Abstract
Background
Cordyceps is a traditional Chinese herb that produces various biopharmaceutical effects, including immune-enhancing effects. In this study, we prepared a Cordyceps mycelium culture extract (Paecilomyces hepiali, CBG-CS-2) to confirm its efficacy in enhancing the immune system and to evaluate its safety in healthy adults.
Methods
Healthy adults were divided into the intervention group (n = 39), who were given 1.68 g/day of CBG-CS-2 in capsules, and the control group (n = 40) for 8 weeks. The activities of natural killer (NK) cells and serum levels of monocyte-derived mediators were assessed initially for a baseline measurement and after 8 wks.
Results
The CBG-CS-2 group showed a significant 38.8 ± 17.6% enhancement from the baseline of NK cell cytotoxic activity relative to the placebo group after the administration of the capsules for 8 wks. (P < 0.019).
Conclusion
The results suggest that the immune system functions well with CBG-CS-2 supplementation, perhaps with less accompanying inflammation. Thus, CBG-CS-2 is safe and effective for enhancing cell-mediated immunity in healthy adults.
Trial registration
This study was registered at Clinical Trials.gov (NCT 02814617).
Similar content being viewed by others
Background
Cordyceps is a natural substance that has traditionally been used as a Chinese medicine in East Asian countries. In particular, Cordyceps sinensis (CS) is largely known as a traditional Chinese medicine [1]. However, its use as a popular medicine is limited due to difficulties in the mass gathering of natural CS. It is a type of wild mushroom grown at 3000~4000 m above sea level around the Himalaya Mountains in Tibet and the high regions in China, and until recently, it was impossible to artificially cultivate due to the difficult growth conditions [2]. In recent years, however, it has become possible to artificially cultivate CS through the development of artificial production technologies that overcome both the decrease in Cordyceps supply and the heterogeneity in its composition [3,4,5]. Recently, there have been some empirical studies on the effectiveness of mass-produced Cordyceps, and several trials have been conducted in various fields, such as medicine, cosmetics, and functional foods [4, 6,7,8,9]. The mycelial cultured CS mycelia (P. hepiali) products include strong bioactive substances, such as nucleosides and polysaccharides (PS), which are part of the bioactive substances of natural CS. Thus, it has been recognized that the bioactivities of mycelial cultured CS are very similar to those of natural Cordyceps [10,11,12,13]. In general, the Paecilomyces hepiali (P. hepiali) that is commonly included in natural CS from Tibet is known as an endoparasitic fungus. The genome sequence of P. hepiali is the medical compound produced using fungi, and there are some trials where it is being applied and developed in different fields. The main components of CS, such as polysaccharides, cordycepin, adenosine, cordycepic acid, nucleosides, and ergosterol, are known to be important bioactive substances with medical relevance [14,15,16]. There have been several reports on the health functionality of these bioactive substances, which include important functions such as immunomodulatory, anticancer, antimetastatic, anti-oxidant, anti-inflammatory, antimicrobial, lipid metabolism improvement, hypoglycemic, anti-aging, and liver and renal recovery functions [11, 17,18,19,20,21,22,23,24,25,26,27,28,29].
In our preclinical study, animal testing in an immunosuppressed mouse model (mitomycin C-treated mice; MMC) verified the immunomodulatory effect of a mycelium extract from P. hepiali isolated from CS (CBG-CS-2) [30, 31]. We also verified that the extract helped reduce a gut immunity suppressive state by controlling the expression of the immune response genes and increased both the NK-cell activity and production of nitric oxide (NO). In addition, the effects of controlling the anti-oxidant, antifatigue, and anti-inflammatory functions, including immunomodulation, were identified by removing free radicals based on the suppression of the excessive expression of inducible nitric oxide synthase (iNOS) and the excessive production of the TNF-α inflammatory mediators [30,31,32]. However, in our preclinical studies, the effectiveness and safety of CBG-CS-2 for an immunoregulatory effect were not evaluated, although the mechanism and effectiveness of the immunomodulating effect of CBG-CS-2 separated and cultivated from P. hepiali from CS were verified.
