bibi S, Shah SI. Hypoglycemic and hypocholesterolemic activity of leave of few medicinal plants against steptozotocin induced hyperglycemia. Pak J Pharm Sci. 2016;29(6):2065–70.
Ali BH. The effect on plasma glucose, insulin and glucagon levels of treatment of diabetic rats with the medicinal plant Rhazya stricta and with glibenclamide, alone and in combination. J Pharm Pharmacol. 1997;49(10):1003–7.
Baeshin NA, Yaghmoor SS, Ashmaoui HM, Al, Kumosani TA, Saini KS. The Indole-alkaloid fraction of Rhazya stricta improves key biochemical parameters associated with metabolic syndrome in rats. Botholia J 2014;44(5):358–371.
Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2011;29(2):116–22.
Van de Laar FA. Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vasc Health Risk Manag. 2008;4(6):1189–95.
Holst JJ. Treatment of type 2 diabetes mellitus with agonists of the GLP-1 receptor or DPP-IV inhibitors. Expert Opin Emerg Drugs. 2004;9(1):155-66. https://doi.org/10.1517/eoed.9.1.155.32952.
Deacon CF. Circulation and degradation of GIP and GLP-1. Horm Metab Res. 2004 Nov 18;36(11/12):761–5.
Green BD, Irwin N, Duffy NA, Gault VA, O’Harte FPM, Flatt PR. Inhibition of dipeptidyl peptidase-IV activity by metformin enhances the antidiabetic effects of glucagon-like peptide-1. Eur J Pharmacol. 2006;547(1–3):192–9 Available from: https://www.sciencedirect.com/science/article/pii/S001429990600776X.
Demuth H-U, McIntosh CHS, Pederson RA. Type 2 diabetes—therapy with dipeptidyl peptidase IV inhibitors. Biochim Biophys Acta. 2005;1751(1):33–44.
Vella A. Mechanism of action of DPP-4 inhibitors - new insights. J Clin Endocrinol Metab. 2012;97(8):2626–8.
Meakin PJ, Harper AJ, Hamilton DL, Gallagher J, McNeilly AD, Burgess LA, et al. Reduction in BACE1 decreases body weight, protects against diet-induced obesity and enhances insulin sensitivity in mice. Biochem J. 2012;441(1):285–96.
Guglielmotto M, Aragno M, Tamagno E, Vercellinatto I, Visentin S, Medana C, et al. AGEs/RAGE complex upregulates BACE1 via NF-κB pathway activation. Neurobiol Aging. 2012;33(1) Available from: http://www.elsevier.com/copyright.
Meakin PJ, Jalicy SM, Montagut G, Allsop DJP, Cavellini DL, Irvine SW, et al. Bace1-dependent amyloid processing regulates hypothalamic leptin sensitivity in obese mice. Sci Rep. 2018;8(1):1–16.
Coimbra JRM, Marques DFF, Baptista SJ, Pereira CMF, Moreira PI, Dinis TCP, et al. Highlights in BACE1 inhibitors for Alzheimer’s disease treatment. Front Chem. 2018:1–10. Available from: https://www.frontiersin.org/article/. https://doi.org/10.3389/fchem.2018.00178/full.
Andersen ML, Winter LMF. Animal models in biological and biomedical research – experimental and ethical concerns. Acad Bras Cienc. 2017:1–14.
Chatzigeorgiou A, Halapas A, Kalafatakis K, Kamper EF. The use of animal models in the study of diabetes mellitus. Vivo. 2009;23(2):245–58.
Chen D, Wang MW. Development and application of rodent models for type 2 diabetes. Diabetes Obes Metab. 2005;7(4):307–17.
Mahmood R, Malik F, Shamas S, Ahmed T, Kausar M, Rubnawaz S, Ashfaq M, Hussain S, Green BD, Mirza B. Pharmacological evaluation of Rhazya stricta root extract. Bol Latinoam Caribe Plant Med Aromat. 2020;19(2):188–206.
Fujiwara K, Tsuru D. New chromogenic and Fluorogenic substrates for Pyrrolidonyl peptidase. J Biochem. 1978;83:1145–9.
Saleem S, Jafri L, Ul-Haq I, Chang LC, Calderwood D, GreenBD MB. Plants Fagonia cretica L. and Hedera nepalensis K. Koch contain natural compounds with potent dipeptidyl peptidase-4 (DPP-4) inhibitory activity. J Ethnopharmacol. 2014;28(156):26-32. https://doi.org/10.1016/j.jep.2014.08.017.
Panwar H, Calderwood D, Grant IR, Grover S, Green BD. Lactobacillus strains isolated from infant faeces possess potent inhibitory activity against intestinal alpha- and beta-glucosidases suggesting anti-diabetic potential. Eur J Nutr. 2014;53(7):1465–74.
