Abstract
Background
Cancer is one of the most frequently occurring diseases and is the second leading cause of death worldwide. In this study, anthraquinone derivatives (Compounds 1–5) were evaluated for their anti-cancer potential against various skin and breast cancer cell lines to assess whether these anthraquinone derivatives may serve as a lead for the augmentation of anti-cancer drug.
Methods
Anthraquinone derivatives, 2-methyl-1,3,6-trihydroxy-9,10-anthraquinone-3-O-(6′-O-acetyl)-α-rhamnosyl(1 → 2)-β-glucoside (Comp 1), 2-methyl-1,3,6-trihydroxy-9,10-anthraquinone (Comp 2), and alizarin (Comp 3) were isolated from the dichloromethane fraction of the roots of Rubia philippinensis., whereas ethyl acetate fraction yielded xanthopurpurin (Comp 4) and lucidin-ω-methyl ether (Comp 5). Structures of all the isolated compounds were determined by spectral data analysis. All isolated compounds (Comp 1–5) were assessed for cytotoxicity by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay against four different cancer cell lines, i.e. human melanoma (SK-MEL-5), murine melanoma (B16F10), and human breast adenocarcinoma (MCF7 and MDA-MB-231).
Results
Significant activity of the compounds 4 and 5 was observed against the breast cancer cell line MDA-MB-231 with IC50 values of 14.65 ± 1.45 and 13.03 ± 0.33 μM, respectively. Encouragingly, IC50 values of 67.89 ± 1.02 and 79.01 ± 0.03 μM against normal kidney epithelial cells (MDCK) were also obtained for compounds 4 and 5, respectively, which indicated very low toxicity and favorable selectivity indices for compounds 4 and 5 in the range of 1.85 to 3.95 and 2.11 to 6.06 against skin cancer cell lines (SK-MEL-5, and B16F10), and breast cancer cell lines (MCF7 and MDA-MB-231), respectively.
Conclusion
Our results suggested that the compounds 4 (xanthopurpurin) and 5 (lucidin-ω-methyl ether) showed high selective toxicity towards breast cancer cells at lower concentrations without showing toxicity towards normal cells, thus could be of potential as new lead molecules in cancer treatment.
Similar content being viewed by others
Background
Cancer is one of the most frequently occurring diseases and is the second leading cause of death worldwide, while chemotherapy is most extensively used among a wide range of anti-cancer therapies, and its high toxicity, being expensive as well as activating alternative cell signaling pathways are limiting its applications [1]. For centuries to date, being safe, low cost and easily accessible, medicinal herbs are viewed as the main sources of new drugs to treat cancer worldwide while various pharmacological studies continue to validate their uses [1]. Moreover, herbal medicines are widely assumed in complementary and alternative medicine especially in cancer patients with poor socioeconomic condition. Mounting evidences suggest that plants possessing anticancer properties, such as Soymida fembrifuga (Miliaceae), Tinospora cordifolia (Menispermaceae), Lavandula bipinnata (Lamiaceae), Helicteres isora (Sterculiaceae), Urtica membranacea (Urticaceae), Artemesia monosperma (Asteraceae), and Origanum dayi post (Labiatae) etc., are the source of alternative medicine for cancer therapy in various regions of the globe [2,3,4]. However, a large number of plant species remain to be screened for their therapeutic potential; consequently, they can be used as a continual source of new medicines for present and future health problems of humans, including cancer.
Rubia philippinensis is a rambling and low climbing perennial herb that grows in the Southern part of Vietnam. Local communities have long utilized this medicinal plant to treat ordinary ailments such as wounds, inflammation, and skin infections. Previous investigations of the species have resulted in the purification of arborinane triterpenoids, which show promising effects on the prevention and treatment of atherosclerosis [5]. Additionally, rubiarbonone C, a popular chemical entity isolated from R. philippinensis, has been shown to inhibit abnormal proliferation and migration of vascular smooth muscle cells, which plays an important role in the pathophysiology of atherosclerosis. The mechanism by which rubiarbonone C regulates vascular remodeling was further clarified through focal adhesion kinase (FAK), MAPK, and STAT3 Tyr705 [6]. In searching for bioactive components from R. philippinensis, in this study, derivatives of anthraquinone were isolated as the major compounds.
Anthraquinones possessing three benzene rings represent a class of compounds belonging to quinone family. The divergence of the anthraquinone molecules relies on the nature and the setting of the substituents. Anthraquinones display a number of biological functions, including laxative [7], diuretic [8], phytoestrogen [9], anti-platelet [10], anti-fungal [11], anti-viral [12], and anti-cancer properties [13]. Moreover, they have a significant industrial potential of being used as textile dyes, food colorants and bugs repellents.