The objectives of this study were to confirm the immunoregulatory efficacy and safety of CBG-CS-2 separated and cultivated from P. hepiali from CS in healthy Korean adults.
Methods
Participants
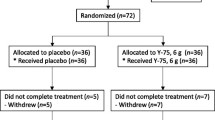
The study subjects were recruited and selected at the Clinical Trials Center for Functional Foods (CTCF2) at Chonbuk National University Hospital from September to November 2015. A total of 80 healthy male and female subjects agreed to participate in this study. A total of 80 participants were randomly assigned into one of the study groups (40 subjects each) using a computer-generated random number table by the Randomization program of the version 9.2 SAS® system (SAS Institute, Cary, NC, USA).
The criteria for the selection and exclusion of participants in this study are described below.
The selection guidelines were as follows:
1) Male and female adult participants aged 20~75 at the time of the screening test.
2) Participants who showed a slight decrease in immunity and had a peripheral blood white blood cell (WBC) level of 3*103 ~ 7*103 cells/μl measured during the screening test.
3) Participants who fully understood the test and decided to participate of their own free will and agreed to the written consent document.
The exclusion criteria were as follows:
1) Male and female adult participants who had a BMI less than 18.5 kg/m2 at the time of the screening test.
2) Participants who had a family history of medicinally or clinically significant hypersensitivity reactions.
3) Participants with thyroid or hypophyseal disorder.
4) Participants with acute severe cardiovascular diseases such as cardiac insufficiency, myocardial infarction, or stroke.
5) Participants with immunological disease, liver or renal failure.
6) Participants who had specific diseases such as a malignant tumor, lung disease, leukemia, collagenosis, multiple sclerosis, allergic skin disease, and other autoimmune disorders or a history thereof.
7) Participants diagnosed with diabetes.
8) Participants who had a “gastroesophageal reflux disease”, such as Crohn’s disease.
9) Participants who, within 2 weeks from the first intake day, had ingested medicine, Chinese medicine, or a health functional food that can affect the immunomodulatory effect of the test product. In the case of health functional foods, it was possible to participate in this program after a one-week wash out period.
10) Participants who had a history of treatment with anti-psychotics within 2 months of the screening test.
11) Participants who had a history of treatment for alcoholism or drug abuse.
12) Those who participated in other clinical studies within 2 months prior to the date of the first clinical test product intake.
13) Participants who had an alanine transaminase (ALT) or aspartate transaminase (AST) plasma level more than three times the guideline of the organization.
14) Female participants who were pregnant or breastfeeding or planned to become pregnant during the test period.
15) Participants who had disqualifying results in the diagnostic test and were inappropriate for other reasons.
All subjects gave written informed consent before entering the study. The study was conducted according to the Declaration of Helsinki. The study protocol was approved by the Functional Food Institutional Review Board (FFIRB) of Chonbuk National University Hospital (FFIRB number 2015–02-010) on August 25, 2015. This study was registered on June 28, 2016 after the termination of the study sub heading: The efficacy and safety of CS mycelium culture extract (P. hepiali, CBG-CS-2) on the promotion of immunity.
Study design
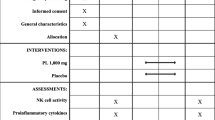
This study was an 8 week, randomized, double blind, and placebo-controlled clinical trial that was performed according to a computer-generated randomization schedule designed to assign subjects to the CBG-CS-2 or placebo group. Neither the investigators nor the subjects knew the randomization code or the results of the NK cell activity levels until after statistical analysis was complete. Subjects attended a screening visit (within 4 weeks), at which inclusion and exclusion criteria were assessed. The enrolled subjects were scheduled for their first visit, and subjects were randomly assigned to one of two groups, either the CBG-CS-2 (n = 40) or placebo group (n = 40). Subjects received either the CBG-CS-2 or placebo capsules every week, and all of the subjects were instructed to take either two CBG-CS-2 capsules (twice per day) or two placebo capsules (twice per day) per day (1.68 g/day) after breakfast and dinner for 8 weeks.
