Abstract
Background
Pyrola decorata H. Andres, is exclusively distributed in China and a source of traditional Chinese herbal medicine Luxiancao for more than 2000 years. Here, we evaluated the antioxidant and cytoprotective effects of P. decorata and its five phenolic components (protocatechuic acid, gallic acid, hyperoside, 2′′-O-galloylhyperin, and quercetin), and discussed their antioxidant chemistry.
Methods
A lyophilized aqueous extract of P. decorata (LAEP) was prepared and analyzed with high-performance liquid chromatography (HPLC). LAEP and its five phenolic components were comparatively investigated using five antioxidant assays, including ferric-reducing antioxidant power, cupric ion-reducing antioxidant capacity, 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide radical (PTIO•)-scavenging, 1,1-diphenyl-2-picryl-hydrazl radical (DPPH•)-scavenging, and 2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid) radical (ABTS+•)-scavenging activities. The reaction products of the five phenolic components with 4-methoxy-2,2,6,6-tetramethylpiperidine-1-oxyl radical (4-methoxy-TEMPO•) were determined with ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (UPLC-ESI-Q-TOF-MS/MS) analysis. LAEP and its five phenolic components were incubated with bone marrow-derived mesenchymal stem cells (bmMSCs) subjected to oxidative stress to demonstrate their cytoprotective effects with a flow cytometry assay.
Results
In the five antioxidant assays, LAEP and its five phenolic components dose-dependently increased the radical-scavenging (or reducing power) activities. However, the IC50 values of hyperoside were consistently higher than those of 2′′-O-galloylhyperin. UPLC-ESI-Q-TOF-MS/MS analysis results indicated that the five phenolics could yield dimer products in the presence of 4-methoxy-TEMPO• via the radical adduct formation (RAF) pathway. Flow cytometry assay results confirmed the cytoprotective activity of LAEP and its five phenolic components toward stressed bmMSCs. In particular, 2′′-O-galloylhyperin could more effectively reduce the percentage of damaged bmMSCs than hyperoside.
Conclusion
LAEP and its five phenolic components may undergo redox-based pathways (such as electron transfer and H+ transfer) and covalent-based pathway (i.e., RAF) to exhibit antioxidant activity. One consequence of RAF is the generation of phenolic-phenolic dimer. In both organic and aqueous media, 2′′-O-galloylhyperin exhibited higher redox-based antioxidant levels (or cytoprotective levels) than those with hyperoside. The differences could be attributed to 2′′-O-galloylation reaction.
Similar content being viewed by others
Background
Pyrola L. family comprises about 30 plant species, including P. japonica [1], P. incarnata Fisch [2], P. decorata H. Andres [3], and P. renifolia Maxim [4]. These plants are mainly distributed in temperate and cold temperate zones of the northern hemisphere [5]. P. decorata, however, is exclusively distributed in China [6].
The dried whole herb of P. decorata can act as the resource of Chinese herbal medicine Luxianao [7]. Luxianao however has been used in traditional Chinese medicine (TCM) for more than 2000 years. Modern pharmacological investigation has suggested that Luxiancao (or its bioactive components) exhibit anti-osteoarthritis [8], anti-inflammatory [8], hepatoprotective [9], and anti-influenza [10] properties. These pharmacological effects are known to be closely associated with its antioxidant and cytoprotective actions [4, 11,12,13]. However, the antioxidant mechanisms and cytoprotective effects of Luxiancao (P. decorata) have not been reported yet, regardless that its extract has been evaluated for the antioxidant capacity using chemical approach [4].
To investigate the antioxidant mechanisms, the study tried to firstly analyze the antioxidant phenolics of P. decorata using HPLC technology. These phenolics were further measured for the radical adduct formation (RAF) possibility using ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (UPLC-ESI-Q-TOF-MS/MS) technology. Especially, two flavonoids hyperoside (i.e., hyperin) and 2′′-O-galloylhyperin were comparatively analyzed in the study, this is because that: (1) they have been demonstrated to co-exist in P. decorata [4, 14, 15]; (2) Essentially, 2′′-O-galloylhyperin is a galloylation ester of hyperoside at 2′′-O-position (Fig. 1). Thereby, the comparison between them can be used to analyze the antioxidant structure-activity relationship of 2″-O-galloylation modification.
To explore the cytoprotective effects of P. decorata, bone marrow-derived mesenchymal stem cells (bmMSCs) were isolated from bone marrow of femur and tibia of rats [16, 17]. bmMSCs are known to possess characteristics of stem cells, including self-renewal and multi-directional differentiation properties [18], and bmMSC transplantation has been applied to treat heart diseases [19], spinal cord injuries [20] and diabetes [21]. During the process of diffusion and differentiation, chemical or physical factors, such as radiation and iron overload, could produce reactive oxygen species (ROS), resulting in the oxidative stress-induced damage to bmMSCs [22, 23]. The oxidative damage however has limited bmMSCs in transplantation engineering application. The study tried to use flow cytometric assay to evaluate the possible cytoprotective effect of P. decorata on oxidative stressed bmMSCs.
Apparently, the evaluation of the cytoprotective effects of this plant may improve our understanding of the beneficial properties of Luxiancao, and provide important information on its phenolics in MSCs transplantation engineering. The conclusion of the antioxidant structure-activity relationship of hyperoside and 2′′-O-galloylhyperin can also be used to explain the antioxidant difference between myricetin-3-O-rhamnopyranoside and myricetin-3-(2′′-O-galloyl)-O-rhamnopyranoside [24].