As a part of continuous attempts to probe the potential nature-derived drug templates for the treatment of cancer [5, 14], the current study delineates the isolation and characterization of five anthraquinone derivatives (compound 1–5) from R. philippinensis. These compounds were evaluated for their anti-cancer potential against various skin cancer cells (SK-MEL-5 and B16F10) and breast cancer cells (MCF7 and MDA-MB-231) to assess whether these anthraquinone derivatives may serve as a lead for the development of anti-cancer drugs.
Methods
Plant materials
Root samples of Rubia philippinensis were procured from Bidoup-Nui Ba National Park, Lamdong province, Vietnam and identified by the expert Dr. Phuong Thien Thuong at the Department of Pharmaceutical Analysis and Herbal Standardization, NIMM, Hanoi, Vietnam. An authenticated root voucher sample was deposited at the laboratory of the NIMM (VDL20140801) and at the Pharmacognosy Laboratory, College of Pharmacy, Chungnam National University (CNU1409), Daejeon, Korea.
Extraction, isolation, and characterization of anthraquinone derivatives
Anthraquinones were isolated from the root samples of R. philippinensis by chromatographic techniques. In brief, the ethanol extract of R. philippinensis (150 g) was suspended in H2O (1.5 L) and sequentially partitioned with CH2Cl2 (2 L × 3) and EtOAc (2 L × 3) to yield the CH2Cl2 and EtOAc extracts. The CH2Cl2-soluble fraction (50 g) was loaded into silica gel VLC and eluted with n-hexane-EtOAc (20:1, 10:1, 5:1, 3:1, 2:1) and CHCl3-MeOH (8:1) to afford six fractions (D-1 → D-6). Fraction D-4 (6.1 g) was divided into 10 sub-fractions (D-4-1 → D-4-10) using MPLC with a step-wise gradient of Acetone-H2O (60:40, 72:28, 75:25, 95:5, 100:0, each 1.5 L). Xanthopurpurin (4) (tR 33.5 min, 28 mg) and lucidin-ω-methyl ether (5) (tR 36.0 min, 31 mg) were obtained from D-4-4 (320 mg) by HPLC eluting with MeCN-H2O (54.5:45.5, 4 mL/min, UV 360 nm). The EtOAc fraction (14.0 g) was subjected to silica gel VLC and eluted with n-hexane/EtOAc/MeOH (2:1:0.2) and CHCl3-MeOH (8:1, 5:1, 3:1, 0:1) to yield five fractions (EA-1 → EA-5). Eight sub-fractions (EA-1-1 → EA-1-8) were collected from fraction EA-1 (1.8 g) by utilizing MPLC, eluting with MeOH-H2O (10:90, 50:50, 67:33, 80:20, 100:0, each 500 mL). Alizarin (3) (tR 44.0 min, 2 mg) was isolated from EA-1-5 (100 mg) by HPLC eluting with MeCN-H2O (44.5:55.5, 4 mL/min, UV 360 nm). Two sub-fractions EA-1-6 and EA-1-7 were combined (EA-1-6,7; 500 mg), then purified one more time by MPLC, eluted with Acetone-H2O (40:60, 60:40, 100:0, each 500 mL) to afford 2-methyl-1,3,6-trihydroxy-9,10-anthraquinone (2) as orange crystals (200 mg). Fraction EA-4 (2.6 g) was separated by MPLC applying mixtures of solvent MeOH-H2O (23:77, 37:63, 47:53, 52:48, 60:40, 67:33, 100:0, each 400 mL) to yield 11 sub-fractions (EA-4-1 → EA-4-11). 2-methyl-1,3,6-trihydroxy-9,10-anthraquinone-3-O-(6’-O-acetyl)-α-rhamnosyl(1 → 2)-β-glucoside (1) (tR 40 min, 800 mg) was purified from sub-fractions EA-4-8 (706 mg) and EA-4-9 (410 mg) by HPLC utilizing MeOH-H2O (65:35, 6 mL/min, UV 254 nm).
2-methyl-1,3,6-trihydroxy-9,10-anthraquinone-3-O-(6’-O-acetyl)-α-rhamnosyl(1 → 2)-β-glucoside (1): yellow powder, 1H NMR (300 MHz, DMSO-d6): δH 13.28 (1H, s, OH-1), 8.08 (1H, d, J = 8.4 Hz, H-8), 7.45 (1H, d, J = 2.4 Hz, H-5), 7.40 (1H, s, H-4), 7.20 (1H, dd, J = 8.4, 2.4 Hz, H-7), 5.45 (1H, d, J = 6.9 Hz, Glu-H-1′), 5.28 (1H, d, J = 0.9 Hz, Rha-H-1″), 2.15 (3H, s, CH3–2), 1.93 (3H, s, OAc-6′), 1.09 (3H, d, J = 6.3 Hz, Rha-CH3–6″). 13C NMR (150 MHz, DMSO-d6) δC Aglycone: 164.2 (C-1), 120.5 (C-2), 160.0 (C-3), 105.2 (C-4), 135.4 (C-4a), 112.8 (C-5), 161.3 (C-6), 121.6 (C-7), 129.7 (C-8), 124.1 (C-8a), 186.3 (C-9), 110.7 (C-9a), 181.8 (C-10), 131.9 (C-10a), 8.7 (CH3–2). Glucose: 97.3 (C-1′), 76.3 (C-2′), 77.0 (C-3′), 70.0 (C-4′), 74.0 (C-5′), 63.3 (C-6′), 170.3 (OAc-6′), 20.4 (OAc-6″). Rhamnose: 100.2 (C-1″), 70.3 (C-2″), 70.5 (C-3″), 72.0 (C-4″), 68.5 (C-5″), 18.1 (C-6″).