During the intervention period of 8 weeks, subjects were asked to continue their usual diets and activity and to not ingest any other functional foods or dietary supplements. Anthropometric and biochemical parameters, vital signs, and nutrient intake were measured before and after the intervention period. Every week, the subjects were asked to report any adverse events or changes in training, lifestyle, or eating patterns and to assess their capsule-dosing compliance.
A CONSORT checklist for the reporting of this study can be found in Additional file 1.
Preparation of test materials
Cordyceps mycelium extract (Paecilomyces hepiali, CBG-CS-2) was provided by Chebeigen, Inc. (Jeonju, Republic of Korea), and CS mycelium culture extracts were prepared as described previously [33]. The main components of the mycelium culture extract were as follows: 32% cordyceps polysaccharide, 7.3% cordycepic acid, 0.13% adenosine, and 0.001% cordycepin [33]. Main component profiles in the CBG-CS-2 were analyzed by high-performance liquid chromatography (HPLC) according to the Korean Food and Drug Administration (KFDA) guidelines, and these cordyceps polysaccharide profiles are shown in (Table 1). The extract was administered as a dark brown powder composed of 85.36% CS mycelium culture extract powder peel, 12.64% microcrystalline cellulose, and 2.0% hydroxylpropyl methylcellulose. The placebo was composed of 84.0% microcrystalline cellulose, 14.2% hydroxylpropyl methylcellulose, and 0.6% orange yellow.
Biochemical analyses
The efficacy evaluation and safety evaluation parameters before the baseline (Week 0) of the human study and after eight weeks of participation were analyzed prior to participation in this human study for all of the subjects. The fasting blood samples of all subjects were collected and centrifuged (Hanil Science Industrial Co., Ltd. Seoul, Korea) at 3000 rpm for 20 min while keeping an empty stomach for more than 12 h from the day before the blood collection, and they were kept frozen at − 80 °C until the analysis. The blood samples were analyzed using a Hitachi 7600–110 analyzer (Hitachi High-Technologies Corporation, Tokyo, Japan). The primary outcomes of this clinical trial measured the changes in natural killer cell activity [28, 34], and the secondary outcomes measured cytokines (IFN-γ and TNF-α, IL-1β, IL-2, IL-4, IL-10, and IL-12). The assessment of the safety parameters was performed through measuring an electrocardiogram (ECG) as well as laboratory tests (WBCs, RBCs, hemoglobin, hematocrit, platelets, total bilirubin, γ-glutamyl transferase (GGT), ALT, AST, alkaline phosphatase (ALP), TC, TG, LDL-C, HDL-C, fasting blood glucose, total protein, albumin, blood urea nitrogen (BUN), creatinine, creatine kinase, lactate dehydrogenase, Na, K, Cl, Ca, and P). Vital signs (systolic blood pressure, diastolic blood pressure, and pulse) of the study subjects were measured at every visit.
Statistical analysis
All statistical processing was analyzed using SAS version 9.2 (SAS Institute, Cary, NC, USA). All data were expressed as the mean ± standard deviation (SD) for continuous variables and as a frequency (%) for categorical variables. In this study, the intention-to-treat (ITT) population visited at least once and included performing an analysis of the efficacy and safety of those who had undergone measurements on the main evaluation variables after taking the CBG-CS-2 and placebo products. The data on the efficacy evaluation were based on the per-protocol (PP) group as the main analysis target, but a further analysis of the ITT group was implemented for its efficacy. To detect a 7.4% (SD 11.4%) difference in NK-cell activity change between groups with a power of 80% with a two-tailed alpha level of 0.05, a sample size of 40 per group (N = 80) was needed [35]. In the case of the categorical variables, the significance test was applied with the Chi-square test (Fisher’s exact test). Additionally, the baseline and endpoint comparison of the investigation results of the evaluation variables in each group were tested by applying the paired t-test. The mean comparison between the two groups was tested with the independent sample t-test for independent samples, and the outcome variables for repeated measurement of the intake groups were applied with a linear mixed model within the intake groups and between the intake groups. The significance was statistically significant at the level of p < 0.05.