Methods
Reagents and materials
Protocatechuic acid (C7H6O4, CAS NO. 99–50-3, M.W. 154.1, purity 99%, Additional file 1), gallic acid (C7H6O5, CAS NO. 149–91-7, M.W. 170.1, purity 99%, Additional file 2), hyperoside (C21H20O12, CAS NO. 482–36-0, M.W. 464.4, purity 99%, Additional file 3), 2′′-O-galloylhyperin (C28H24O16, CAS NO. 53209–27-1, M.W. 616.5, purity 99%, Additional file 4), and quercetin (C15H10O7, CAS NO. 117–39-5, M.W. 302.2, purity 99%, Additional file 5) were obtained from Chengdu Biopurify Phytochemicals Ltd. (Chengdu, China). The compounds 2,9-dimethyl-1,10-phenanthroline (neocuproine), 1,1-diphenyl-2-picryl-hydrazl radical (DPPH•), ± − 6-hydroxyl-2,5,7,8-tetramethlychromane-2-carboxylic acid (Trolox), and 2,4,6-tripyridyl triazine (TPTZ) were purchased from Sigma-Aldrich Shanghai Trading Co., Ltd. (Shanghai, China), while 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide radical (PTIO•) was supplied by TCI Chemical Co. Ltd. (Shanghai, China). We purchased 2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid) diammonium salt ([NH4]2ABTS) from Amresco Chemical Co. Ltd. (Solon, OH, USA), and 4-methoxy-2,2,6,6-tetramethylpiperidine-1-oxyl radical (4-methoxy-TEMPO•) from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Dulbecco’s modified Eagle’s medium (DMEM), trypsin, and fetal bovine serum (FBS) were procured from Gibco (Grand Island, NY, USA) and CD44 was supplied by Wuhan Boster Co., Ltd. (Wuhan, China). Annexin V/propidium iodide (PI) assay kit was obtained from Abcam (Cambridge, UK), and acetonitrile, from Merck Serono Co., Ltd. (Shanghai, China). Methanol, acetonitrile, high-performance liquid chromatography (HPLC)-grade water, and all other chemicals used were of analytical grade.
Preparation of lyophilized aqueous extract of P. decorata (LAEP)
Dried P. decorata (Luxiancao) was purchased from Guangzhou Caizhilin Pharmaceutical Co., Ltd. (Guangzhou, China). It was identified by Dr. Guangtian Peng in School of Pharmaceutical Sciences, Guangzhou University of Chinese Medicine. A voucher specimen (No. GUCM010060) was deposited at Medicinal Plant Herbarium of Guangzhou University of Chinese Medicine (Additional file 6). P. decorata was subjected to extraction based on the guidance of the manufacturer (Fig. 2). In brief, the newly purchased P. decorata was decocted twice using 50-fold distilled water at 100 °C for 5 min. The extract was concentrated then lyophilized at − 60 °C under vacuum (1 Pa) for 24 h to obtain a lyophilized aqueous extract of P. decorata (LAEP). LAEP was stored at 5 °C until further use.
HPLC analysis for phenolic components in LAEP
The LAEP powder was dissolved in methnol at 15 mg/mL and then filtered through a 0.45 μm nylon membrane. The filtrate was analyzed using HPLC on Shimadzu LC-20A (Shimadzu Co., Kyoto, Japan) equipped with Agilent 5 TC-C18 (250 mm × 4.6 mm, 5 μm) (Agilent Technologies Inc., Palo Alto, CA, USA). The mobile phase comprised A (0.1% formic acid in water) and B (acetonitrile) with a gradient elution as follows: 0–10 min (10–20% B), 10–20 min (20–30% B), and 20–30 min (30–50% B). The flow rate, injection volume, and column temperature were 1.0 mL/min, 10 μL, and 35 °C, respectively, and absorption was measured at 280 nm wavelength. Protocatechuic acid, gallic acid, hyperoside, 2′′-O-galloylhyperin, and quercetin were identified by comparing their retention times with those of authentic samples.
Metal-reducing assays
The metal-reducing assays included ferric-reducing antioxidant power (FRAP) and cupric ion (Cu2+)-reducing antioxidant capacity (CUPRAC). The FRAP assay was carried out as per the method of Benzie and Strain [25]. In brief, the FRAP reagent was freshly prepared by mixing 10 mM TPTZ, 20 mM FeCl3, and 0.25 M acetate buffer (pH 3.6) at a ratio of 1:1:10. LAEP (5 mg/mL, x = 2–10 μL) or its five phenolic components (0.25 mg/mL, x = 2–10 μL) were added to (20 − x) μL of methanol and treated with 80 μL of FRAP reagent. After incubation for 30 min, the absorbance of the mixture was measured at 593 nm wavelength (A593nm) using acetate buffer as the blank on a microplate reader (Multiskan FC, Thermo Scientific, Shanghai, China). The relative reducing antioxidant power of the sample as compared to the maximum absorbance was calculated by the following formula:
Where Amin is the lowest A593nm value in the experiment, A is the A593nm value of the reaction mixture with sample, and Amax is the greatest A593nm value in the experiment.
The CUPRAC assay was conducted as per the method of Apak [26]. In brief, 12 μL CuSO4 aqueous solution (10 mM), 12 μL neocuproine methanol solution (7.5 mM), and (75 − x) μL CH3COONH4 buffer solution (100 mM, pH 7.4) were added to test tubes and incubated with different volumes of samples (10 mg/mL for LAEP or 0.5 mg/mL for the five phenolic components, x = 2–10 μL). The total volume was adjusted to 100 μL with buffer and the reactants were vigorously mixed. Absorbance against a buffer blank was measured at 450 nm wavelength using the microplate reader after 30 min. The relative reducing power of the sample as compared with the maximum absorbance was calculated using the formula presented for FRAP assay, wherein Amin is the lowest A450nm value in the experiment, A is the A450nm value of the reaction mixture with sample, and Amax is the greatest A450nm value in the experiment.