Cox CJ, Choudhry F, Peacey E, Perkinton MS, Richardson JC, Howlett DR, et al. Dietary (−)-epicatechin as a potent inhibitor of βγ-secretase amyloid precursor protein processing. Neurobiol Aging. 2015;36(1):178–87.
Gillespie AL, Calderwood D, Hobson L, Green BD. Whey proteins have beneficial effects on intestinal enteroendocrine cells stimulating cell growth and increasing the production and secretion of incretin hormones. Food Chem. 2015;189:120–8.
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010;8(6).
Matias M, Silvestre S, Falcao A, Alves G. Consideration and pitfalls in selecting the drug vehicle for evaluation of new drug candidate: focus on in vivo pharmaco-toxicological assay based on the rotarod performance test. J Pharm Pharm Sci. 2018;21:110–8.
Anwar M, Waqar MA, Khan FA, Tariq WUZ, Ahmed S, Mushtaq S, et al. Manual of Laboratory Medicines. 3rd Edition. Armed Forces Institute of Pathology Rawalpindi-Pakistan 2005.
Siporin C, Cooney JJ. Inhibition of glucose metabolism by n-hexadecane in Cladosporium ( Amorphotheca) resinae. J Bacteriol. 1976;128(1):235–41.
Tan DC, Kassim NK, Ismail IS, Hamid M, Bustamam MSA. Identification of antidiabetic metabolites from Paederia foetida L. Twigs by Gas Chromatography-Mass Spectrometry-based Metabolomics and Molecular Docking Study. Biomed Res Int. 2019:29. https://doi.org/10.1155/2019/7603125.
Asghar SF, Choudahry MI, Habib-Ur R, Atta-Ur R. Gas chromatography- mass spectrometry (GC-MS) analysis of petroleum ether extract (oil) and bio-assays of crude extract of Iris germanica. Int J Genet Mol Biol. 2011;3(7):95–100.
Rocha MP, Campana PRV, Scoaris D-D-O, Lúcia-de-Almeida V, JCD L, Silva AF, Pieters L, Silva CG. Biological activities of extracts from Aspidosperma Subincanum Mart. and in-Silico prediction for inhibition of Acetylcholinesterase. Phytother Res. 2018;32(10):2021–33. https://doi.org/10.1002/ptr.6133.
Pereira ASP, Haan HD, Peña-García J, Moreno MM, Pérez-Sánchez H, Apostolides Z. Exploring African medicinal plants for potential anti-diabetic compounds with the DIA-DB inverse virtual screening web server. Molecules. 2019;24:2002. https://doi.org/10.3390/molecules24102002.
Kumar SR, Yamauchi I, Narayan B, Katsuki A, Hosokawa M, Miyashita K. Squalene modulates fatty acid metabolism: enhanced EPA/DHA in obese/diabetic mice (KK-Ay ) model. Eur J Lipid Sci. 2016;118(12):1935–41.
Amarowicz R. Editorial. Squalene; A natural antioxidant? Eur J Lipid Sci Technol. 2009;111:411–2. https://doi.org/10.1002/ejlt.200900102.
Cajuday LA, Amparado EA. Hypoglycemic property of Telosma procumbens (Blanco) Merr. (Apocynaceae) in normal and alloxan-induced diabetic juvenile mice (Mus musculus). J Phytopharm. 2014;3(2):113–7.
Naquvi KJ, Ahamad J, Mir SR, Ali M, Shuaib M. Review on role of natural Αlpha-Glucosidase inhibitors for Management of Diabetes Mellitus. Int J Biomed Res. 2011;2(6):374–80 Available from: http://ssjournals.com/index.php/ijbr/article/view/665.
Sharma A, Paliwal G, Upadhyay N, Tiwari A. Therapeutic stimulation of GLP-1 and GIP protein with DPP-4 inhibitors for type-2 diabetes treatment. J Diab Metab Disord. 2015;14(15):1–8.
Meakin PJ, Mezzapesa A, Benabou E, Haas ME, Bonardo B, Grino M, et al. The beta secretase BACE1 regulates the expression of insulin receptor in the liver. Nat Commun 2018;9(1306):1–14. Available from: https://doi.org/10.1038/s41467-018-03755-2.
Dawood KM. An update on benzofuran inhibitors: a patent review. Exp Opinion Ther Patents. 2019;29(11).
Irfan A, Tahir OA, Umer M, Ahmad S, Kousar H. A review on biological studies of Quinoxaline derivatives. World J Pharm Pharmaceu Sci. 2017;6(2):11–30.