Results
Demographic and clinical characteristics of participants
Ninety-seven participant were assessed for eligibility, and a total of 80 participants (mean age = 47.6 ± 5.9 years, 52 males and 28 females, mean body mass index = 24.3 ± 3.0 kg/m2, NK-cell activity mean = 35.8 ± 17.7%) met the inclusion criteria. Seventeen participants were excluded from the current analysis because they did not meet all of the inclusion criteria. Thus, a total of 79 participants completed the study (Fig. 1). One subject from the CBG-CS-2 group failed to complete the study. One subject was excluded because of the lack of compliance. As a result, 79 subjects (CBG-CS-2 group = 39 and placebo group = 40) remained. There were no significant differences between groups in the baseline characteristics such as age, sex, height, weight, body mass index, NK cell activity, IL-1ß, IL-2, 4, 10, 12, INF-γ, TNF-α, alcohol intake, and smoking status (Table 2). The compliance rates, which were based on pill count, were 94.5 ± 7.0% and 94.6 ± 6.1% in the placebo and CBG-CS-2 groups, respectively.
Safety analysis
Safety outcomes were assessed by assessing adverse events during the overall clinical study period. The laboratory tests (blood and urinalysis), ECG, vital signs, and anthropometric parameters (data not shown) were evaluated. At every visit, information about symptoms or adverse events was recorded, but no serious adverse events were reported during the study period. The parameters of the safety assessments were in the normal range, and no subjects withdrew because of adverse events.
Efficacy evaluation
Changes in the NK-cell activity and cytokines (IFN-γ, TNF-α, IL-1β, IL-2, IL-4, and IL-10, and IL-12) during the overall intervention period are shown in Table 3.
The rate of change in NK-cell activity increased up to 38.8 ± 17.6% in the CBG-CS-2 group but decreased by 35.5 ± 19.9% in the placebo group. A significant difference between these two groups was observed (P = 0.019). Additionally, the CBG-CS-2 and placebo groups showed increases in the level of IFN-γ of 11.4 ± 7.1% and 10.7 ± 6.7%, respectively. However, there was no significant difference between these two groups. In addition, with respect to the levels of TNF-α and IL-12, the CBG-CS-2 group showed a tendency toward increasing levels, but there was no significant difference between the two groups. Furthermore, there were no significant differences in the levels of other cytokines such as IL-1β, IL-2, IL-4, and IL-10.
Discussion
In this study, we performed an 8-week clinical trial to evaluate the immunoregulatory function and safety of a mycelium extract of Cordyceps (CBG-CS-2; 1.43 g/day) separated and cultivated from P. hepiali derived from natural CS picked in Tibet. In healthy Korean test subjects, the NK-cell activity increased significantly after CBG-CS-2 was taken for 8 weeks. In the CBG-CS-2 group, the changes in NK-cell activity represented a higher immune regulatory effect than that observed in the placebo group. Additionally, we verified that CBG-CS-2 had an immune pharmacology efficacy in our in vitro and in vivo tests. In particular, the oral administration of CBG-CS-2 for 28 days in an immune-suppressed mouse model (mitomycin C-treated mice: MMC) helped reduce a gut immunity suppressive state in Peyer’s patches [33] and increased splenocyte proliferation in C57BL6 mice. Furthermore, we verified that it increased the generation of IL-12, which is an important cytokine in the T-helper cell 1 (Th1 cell) reaction related to cell-mediated immunity. In addition, it can activate the release of interferon (IFN)-γ through activating immunocytes such as dendritic cells and macrophages and has a clear immune regulatory effect caused by increasing tumor necrosis factor-alpha (TNF-α) and enhancing NK-cell activity [30, 31]. It is believed that this mechanism promotes the proliferation of splenocytes caused by the administration of CBG-CS-2 and that this increases the expression of IL-12, IFN-γ, and TNF-α by stimulating a T-helper cell-type immune reaction in mouse splenocytes. In addition, the extract improved cell-mediated immunity by increasing the NK-cell activity [31]. However, there were significant changes in the serum cytokines, including IL-12 and IFN-γ, in the clinical trial compared with our previous animal