Free radical-scavenging assays in vitro
We performed three different free radical-scavenging assays, including 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide radical (PTIO•)-scavenging, 2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid) radical (ABTS+•)-scavenging, and 1,1-diphenyl-2-picryl-hydrazl radical (DPPH•)-scavenging assays. The PTIO•-scavenging assay was conducted based on our previously published method [27]. The experimental procedures are briefly described as follows: PTIO• radical was dissolved in phosphate buffers (0.1 mM, pH 4.5 and 7.4) to prepare a PTIO• solution; LAEP (1 mg/mL at both pH 4.5 and 7.4) and its five phenolic components (0.5 mg/mL at pH 4.5 or 0.3 mg/mL at pH 7.4) were prepared using methanol. Various volumes (x = 2–10 μL) of samples were mixed with phosphate buffers at pH 4.5 and 7.4 and treated with PTIO• solution (80 − x μL). After incubation for 3 h, the product mixture was analyzed by measuring the absorbance at 560 nm on microplate reader against a buffer blank. The PTIO• inhibition percentage was calculated as follows:
Where A0 is the absorbance at 560 nm of the control without the sample, and A is the absorbance of the reaction mixture with the sample. The above experiment was repeated using phosphate buffers at different pH (including pH 4.5 and 7.4).
The ABTS+•-scavenging activity was evaluated according to the previous method [28]. ABTS+• was produced by mixing 0.2 mL of ABTS diammonium salt (7.4 mmol/L) with 0.2 mL of potassium persulfate (2.6 mmol/L). The mixture was incubated in the dark at room temperature (27 °C) for 12 h to complete the radical generation process before dilution with distilled water (at a ratio of approximately 1:20); the absorbance at 734 nm was 0.35 ± 0.01. To determine the scavenging activity, the x μL sample (x = 2–10 μL, 1 mg/mL for LAEP or 0.05 mg/mL for the five phenolic components) was added to (20 − x) μL of distilled water and treated with 80 μL of ABTS+• reagent. The absorbance at 734 nm was measured 3 min after initial mixing using distilled water as the blank. The percentage of ABTS+•-scavenging activity was calculated using the formula presented for PTIO•-scavenging assay, wherein A0 is the absorbance at 734 nm of the control (reaction system without sample) and A is the absorbance at 734 nm of the reaction mixture with the sample.
The DPPH• radical-scavenging activity was determined as previously described [29]. Briefly, 80 μL of DPPH•-methanolic solution (0.1 mol/L) was mixed with x μL sample-methanolic solution (for LAEP: x = 1–5 μL, the concentration was 2.5 mg/mL; for the five phenolics: x = 4–20 μL, the concentration was 0.01 mg/mL) and (20 – x) μL methanol. The mixture was maintained at room temperature for 30 min, and the absorbance was measured at 519 nm on microplate reader against a buffer blank. The percentage of DPPH•-scavenging activity was calculated based on the formula presented for PTIO•-scavenging assay, wherein A0 is the absorbance at 519 nm of the reaction system without sample and A is the absorbance at 519 nm of the reaction mixture with sample.
Ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (UPLC−ESI − Q − TOF − MS/MS) analysis
UPLC−ESI − Q − TOF − MS/MS analysis of reaction products of 4-methoxy-TEMPO• with the five phenolic components was conducted as per the method described in our previous report [30]. The methanolic solutions of phenolic components were mixed with a solution of 4-methoxy-TEMPO• in methanol at a molar ratio of 1:2, and the resulting mixtures were incubated for 24 h at room temperature. The product mixtures were filtered through a 0.22-μm filter and analyzed with a UPLC-ESI-Q-TOF-MS/MS system equipped with a C18 column (2.0 mm i.d. × 100 mm, 2.2 μm, Shimadzu Co., Kyoto, Japan). The mobile phase used for elution comprised a mixture of acetonitrile (phase A) and 0.1% formic acid in water (phase B). The column was eluted at a flow rate of 0.2 mL/min with the following gradient elution program: 0–2 min, maintained at 30% B; 2–10 min, 30–0% B; and 10–12 min, 0–30% B. The sample injection volume was set at 5 μL, while the column temperature was 35 °C.
The Q-TOF-MS/MS analysis was conducted on a Triple TOF 5600plus mass spectrometer (AB SCIEX, Framingham, MA, USA) equipped with an ESI source in the negative ionization mode. The scan range was set at 100–2000 Da. The system was run with the following parameters: ion spray voltage, − 4500 V; ion source heater, 550 °C; curtain gas (CUR, N2), 30 psi; nebulizing gas (GS1, air), 50 psi; Tis gas (GS2, air), 50 psi. The declustering potential (DP) was set at − 100 V, whereas the collision energy (CE) was set at − 40 V at a collision energy spread (CES) of 20 V. The RAF products were quantified by extracting corresponding formula (e.g., [C14H10O8-H]− for protocatechuic acid-protocatechuic acid dimer and [C14H10O10-H]− for gallic acid-gallic acid dimer) from the total ion chromatogram and integrating the corresponding peak using PeakView 2.0 software (AB Sciex, Framingham, MA, USA).