2-methyl-1,3,6-trihydroxy-9,10-anthraquinone (2): orange crystal, 1H NMR (300 MHz, DMSO-d6): δH 13.31 (1H, s, OH-1), 8.05 (1H, d, J = 8.4 Hz, H-8), 7.43 (1H, d, J = 2.4 Hz, H-5), 7.20 (1H, s, H-4), 7.20 (1H, dd, J = 8.4, 2.4 Hz, H-7), 2.05 (3H, s, CH3–2). 13C NMR (75 MHz, DMSO-d6) δC 162.3 (C-1), 117.4 (C-2), 163.1 (C-3), 107.1 (C-4), 135.2 (C-4a), 112.5 (C-5), 162.1 (C-6), 121.3 (C-7), 129.4 (C-8), 124.7 (C-8a), 185.8 (C-9), 108.6 (C-9a), 182.0 (C-10), 131.8 (C-10a), 8.1 (CH3–2).
Alizarin (3): brownish red powder, 1H NMR (300 MHz, DMSO-d6) δH 7.51 (1H, d, J = 8.1, H-4), 7.18 (1H, d, J = 8.1, H-3). 13C NMR (150 MHz, DMSO-d6) δC 151.0 (C-1), 153.3 (C-2), 120.8 (C-3), 121.3 (C-4), 126.5 (C-5), 134.0 (C-6), 135.1 (C-7), 126.7 (C-8), 188.8 (C-9), 180.5 (C-10), 123.5 (C-4a), 132.9 (C-10a), 133.7 (C-8a), 116.2 (C-9a).
Xanthopurpurin (4): orange powder, 1H NMR (300 MHz, DMSO-d6) δH 7.79 (1H, d, J = 2.1, H-4), 7.27 (1H, d, J = 2.1, H-3). 13C NMR (75 MHz, DMSO-d6) δC 164.7 (C-1), 107.6 (C-2), 165.5 (C-3), 108.4 (C-4), 126.7 (C-5), 134.5 (C-6), 134.3 (C-7), 126.2 (C-8), 185.6 (C-9), 181.6 (C-10), 134.7 (C-4a), 132.7 (C-10a), 132.8 (C-8a), 109.1 (C-9a).
Lucidin-ω-methyl ether (5): orange powder, 1H NMR (300 MHz, CDCl3) δH 13.24 (1H, s, OH-1), 4.89 (2H, s, CH2OCH3–2), 3.55 (3H, s, CH2OCH3–2). 13C NMR (75 MHz, CDCl3) δC 162.0 (C-1), 114.5 (C-2), 164.2 (C-3), 109.7 (C-4), 127.5 (C-5), 134.3 (C-6), 134.2 (C-7), 126.8 (C-8), 187.0 (C-9), 182.3 (C-10), 133.6 (C-4a), 134.2 (C-10a), 133.6 (C-8a), 109.9 (C-9a), 69.0 (CH2OCH3–2), 59.5 (CH2OCH3–2).
Cell culture and cell viability assay
The potential cytotoxicity of the isolated anthraquinone derivatives was studied against various cancer cell lines, including SK-MEL-5 (human melanoma), B16F10 (murine melanoma) MCF7 (human breast adenocarcinoma), and MDA-MB-231 (human breast adenocarcinoma) and the normal cell line MDCK (normal kidney epithelial) using the MTT assay [15]. All cell lines were cultured in DMEM medium supplemented with 10% foetal bovine serum (FBS) and streptomycin–penicillin (100 μg/ml each; Hyclone) in a 5% CO2 humidified incubator. An MTT assay was employed to determine the percentage of the viability of various cancer cells as well as MDCK cells. All cells were first cultured in 96-well plates (1 × 105 cells/mL for all cancerous cells and 5 × 105 cells/mL for MDCK cells) for 24 h, and treated with indicated concentration of isolated compounds (6.25–100 μM for cancerous cells and 6.25–400 μM for MDCK cells). Various dilutions of stock culture were made in the culture medium to get the final concentration of the sample with a 0.1% of DMSO concentration, including the control. After 24 h incubation, MTT reagent was added to each well and the plate was incubated at 37 °C for 1 h. After removing the medium, the plate was washed twice with PBS (pH 7.4). The intracellular insoluble formazan was dissolved in 100% DMSO. A microplate reader was used to measure the absorbance of each cell line at 570 nm, and the percentage of cell viability was calculated. The absorbance value for the average of wells of cells treated with each test sample concentration was expressed as a percentage of this control and the IC50 values for each sample on each cell line were calculated. The anti-cancer drug oxaloplatin was used as a positive control.