test, in which only the NK-cell activity was increased, that is, the NK-cell activity increased without any changes in the levels of cytokines. This might be due to the measurement focusing on changes in the activity of immune reactions after applying the K562 cells to trigger an immune reaction in peripheral blood mononuclear cells (PBMCs) by separating the immunocytes from blood. Kang et al. [28] showed that the intake of C. militaris for 4 weeks by healthy adults increased both the NK-cell activity and levels of the cytokines IL-12 and IFN-γ; these findings differ from the results of this study. It is known that NK-cells in the human body play a role in innate immunity by detecting and killing virus-infected cells, tumor cells, and abnormal cells and that activated NK-cells promote the release of cytokines such as IFN-γ and TNF-α [36, 37]. Kuo et al. [38] observed that in healthy adults, the processing of LPS in immunocytes (BALF cells) sampled from bronchial tubes after taking CS decreased Th2 cytokines and increased the release of Th1 cytokines (IL-12 and IFN-γ). This dynamic controls immune functions based on the balance between Th1 and Th2 cytokines. Additionally, they reported that in healthy people and leukemia patients, the application of Cordyceps increased only the activity of NK-cells [39]. These results are similar to those obtained in the current study. This finding of a change only in NK-cell activity might be because our study investigated changes in serum cytokine indices in healthy participants without any external stimulation or triggering factors. In addition, because it plays a role in maintaining homeostasis in healthy human subjects, it is believed that the extract does not affect cytokine levels [27]. Shin et al. [14] and Kang et al. [28] showed that the major components of C. militaris, cordycepin and adenosine, contributed to controlling both the phenotype transition of macrophages as well as the inflammatory and immunomodulatory effects. It is expected that the major components of CBG-CS-2, Cordyceps polysaccharides and adenosine, positively contributed to the immunoregulatory effects even though the extract showed a low cordycepin content compared to that of C. militaris. Additionally, it has been shown that simple and protein-bound polysaccharides (PS) separated from Cordyceps had excellent immunomodulatory effects [40, 41]. In particular, it was reported that PS played a role in increasing innate immune and cell-mediated immune responses as a polymer [29, 42]. Additionally, these compounds showed immunomodulatory and tumor growth inhibition effects by increasing the phagocytic activity of macrophages, the proliferation of splenocytes, and the levels of NK-cell activity, IFN-γ, and TNF-α. Therefore, we believe that the major components of CBG-CS-2 in this study, Cordyceps PS and adenosine, play an important role in presenting immune reactions as a trigger and induce an immunomodulatory effect by enhancing both the NK-cell activity and phagocyte reactions via the activation of macrophages.
There are some limitations to this study. We likely did not find differences between the CBG-CS-2 and placebo groups in the immune index of the Th1 cytokine cluster because the cytokine index was measured without any triggering factors, which can cause specific immune reactions. Additionally, we used blood samples from healthy adult participants who had no specific diseases.
This study was registered at Clinical Trials.gov on June 2016 (NCT 02814617).
Conclusion
It will be necessary to investigate changes in immune-related cytokines in participants with depressed immune responses and to precisely design a challenge test that can externally stimulate immune depression. Furthermore, a long-term study will also be required. In summary, it is expected that the continuous dose of CBG-CS-2 obtained from CS increases Th1 immune responses and activates NK-cells, which results in immunomodulatory effects by improving cell-mediated immunity.
Abbreviations
- CBG-CS-2:
-
Mycelium extract of Cordyceps
- CS:
-
Cordyceps sinensis
- iNOS:
-
Inducible nitric oxide synthase
- MMC:
-
Mitomycin C-treated mice
- NK-cell:
-
Natural killer
- P. hepiali :
-
Paecilomyces hepiali
- PS:
-
Polysaccharides
References
Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52.