Protective effect on bmMSCs against •OH-induced oxidative stress (flow cytometric assay)
Eight male Sprague-Dawley rats (4 weeks old, 60-80 g) were obtained from the Experimental Animal Center of Guangzhou University of Chinese Medicine (Guangzhou, China) and housed in there at 25 ± 2 °C, and exposed to a 12 h/12 h light–dark cycle with free access to food and water. Then, four Sprague-Dawley mice were euthanized by cervical dislocation to obtain enough bone marrow-derived mesenchymal stem cells (bmMSCs) from the bone marrow of femur and tibia of the rats.
The bmMSCs were cultured as previously described with some modifications [31]. Briefly, the cells were collected by gradient centrifugation at 900×g for 30 min on 1.073 g/mL Percoll. The prepared cells were detached by treatment with 0.25% trypsin and transferred into culture flasks at a density of 1 × 104 cells/cm2. The cells were evaluated for homogeneity through the detection of CD44 expression via cytometry, and seeded at 1 × 106 cells/well into 12-well plates. After adherence for 24 h, bmMSCs were divided into control, model, and sample groups. In the control group, bmMSCs were incubated for 24 h in DMEM, while the cells from the model and sample groups were incubated in the presence of FeCl2 (100 μM) and then treated with by H2O2 (50 μM). The cells from the sample group were incubated with 3 μg/mL of sample.
After incubation for 10 min, the mixture of FeCl2 and H2O2 was removed. The cells were washed twice with 0.1 mol/L cold phosphate-buffered saline (PBS) and resuspended in a binding buffer at a concentration of 1 × 106 cells/mL. About 100 μL of the solution (1 × 105 cells) was transferred to a 5 mL culture tube and treated with 5 μL of fluorescein isothiocyanate (FITC)-Annexin V and 5 μL PI. The cells were vortexed and incubated for 15 min in the dark and treated with 400 μL of binding buffer, followed by analysis with flow cytometry (Accuri C6, Franklin Lakes, BD, USA) with standard software.
Statistical analysis
Each experiment was performed in triplicates; the data were recorded as mean ± standard deviation (SD). The dose-response curves were plotted using Origin 2017 professional software (OriginLab, Northampton, MA, USA). The IC50 value was defined as the final concentration of 50% radical inhibition (or relative reducing power). Statistical comparisons were carried out with one-way analysis of variance (ANOVA) to detect significant differences using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA) for Windows. A value of p < 0.05 was considered statistically significant.
Results
Preparation and HPLC analysis of LAEP
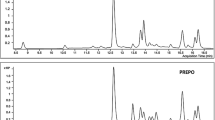
The appearance of LAEP was a brown powder (Additional file 7), and its yield was calculated as 6.87%. The prepared LAEP was analyzed with HPLC and found to exhibit at least five different phenolic components, including protocatechuic acid, gallic acid, hyperoside, 2′′-O-galloylhyperin, and quercetin at 0.55, 0.31, 0.16, 0.12, and 0.08%, respectively. The retention times of these compounds were 5.369 min, 8.168 min, 18.062 min, 18.663 min, and 27.670 min, respectively (Fig. 3).
Metal-reducing assays
In the study, two metal-reducing assays (i.e., FRAP assay and CUPRAC assay) were carried out to demonstrate the reducing power of antioxidants. As illustrated in Additional file 8, in FRAP assay, LAEP and its five phenolic components respectively increased the FRAP percentages in a dose-dependent manner. According to the IC50 values in Table 1, their Fe3+-reducing levels roughly increased in the order of protocatechuic acid < quercetin < gallic acid < hyperoside < 2“-O-galloylhyperin. In CUPRAC assay, LAEP and its five phenolic components respectively increased the CUPRAC percentages in a dose-dependent manner, and their order of Cu2+-reducing levels increased in the order of protocatechuic acid < gallic acid < quercetin < hyperoside < 2”-O-galloylhyperin (Table 1).
Free radical-scavenging assays in vitro
As shown in Additional file 8, LAEP and its five phenolic components could successfully scavenge PTIO• at pH 4.5 and pH 7.4. However, according to the IC50 values in Table 1, their relative PTIO•-scavenging levels at the same pH value were different from each other. Also, each of the five phenolic components exhibited different PTIO•-scavenging levels between pH 4.5 and pH 7.4.
The DPPH•-scavenging and ABTS+•-scavenging assays have been widely used in antioxidant studies. As seen in Additional file 8, LAEP and its five phenolic components concentration-dependently increased the DPPH•-scavenging and ABTS+•-scavenging percentages. The IC50 values were detailed in Table 1.
Ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (UPLC−ESI − Q − TOF − MS/MS) analysis
Each of five phenolic components (i.e., protocatechuic acid, gallic acid, hyperoside, 2″-O-galloylhyperin, and quercetin) in LAEP was incubated with 4-methoxy-TEMPO• for 24 h and analyzed with UPLC-ESI-Q-TOF-MS/MS. The chromatographic peaks, primary MS spectra (molecular ion peak), and secondary MS spectra were shown in Fig. 4.