Bano B, Abbasi S, Khan JAJ, Hussain S, Rasheed S, Perveen S, Khan KM, Choudhary MI. Antiglycation activity of Quinoline derivatives- A new therapeutic class for the management of Type-2 Diabetes complications. Med Chem. 2014;11(1):60–8. https://doi.org/10.2174/1573406410666140526151254.
Heydari Z, Mohammadi-Khanaposhtani M, Imanparast S, Faramarzi MA, Mahdavi M, Ranjbar PR, Larijani B. Pyrano[3,2-c]quinoline derivatives as new class of α-Glucosidase inhibitors to treat type 2 diabetes: synthesis, in vitro biological evaluation and kinetic study. Med Chem. 2019;15(1):8–16. https://doi.org/10.2174/1573406414666180528110-104.
Altaf AA, Shahzad A, Gul Z, Rasool N, Badshah A, Lal B, Khan E. A review on the medicinal importance of Pyridine Derivatives. J Drug Design Med Chem. 2015;1(1):1–11 doi:https://doi.org/10.11648/j.jddmc.20150101.1.
Chandrasekhar R, Nanjan MJ. Sydnones: a brief review. Mini Rev Med Chem. 2012;12(13):1359–65. https://doi.org/10.2174/13895575112091359.
Naz S, Zahoor M, Umar MN, Ali B, Ullah R, Shahat AA, Mahmood HM, MUK S. Enzyme inhibitory, antioxidant and antibacterial potentials of synthetic symmetrical and unsymmetrical Thioureas. Drug Des Devel Ther. 2019;13:3485–95. https://doi.org/10.2147/DDDT.S225311.
Yin J, Ye J, Jia W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharm Sin B. 2012;2(4):327–34.
Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279:42351–4. https://doi.org/10.1074/jbc.R400019200.
DCCT. The diabetes control and complications trial research group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86.
Niedowicz D, Daleke D. The role of oxidative stress in diabetic complications. Cell Biochem Biophys. 2005;43:289–330.
Coleman MD, Fernandes S, Khanderia L. A preliminary evaluation of a novel method to monitor a triple antioxidant combination (vitamins E, C and [alpha]-lipoic acid) in diabetic volunteers using in vitro methaemoglobin formation. J Environ Toxicol Pharmacol. 2003;14:69–75.
Ozkan Y, Yilmaz OK, Ihsan AO, Yasemin E. Effects of triple antioxidant combination (vitamin E, vitamin C and a-lipoic acid) with insulin on lipid and cholesterol levels and fatty acid composition of brain tissue in experimental diabetic and non-diabetic rats. Cell Biol Int. 2005;29:754–60.
Kaur C, Kapoor HC. Antioxidants in fruits and vegetables ± the millennium’s health. Int J Food Sci Technol. 2001;36:703–25.
Autore G, Caruso A, Marzocco S, Nicolaus B, Palladino C, Pinto A, Popolo A, Sinicropi MS, Tommonaro G, Saturnino C. Acetamide derivatives with antioxidant activity and potential anti-inflammatory activity. Molecules. 2010;15:2028–38. https://doi.org/10.3390/molecules150-32028.
Baeshen NA, Lari SA, Aldoghaither HA, Elkady AI. Biochemical evaluation of the effect of Rhazya stricta aqueous leaves extract in liver and kidney functions in rats. Nat Sci. 2010;8(4):136–42.
Acknowledgements
National Institute of Health, Islamabad is acknowledged for guidance and support.
Funding
This study was funded by the Higher Education Commission of Pakistan through the International Research Support Initiative Program (1–8/HEC/HRD/2016/6291). Funds were provided to the first author and have been utilized for experimentation, analysis and interpretation of data. Open access funding provided by Royal Institute of Technology.
Author information
Authors and Affiliations
Contributions
BM, RM, WKK designed this study; RM, TA, SR, WKK collected plant material, prepared crude extract and did fractionation; FM, FR supervised all experimental work and contributed in data analysis; SH, RM, MA, OK, DC, FR performed laboratory work, data interpretation/analysis, and writing/editing the manuscript; HA helped in animal studies and histopathological examination; BDG, GAR made contribution in GC-MS analysis, interpretation, and manuscript editing; BM supervised whole of the study and BM, FR, WKK finalized the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Study protocols were approved by the Institutional Animal Use and Care Committee (IAUCC) of the Nation Institute of Health, Islamabad, Pakistan, and all institutional procedures and protocols (NIH guidelines) regarding the animal handling and care were followed.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mahmood, R., Kayani, W.K., Ahmed, T. et al. Assessment of antidiabetic potential and phytochemical profiling of Rhazya stricta root extracts. BMC Complement Med Ther 20, 293 (2020). https://doi.org/10.1186/s12906-020-03035-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-020-03035-x