Panda AK, Swain KC. Traditional uses and medicinal potential of Cordyceps sinensis of Sikkim. J Ayurveda Integr Med. 2011;2(1):9–13.
Yin D, Tang X. Advances in the study on artificial cultivation of Cordyceps sinensis. Zhongguo Zhong Yao Za Zhi. 1995;20(12):707–9 inside back cover.
Shashidhar GM, Giridhar P, Manohar B. Functional polysaccharides from medicinal mushroom Cordyceps sinensis as a potent food supplement: extraction, characterization and therapeutic potentials - a systematic review. RSC Adv. 2015;5(21):16050–66.
Ko YF, Liau JC, Lee CS, Chiu CY, Martel J, Lin CS, Tseng SF, Ojcius DM, Lu CC, Lai HC, et al. Isolation, culture and characterization of Hirsutella sinensis Mycelium from Caterpillar fungus fruiting body. PLoS One. 2017;12(1):1–21.
Lo HC, Hsu TH, Tu ST, Lin KC. Anti-hyperglycemic activity of natural and fermented Cordyceps sinensis in rats with diabetes induced by nicotinamide and streptozotocin. Am J Clin Med. 2006;34(5):819–32.
Xiao JH, Li Y, Xiao Y, Zhong JJ. Advance and prospect of studies on bioactivity and mechanism of Cordyceps fungi. Zhongguo Zhong Yao Za Zh. 2013;38(5):640–7.
Yan WJ, Li TH, Lao JH, Song B, Shen YH. Anti-fatigue property of Cordyceps guangdongensis and the underlying mechanisms. Pharm Biol. 2013;51(5):614–20.
Yu Y, Wang W, Wang L, Pang F, Guo L, Song L, Liu G, Feng C. Draft genome sequence of Paecilomyces hepiali, isolated from Cordyceps sinensis. Genome Announc. 2016;4(4):1–2.
Wang BJ, Won SJ, Yu ZR, Su CL. Free radical scavenging and apoptotic effects of Cordyceps sinensis fractionated by supercritical carbon dioxide. Food Chem Toxicol. 2005;43(4):543–52.
Chen J, Zhang W, Lu T, Li J, Zheng Y, Kong L. Morphological and genetic characterization of a cultivated Cordyceps sinensis fungus and its polysaccharide component possessing antioxidant property in H22 tumor-bearing mice. Life Sci. 2006;78(23):2742–8.
Ghatnur SM, Parvatam G, Balaraman M. Culture conditions for production of biomass, adenosine, and Cordycepin from Cordyceps sinensis CS1197: optimization by desirability function method. Pharmacogn Mag. 2015;11(Suppl 3):S448–56.
Xu H, Li S, Lin Y, Liu R, Gu Y, Liao D. Effectiveness of cultured Cordyceps sinensis combined with glucocorticosteroid on pulmonary fibrosis induced by bleomycin in rats. Zhongguo Zhong Yao Za Zhi. 2011;36(16):2265–70.
Shin S, Moon S, Park Y, Kwon J, Lee S, Lee CK, Cho K, Ha NJ, Kim K. Role of Cordycepin and adenosine on the phenotypic switch of macrophages via induced anti-inflammatory cytokines. Immune Netw. 2009;9(6):255–64.
Yue K, Ye M, Lin X, Zhou Z. The artificial cultivation of medicinal Caterpillar fungus, Ophiocordyceps sinensis (ascomycetes): a review. Int J Med Mushrooms. 2013;15(5):425–34.
Kai Z, Yongjian L, Sheng G, Yu L. Effect of Dongchongxiacao (Cordyceps) therapy on contrast-induced nephropathy in patients with type 2 diabetes and renal insufficiency undergoing coronary angiography. J Tradit Chin Med. 2015;35(4):422–7.