Results of UPLC-ESI-Q-TOF-MS/MS analysis. a Chromatogram of proto catechuic acid-protocatechuic acid dimer after the extraction of [C14H10O8-H]−.a1 Primary MS spectra of protocatechuic acid-protocatechuic acid dimer. a2 Secondary MS spectra of protocatechuic acid-protocatechuic acid dimer. b Chromatogram of gallic acid-gallic acid dimer after the extraction of [C14H10O10-H]−. (B1) Primary MS spectra of gallic acid-gallic acid dimer. c Chromatogram of hyperoside-hyperoside dimer after the extraction of [C42H38O24-H]−. c1 Primary MS spectra of hyperoside-hyperoside dimer. c2 Secondary MS spectra of hyperoside-hyperoside dimer. d Chromatogram of 2′′-O-galloylhyperin-2′′-O-galloylhyperin dimer after the extraction of [C56H46O32-H]−. d1 Primary MS spectra of 2′′-O-galloylhyperin-2′′-O-galloylhyperin dimer. d2 Secondary MS spectra of 2′′-O-galloylhyperin-2′′-O-galloylhyperin dimer. e Chromatogram of quercetin-quercetin dimer upon extraction of [C30H18O14-H]−. e1 Primary MS spectra of quercetin-quercetin dimer. e2 Secondary MS spectra of quercetin-quercetin dimer
Protective effect on bmMSCs against •OH-induced oxidative stress (flow cytometric assay)
As shown in Fig. 5, both LAEP and its five phenolic components had significantly (p < 0.05) decreased the percentages of damaged cells compared with the model group. In particular, the percentage of damaged cells was significantly (p < 0.05) lower in 2′′-O-galloylhyperin-treated group than in hyperoside-treated group.
LAEP and its five phenolic components protect bmMSCs against Fenton-induced apoptosis. The percentage of damaged cells was assessed using a flow cytometric assay. Experiments were performed with three different batches of cells and each batch was tested in triplicates. Data are calculated as the mean ± SD values. LAEP means the lyophilized aqueous extract of P. decorata. The percentages of damaged cells with different superscripts (a, b, c, d, e, or f) are significantly different (p < 0.05)
Discussion
In the study, five phenolic components have been found in P. decorata, including two phenolic acids (protocatechuic acid and gallic acid), and three flavonoid glucosides (hyperoside, 2′′-O-galloylhyperin, and quercetin) (Fig. 3). The detection of two phenolic acids undoubtedly has added new information of phytochemistry of P. decorata species [14, 15]. Regardless that the five phenolic components have also been expressed in P. incarnata; however, their contents in LAEP were slightly lower than those in P. incarnata [5, 32, 33]. The difference could be attributed to the species difference, the extraction processes employed. Furthermore, harvest seasons may also influence the contents of phenolics in Pyrola L. plants [33].
The five phenolic components and LAEP thus were further estimated using various antioxidant assays, such as FRAP and CUPRAC assays. The FRAP and CUPRAC assays are based on the electron transfer (ET) reaction at pH 3.6 and 7.4, respectively [34, 35]. Hence, the concentration-dependent increase of LAEP and its five phenolic components in reducing powers suggests that they could be involved in the ET pathway to exert antioxidant effects at pH 3.6 and 7.4.
To investigate whether the ET pathway could mediate radical-scavenging process, LAEP and its five phenolic components were further evaluated with PTIO•-scavenging assay. The PTIO• radical is present as a stable form in aqueous buffers at various pH values, and its scavenging reaction at pH 4.5 was confirmed as a pure ET pathway by cyclic voltammetry [36]. The results demonstrated that LAEP and its five phenolic components could exhibit elevated PTIO•-scavenging activities at pH 4.5, indicative of their abilities to undergo ET reaction to scavenge radicals. However, at pH 7.4, LAEP and its five phenolic components could also effectively scavenge PTIO• radical and their IC50 values at pH 7.4 were lower than those at pH 4.5 (Table 1). These pH-mediated effects suggest the involvement of the H+ transfer pathway in the PTIO•-scavenging process [27].
Besides aqueous buffers, aqueous solution was used as the experimental medium to evaluate the antioxidant properties in the ABTS+•-scavenging assay. Furthermore, an organic medium was used in the DPPH•-scavenging assay. The effectiveness of LAEP and its five phenolic components in the two assays revealed their ability to exert antioxidant actions in both organic and aqueous media.
The five detected phenolic components had different IC50 values in each antioxidant assay (Table 1). In particular, hyperoside showed higher IC50 values than 2′′-O-galloylhyperin in each of antioxidant assay, suggesting that 2′′-O-galloylhyperin had higher antioxidant effects based on ET and H+ transfer pathways than hyperoside. As previously discussed, the sole structural difference between hyperoside and 2′′-O-galloylhyperin is the presence of 2′′-O-galloyl moiety. Hence, the difference in their antioxidant activities could only be attributed to the role of galloylation. The pyrogallol moiety in 2′′-O-galloyl provides additional hydroxyl groups with radical-scavenging and metal-chelating abilities (Fig. 6) [24, 37,38,39,40].
Both ET and H+ transfer pathways are associated with the redox-based antioxidant mechanisms [41,42,43]. Through redox-based antioxidant mechanisms, new radical intermediates may be generated [30, 44]. These radical intermediates (including radical) may covalently link with each other to form a radical adduct. This is called as the radical adduct formation (RAF) mechanism. Dimerization however is one type of RAF. As seen in Fig. 4, protocatechuic acid, gallic acid, hyperoside, 2′′-O-galloylhyperin, and quercetin respectively produced the m/z values 306, 338, 926, 1230, and 602. These m/z values are exactly two units lower than twice their molecular weights of (M.W. 154, 170, 464, 616, and 302). We assumed that the two phenolic molecules were dimerized via one covalent bond. The observation of dimer formation indicates the presence of RAF mechanism in the antioxidant process associated with the five phenolic components in LAEP.
Of these phenolic components, protocatechuic acid belongs to the phenolic acid family, whereas 2′′-O-galloylhyperin is a member of the flavonoid family. According to the MS/MS data, the dimeric products of protocatechuic acid and 2′′-O-galloylhyperin could be fully elucidated, as shown in Fig. 7. These elucidations further supported the presence of RAF mechanism. Furthermore, these elucidations clearly suggest that one consequence of RAF is the generation of phenolic-phenolic dimer. The dimer with covalent linkage, however, could effectively diminish the free radical to terminate the radical chain reaction [11].
Proposed RAF products with MS elucidations of protocatechuic acid (a) and 2″-O-galloylhyperin (b) after mixed with 4-methoxy-TEMPO•. (The MS spectra were in the negative ion model, and the charge imposed by the MS field was not marked. Other linking sites between two phenolic moieties should not be excluded. Other reasonable cleavages should not be excluded in the MS elucidation)
The high resolution MS spectra provided an accurate m/z value for various fragments. For instance, the loss from m/z 151.0044 to 107.0152 was exactly the m/z value of CO2. The experimental m/z value of CO2 was 43.9892 (151.0044–107.0152) (Fig. 4a2 and Fig. 7a), while the calculated m/z value was 43.9899. The relative bias was only 1.6 × 10− 5. In addition, the m/z value from 151.0044 to 123.0099 was equal to 28. This loss was considered to be from N2 or CO. However, the accurate m/z value was 27.9945, indicating that the loss was related to CO (calculated m/z value 27.9949) than to N2 (calculated m/z value 28.0061) (Fig. 4A2 and Fig. 7a). The MS elucidations shown in Fig. 7 suggest the possible presence of other linking sites between two phenolic moieties, and that other reasonable cleavages should not be excluded in the MS elucidation.
To investigate whether LAEP and its five phenolic components could resist the oxidative stress-induced damage in biological system, we incubated bmMSCs damaged with Fenton reagent (an •OH radical generator) in the presence of these compounds in an aqueous buffer. The percentage of damaged cells was characterized via flow cytometry. The results that LAEP and its five phenolic components could significantly (p < 0.05) decrease the percentages of damaged cells could be partly responsible for the pharmacological effect of Luxiancao. Furthermore, P. decorata and its phenolic components could function as therapeutic candidates for oxidative damages in bmMSC transplantation.
As mentioned above, the 2′′-O-galloylhyperin-treated group showed significantly (p < 0.05) higher cellular viability than hyperoside-treated group. Thus, 2′′-O-galloylhyperin could improve the cytoprotective effect of phenolic glycosides in aqueous buffer, consistent with the findings of antioxidant assays in organic and aqueous media. In bmMSC transplantation, galloylation of phenolic glycosides could be considered as a promising agent to improve the effectiveness of cytoprotectors.
Conclusion
P. decorata contains at least five phenolic components, including protocatechuic acid, gallic acid, hyperoside, 2′′-O-galloylhyperin, and quercetin. These compounds may undergo redox-based pathways (such as ET and H+ transfer) and covalent-based pathway (i.e., RAF) to exhibit antioxidant activities. RAF could yield a stable phenolic-phenolic dimer. In comparison with hyperoside, 2′′-O-galloylhyperin showed better redox-based antioxidant activity (or cytoprotective activity) in both organic and aqueous media. The improvement stems from the 2′′-O-galloylation modification, which adds adjacent hydroxyl groups with radical-scavenging and metal-chelating abilities.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- (NH4)2ABTS:
-
2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid) diammonium salt
- 4-methoxy-TEMPO:
-
4-methoxy-2,2,6,6-tetramethylpiperidine-1-oxyl radical
- ABTS+• :
-
2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid) radical
- bmMSCs:
-
Bone marrow-derived mesenchymal stem cells
- MSCs:
-
Mesenchymal stem cells
- CUPRAC:
-
Cupric ions (Cu2+) reducing antioxidant capacity
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DPPH:
-
1,1-diphenyl-2-picryl-hydrazl radical
- ET:
-
Electron transfer
- FBS:
-
Fetal bovine serum
- FRAP:
-
Ferric reducing antioxidant power
- HAT:
-
hydrogen atom transfer
- LAEP:
-
Lyophilized aqueous extract of Pyrola decorata
- PCET:
-
Proton-coupled electron transfer
- PTIO:
-
2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide radical
- RAF:
-
Radical adduct formation
- ROS:
-
Reactive oxygen species
- SD:
-
Standard deviation
- SEPT:
-
Sequential electron-proton transfer
- SPLET:
-
Sequential proton loss single-electron transfer
- TCM:
-
Traditional Chinese medicine
- TPTZ:
-
2,4,6-tripyridyl triazine
- Trolox:
-
(±)-6-hydroxyl-2,5,7,8-tetramethlychromane-2-carboxylic acid
- UPLC-ESI-Q-TOF-MS/MS:
-
Ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry
References
Uesugi T, Nakano M, Selosse M, Obase K, Matsuda Y. Pyrola japonica, a partially mycoheterotrophic Ericaceae, has mycorrhizal preference for russulacean fungi in Central Japan. Mycorrhiza. 2016;26(8):819–29.
Zhang SD, Wang P, Zhang J, Wang W, Yao LP, Gu CB, Efferth T, Fu YJ. 2′-O-galloylhyperin attenuates LPS-induced acute lung injury via upregulation antioxidation and inhibition of inflammatory responses in vivo. Chem Biol Interact. 2019;304:20–7.
Chen YL, Tan CH, Tan JJ, Qu SJ, Jiang SH, Zhu DY. Two new isomeric α-Tetralones from Pyrola decorata. Planta Med. 2008;74(15):1826–8.
Wang DM, He FY, Lv ZJ, Li DW. Phytochemical composition, antioxidant activity and HPLC fingerprinting profiles of three Pyrola species from different regions. PLoS One. 2014;9(5):e96329.
Yao XH, Zhang DY, Zu YG, Fu YJ, Luo M, Gu CB, Li CY, Mu FS, Efferth T. Free radical scavenging capability, antioxidant activity and chemical constituents of Pyrola incarnata Fisch leaves. Ind Crop Prod. 2013;49:247–55.