Kiho T, Ookubo K, Usui S, Ukai S, Hirano K. Structural features and hypoglycemic activity of a polysaccharide (CS-F10) from the cultured mycelium of Cordyceps sinensis. Biol Pharm Bull. 1999;22(9):966–70.
Koh JH, Yu KW, Suh HJ, Choi YM, Ahn TS. Activation of macrophages and the intestinal immune system by an orally administered decoction from cultured mycelia of Cordyceps sinensis. Biosci Biotechnol Biochem. 2002;66(2):407–11.
Yang JY, Zhang WY, Shi PH, Chen JP, Han XD, Wang Y. Effects of exopolysaccharide fraction (EPSF) from a cultivated Cordyceps sinensis fungus on c-Myc, c-Fos, and VEGF expression in B16 melanoma-bearing mice. Pathol Res Pract. 2005;201(11):745–50.
Zhang W, Yang J, Chen J, Hou Y, Han X. Immunomodulatory and antitumour effects of an exopolysaccharide fraction from cultivated Cordyceps sinensis (Chinese caterpillar fungus) on tumour-bearing mice. Biotechnol Appl Biochem. 2005;42(Pt 1):9–15.
Li F, Gao XY, Rao BF, Liu L, Dong B, Cui LQ. Effects of cordyceps sinensis alcohol extractive on serum interferon-gamma level and splenic T lymphocyte subset in mice with viral myocarditis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2006;22(3):321–3.
Yoon TJ, Yu KW, Shin KS, Suh HJ. Innate immune stimulation of exo-polymers prepared from Cordyceps sinensis by submerged culture. Appl Microbiol Biotechnol. 2008;80(6):1087–93.
Ding CG, Tian PX, Jin ZK. Clinical application and exploration on mechanism of action of Cordyceps sinensis mycelia preparation for renal transplantation recipients. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi. 2009;29(11):975–8.
Choi JW, Ra KS, Kim SY, Yoon TJ, Yu KW, Shin KS, Lee SP, Suh HJ. Enhancement of anti-complementary and radical scavenging activities in the submerged culture of Cordyceps sinensis by addition of citrus peel. Bioresour Technol. 2010;101(15):6028–34.
Chen Y, Chen YC, Lin YT, Huang SH, Wang SM. Cordycepin induces apoptosis of CGTH W-2 thyroid carcinoma cells through the calcium-calpain-caspase 7-PARP pathway. J Agric Food Chem. 2010;58(22):11645–52.
Zhu ZY, Chen J, Si CL, Liu N, Lian HY, Ding LN, Liu Y, Zhang YM. Immunomodulatory effect of polysaccharides from submerged cultured Cordyceps gunnii. Pharm Biol. 2012;50(9):1103–10.
Pao HY, Pan BS, Leu SF, Huang BM. Cordycepin stimulated steroidogenesis in MA-10 mouse Leydig tumor cells through the protein kinase C pathway. J Agric Food Chem. 2012;60(19):4905–13.
Kang HJ, Baik HW, Kim SJ, Lee SG, Ahn HY, Park JS, Park SJ, Jang EJ, Park SW, Choi JY, et al. Cordyceps militaris enhances cell-mediated immunity in healthy Korean men. J Med Food. 2015;18(10):1164–72.
Kuo MC, Chang CY, Cheng TL, Wu MJ. Immunomodulatory effect of exo-polysaccharides from submerged cultured Cordyceps sinensis: enhancement of cytokine synthesis, CD11b expression, and phagocytosis. Appl Microbiol Biotechnol. 2007;75(4):769–75.
Jang SH, Kim SH, Lee HY, Jang SH, Jang H, Chae SW, Jung SJ, So BO, Ha KC, Sin HS, et al. Immune-modulating activity of extract prepared from mycelial culture of Chinese Caterpillar mushroom, Ophiocordyceps sinensis (ascomycetes). Int J Med Mushrooms. 2015;17(12):1189–99.