Luo DQ, Yang YZ, Song L, Wang JX. Advances in studies on special plants of Pyrola L. in China. Chinese Traditional and Herbal Drugs. 2004;34(4):107–10 (in Chinese).
Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia, vol. 1. 2015th ed; 2015. p. 324.
Li LF, Liu H, Yang JQ, Shi W, Wu LH. The anti-inflammatory and anti-osteoarthritis activities of Pyrola Decorata extracts. J Am Geriatr Soc. 2015;63(S02):S359–60.
Wang P, Gao YM, Sun X, Guo N, Li J, Wang W, Yao LP, Fu YJ. Hepatoprotective effect of 2′-O-galloylhyperin against oxidative stress-induced liver damage through induction of Nrf2/ARE-mediated antioxidant pathway. Food Chem Toxicol. 2017;102:129–42.
Yang XY, Liu AL, Liu SJ, Xu XW, Huang LF. Screening for neuraminidase inhibitory activity in traditional Chinese medicines used to treat influenza. Molecules. 2016;21(9):1138.
Grover AK, Samson SE. Benefits of antioxidant supplements for knee osteoarthritis: rationale and reality. Nutr J. 2015;15(1):1.
Morris G, Puri BK, Walker AJ, Berk M, Walder K, Bortolasci CC, Marx W, Carvalho AF, Maes M. The compensatory antioxidant response system with a focus on neuroprogressive disorders. Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;95:e109708.
Wang QW, Su Y, Sheng JT, Gu LM, Zhao Y, Chen XX, Chen C, Li WZ, Li KS, Dai JP. Anti-influenza a virus activity of rhein through regulating oxidative stress, TLR4, Akt, MAPK, and NF-kappa B signal pathways. PLoS One. 2018;13(1):e0191793.
Zhang YY, Chen XH, Sun YY, Bi KS. Determination of flavonoid glycosides in Pyrola decorate by reversed-phase high performance liquid chromatography. Chin J Chromatogr. 2007;25(3):367–70.
Zhang YY, Zhang C, Ren R, Liu R. Simultaneous determination of seven major triterpenoids in Pyrola decorata H. Andres by LC-MS method. Pharmazie. 2012;67(10):822–6.
Yu CT, Dai LF, Ma ZX, Zhao HB, Yuan Y, Zhang YF, Bao PF, Su YF, Ma DP, Liu CE, Wu XF, Liu JX, Li YJ, Wang B, Hu M. Effect of Osteoking on the osteogenic and adipogenic differentiation potential of rat bone marrow mesenchymal stem cells in vitro. BMC Complement Altern Med. 2019;19(1):36.
Jacobs FA, van de Vyver M, Ferris WF. Isolation and Characterization of Different Mesenchymal Stem Cell Populations from Rat Femur. Methods Mol Biol. 2019;1916:1333–147.
Yi F, Khan M, Gao HW, Hao F, Sun MY, Zhong LL, Lu CZ, Feng XC, Ma TH. Increased differentiation capacity of bone marrow-derived Mesenchymal stem cells in Aquaporin-5 deficiency. Stem Cells Dev. 2012;21(13):2495–507.
Cai BZ, Wang G, Chen N, Liu YJ, Yin K, Ning CP, Li XD, Yang F, Wang N, Wang Y, Pan ZW, Lu YJ. Bone marrow mesenchymal stem cells protected post-infarcted myocardium against arrhythmias via reversing potassium channels remodelling. J Cell Mol Med. 2014;18(7):1407–16.
Ning GZ, Song WY, Xu H, Zhu RS, Wu QL, Wu Y, Zhu SB, Li JQ, Wang M, Qu ZG. Bone marrow mesenchymal stem cells stimulated with low-intensity pulsed ultrasound: better choice of transplantation treatment for spinal cord injury treatment for SCI by LIPUS-BMSCs transplantation. CNS Neurosci Ther. 2019;25(4):496–508.
Du C, Jiang MD, Wei XL, Qin JP, Xu H, Wang YX, Zhang Y, Zhou DJ, Xue HL, Zheng SM, Zeng WZ. Durable control of autoimmune diabetes in mice achieved by Intraperitoneal transplantation of "neo-islets," three-dimensional aggregates of allogeneic islet and "Mesenchymal stem cells". Stem Cells Transl Med. 2017;6(7):1631–43.
Liu J, Li X, Lin J, Li Y, Wang T, Jiang Q, Chen D. Sarcandra glabra (Caoshanhu) protects mesenchymal stem cells from oxidative stress: a bioevaluation and mechanistic chemistry. BMC Complement Altern Med. 2016;16:423.
Yu ZY, Ma D, He ZC, Liu P, Huang J, Fang Q, Zhao JY, Wang JS. Heme oxygenase-1 protects bone marrow mesenchymal stem cells from iron overload through decreasing reactive oxygen species and promoting IL-10 generation. Exp Cell Res. 2018;362(1):28–42.
Hsieh CY, Chang ST. Antioxidant activities and xanthine oxidase inhibitory effects of phenolic phytochemicals from Acacia confusa twigs and branches. J Agric Food Chem. 2010;58(3):1578–83.
Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–6.
Apak R, Guclu K, Ozyurek M, Bektas Oglu B, Bener M. Cupric ion reducing antioxidant capacity assay for food antioxidants: vitamins, polyphenolics, and flavonoids in food extracts. Methods Mol Biol. 2008;477:163–93.
Li XC. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO•) radical scavenging: a new and simple antioxidant assay In Vitro. J Agric Food Chem. 2017;65(30):6288–97.
Ouyang X, Li X, Lu W, Zhao X, Chen D. A null B-ring improves the antioxidant levels of Flavonol: a comparative study between Galangin and 3,5,7-Trihydroxychromone. Molecules. 2018;23(12):3083.
Li XC, Jiang Q, Wang TT, Liu JJ, Chen DF. Comparison of the antioxidant effects of Quercitrin and Isoquercitrin: understanding the role of the 6″-OH group. Molecules. 2016;21(9):1246.
Liang M, Li X, Ouyang X, Xie H, Chen D. Antioxidant mechanisms of Echinatin and Licochalcone a. Molecules. 2019;24(1):3.
Xie H, Li X, Ren Z, Qiu W, Chen J, Jiang Q, Chen B, Chen D. Antioxidant and Cytoprotective effects of Tibetan tea and its phenolic components. Molecules. 2018;23(2):179.
Yao XH, Zhang DY, Duan MH, Cui Q, Xu WJ, Luo M, Li CY, Zu YG, Fu YJ. Preparation and determination of phenolic compounds from Pyrola incarnata Fisch with a green polyols based-deep eutectic solvent. Sep Purif Technol. 2015;149:116–23.
Yao XH, Zhang ZB, Song P, Hao JY, Zhang DY, Zhang YF. Different harvest seasons modify bioactive compounds and antioxidant activities of Pyrola incarnata. Ind Crop Prod. 2016;94:405–12.
Li X, Chen B, Xie H, He Y, Zhong D, Chen D. Antioxidant structure - activity relationship analysis of five Dihydrochalcones. Molecules. 2018;23(5):1162.
Cekic SD, Baskan KS, Tutem E, Apak R. Modified cupric reducing antioxidant capacity (CUPRAC) assay for measuring the antioxidant capacities of thiol-containing proteins in admixture with polyphenols. Talanta. 2009;79(2):344–51.
Goldstein S, Russo A, Samuni A. Reactions of PTIO and carboxy-PTIO with •NO, •NO2, and •O2 −. J Biol Chem. 2003;278(51):50949–55.
Iglesias J, Pazos M, Lois S, Medina I. Contribution of Galloylation and polymerization to the antioxidant activity of polyphenols in fish lipid systems. J Agric Food Chem. 2010;58(12):7423–31.
Mitjans M, Ugartondo V, Martinez V, Tourino S, Torres JL, Vinardell MP. Role of Galloylation and polymerization in Cytoprotective effects of Polyphenolic fractions against hydrogen peroxide insult. J Agric Food Chem. 2011;59(5):2113–9.
Plumb GW, De Pascual-Teresa S, Santos-Buelga C, Cheynier V, Williamson G. Antioxidant properties of catechins and proanthocyanidins: effect of polymerisation, galloylation and glycosylation. Free Radic Res. 1998;29(4):351–8.
Wang S, Ye K, Shu T, Tang X, Wang XJ, Liu S. Enhancement of Galloylation efficacy of Stigmasterol and β-Sitosterol followed by evaluation of cholesterol-reducing activity. J Agric Food Chem. 2019;67(11):3179–87.
Li XC. Improved Pyrogallol autoxidation method: a reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J Agric Food Chem. 2012;60(25):6418–24.
Klein E, Lukes V. DFT/B3LYP study of the substituent effect on the reaction enthalpies of the individual steps of single electron transfer-proton transfer and sequential proton loss electron transfer mechanisms of phenols antioxidant action. J Phys Chem A. 2006;110(44):12312–20.
Alfonso-Prieto M, Oberhofer H, Klein ML, Rovira C, Blumberger J. Proton transfer drives protein radical formation in helicobacter pylori catalase but not in Penicillium vitale catalase. J Am Chem Soc. 2011;133(12):4285–98.
Lucarini M, Pedulli GF. Free radical intermediates in the inhibition of the autoxidation reaction. Chem Soc Rev. 2010;39(6):2106–19.
Acknowledgements
The authors would be thankful to Dr. Guangtian Peng for the identification of the plant Pyrola decorata H. Andres.
Funding
This work was supported by the National Nature Science Foundation of China (81573558, 81673770) and Guangdong Science and Technology Project (2016A050503039). The funding agencies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript, which are fully the responsibilities of the authors.
Author information
Authors and Affiliations
Contributions
XCL and DFC conceived and designed the experiments; BC performed the chemical experiments; JL and WQ performed the flow cytometric analysis; MSL and QRL analyzed the experimental data; XCL and BC wrote the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Institution Animal Ethics Committee in Guangzhou University of Chinese Medicine (Guangzhou, China) approved the protocols used in this study. Animal study was carried out in strict accordance with the established institutional guidelines on the use of experimental animals. All the animals were euthanized by cervical dislocation. Consent to participate was not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Appearance and analysis certificate of protocatechuic acid.
Additional file 2.
Appearance and analysis certificate of gallic acid.
Additional file 3.
Appearance and analysis certificate of hyperoside.
Additional file 4
Appearance and analysis certificate of 2′′-O-galloylhyperin.
Additional file 5.
Appearance and analysis certificate of quercetin.
Additional file 6
Originate plant and voucher specimen of P. decorate.
Additional file 7
Appearance of LAEP.
Additional file 8.
Dose response curves and IC50 values.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chen, B., Li, X., Liu, J. et al. Antioxidant and Cytoprotective effects of Pyrola decorata H. Andres and its five phenolic components. BMC Complement Altern Med 19, 275 (2019). https://doi.org/10.1186/s12906-019-2698-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-019-2698-y