Jang SH, Park J, Jang SH, Chae SW, Jung SJ, So BO, Ha KC, Sin HS, Jang YS. In vitro stimulation of NK cells and lymphocytes using an extract prepared from mycelial culture of Ophiocordyceps sinensis. Immune Network. 2016;16(2):140–5.
Park SY, Jung SJ, Ha KC, Sin HS, Jang SH, Chae HJ, Chae SW. Anti-inflammatory effects of Cordyceps mycelium (Paecilomyces hepiali, CBG-CS-2) in Raw264.7 murine macrophages. Orient Pharm Exp Med. 2015;15(1):7–12.
Chae SW, Fusako M, Jung SJ, Ha KC, Sin HS, Jang SH, Shin N. Nutrigenomic study on immunomodulatory function of Cordyceps mycelium extract (Paecilomyces hepiali) in Mitomycin C–treated mice. Food Nutr Sci. 2014;5(22):2217–24.
Konjevic G, Jurisic V, Spuzic I. Corrections to the original lactate dehydrogenase (LDH) release assay for the evaluation of NK cell cytotoxicity. J Immunol Methods. 1997;200(1–2):199–201.
Eom SY, Zhang YW, Kim NS. Effect of Keumsa sangwhang (Phellinus linteus) mushroom extracts on the natural killer cell activity in human. Korean J Food sci. 2006;38(5):717–9.
Yoshikai Y, Nishimura H. The role of interleukin 15 in mounting an immune response against microbial infections. Microbes Infect. 2000;2(4):381–9.
Wang R, Jaw JJ, Stutzman NC, Zou Z, Sun PD. Natural killer cell-produced IFN-gamma and TNF-alpha induce target cell cytolysis through up-regulation of ICAM-1. J Leukoc Biol. 2012;91(2):299–309.
Kuo YC, Tsai WJ, Wang JY, Chang SC, Lin CY, Shiao MS. Regulation of bronchoalveolar lavage fluids cell function by the immunomodulatory agents from Cordyceps sinensis. Life Sci. 2001;68(9):1067–82.
Liu C, Lu S, Ji MR. Effects of Cordyceps sinensis (CS) on in vitro natural killer cells. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi. 1992;12(5):267–9.
Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002;60(3):258–74.
Cheung JK, Li J, Cheung AW, Zhu Y, Zheng KY, Bi CW, Duan R, Choi RC, Lau DT, Dong TT, et al. Cordysinocan, a polysaccharide isolated from cultured Cordyceps, activates immune responses in cultured T-lymphocytes and macrophages: signaling cascade and induction of cytokines. J Ethnopharmacol. 2009;124(1):61–8.
Wang M, Meng X, Yang R, Qin T, Li Y, Zhang L, Fei C, Zhen W, Zhang K, Wang X, et al. Cordyceps militaris polysaccharides can improve the immune efficacy of Newcastle disease vaccine in chicken. Int J Biol Macromol. 2013;59:178–83.
Acknowledgements
We thank all of participating researchers and our study staff.
Funding
This study was financially supported by the Ministry of Agriculture, Food, and Rural Affairs (MAFRA)/Korea National Food Cluster grant no. FOODPOLIS 2012–03.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available to protect patient confidentiality but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
All authors participated in this work with their substantive contributions. SJJ carried out the manuscript preparation and statistical analysis. ESJ and EKC had the responsibility of data collection and interaction with subjects for the RCT projects. SWC, HSS and KCH had participated in the biochemical analysis, interpretation of the data, and review of the paper. The manuscript has been read and approved by all of the authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the Declaration of Helsinki. The research protocol was approved by the Institutional Review Board (IRB) of Chonbuk National University Hospital. All participants gave written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Supplements test capsules. (DOCX 49 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jung, SJ., Jung, ES., Choi, EK. et al. Immunomodulatory effects of a mycelium extract of Cordyceps (Paecilomyces hepiali; CBG-CS-2): a randomized and double-blind clinical trial. BMC Complement Altern Med 19, 77 (2019). https://doi.org/10.1186/s12906-019-2483-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-019-2483-y