Statistical analysis
All the results were presented as the mean ± SD following the analysis of one-way ANOVA. A value of p < 0.05 was recognized as significant for the differences. An SPSS version of Windows’ (Chicago, Illinois, USA) was performed for all the analyses.
Results
Identification and characterization of anthraquinone derivatives (Fig. 1)
The 1H NMR data of compound 1 displayed signals of the anthraquinone aglycone, including one aromatic singlet proton δH 7.40 (1H, s, H-4), one ABX ring system δH 8.08 (1H, d, J = 8.4 Hz, H-8), 7.45 (1H, d, J = 2.4 Hz, H-5), 7.20 (1H, dd, J = 8.4, 2.4 Hz, H-7), and one singlet methyl δH 2.15. The glycosidic linkage, meanwhile, contained resonances of two anomeric protons of the sugar moiety at δH 5.45 (glucose), δH 5.28 (rhamnose), one secondary methyl (δH 1.09, rhamnose), and one acetyl group (δH 1.93). The 13C NMR data showed 14 signals of a typical anthraquinone, including two ketones (δC 186.3, 181.8), and resonances for glucose (δC 97.3, 76.3, 77.0, 70.0, 74.0, 63.3), acetoxy (δC 170.3, 20.4), and rhamnose (δC 100.2, 70.3, 70.5, 72.0, 68.5, 18.1) moieties. On the basis of NMR spectroscopic data analyses, the compound was identified as 2-methyl-1,3,6-trihydroxy-9,10-anthraquinone-3-O-(6’-O-acetyl)-α-rhamnosyl(1 → 2)-β-glucoside.
Chemical structures of anthraquinone derivatives, 2-methyl-1,3,6-trihydroxy-9,10-anthraquinone 3-O-(6ʹ-O-acetyl)-α-rhamnosyl(1 → 2)-β-glucoside (compound 1), 2-methyl-1,3,6-trihydroxy-9,10-anthraquinone (compound 2), alizarin (compound 3), xanthopurpurin (compound 4), and lucidin-ω-methyl ether (compound 5) isolated from R. philippinensis
Similar to compound 1, compound 2 also showed resonances of one aromatic singlet proton, one ABX ring system, and one singlet methyl at δH 7.20 (1H, s, H-4); [δH 8.05 (1H, d, J = 8.4 Hz, H-8; 7.43 (1H, d, J = 2.4 Hz, H-5; 7.20 (1H, dd, J = 8.4, 2.4 Hz, H-7)]; and δH 2.05 (3H, s, CH3–2), respectively in 1H NMR spectrum. On the other hand, the skeleton of 14 carbon signals along with two ketonic carbonyls (δC 185.8, 182.0) and one methyl functionality (δC 8.1) was representative of 13C NMR data of an anthraquinone. The 1D NMR of compound 2 resemble closely to those of compound 1, except for the absence of signals belonging to sugar units. In comparison with reference values, compound 2 was determined as 2-methyl-1,3,6-trihydroxy-9,10-anthraquinone. Compound 3, 4, and 5 are also anthraquinone derivatives and their structures were elucidated as alizarin, xanthopurpurin, and lucidin-ω-methyl ether, respectively, based on the NMR data analysis. NMR data of all anthraquinone has been provided in Additional file 1: Figures S1-S5).
Cytotoxicity of anthraquinone derivatives
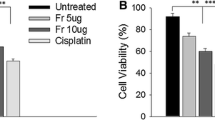
All compounds were tested for cytotoxicity by MTT assay on cell lines SK-MEL-5, B16F10, MCF7, MDA-MB-231, and MDCK cells as a normal cell line, which showed significant cytotoxicity (Table 1, Additional file 1: Figures S6-S10). Our results showed that the IC50 values for cancer cell lines treated ranged from 48.68 ± 0.10 to 91.04 ± 1.88 μM for compound 1; 46.75 ± 1.39 to 79.96 ± 1.14 μM for compound 2; 48.64 ± 0.33 to 98.79 ± 2.10 μM for compound 3, 14.65 ± 1.45 to 23.71 ± 1.71 μM for compound 4, and 13.03 ± 0.33 to 42.79 ± 1.32 μM for compound 5. Regarding the normal cell line MDCK cells, the IC50 values were 192.34 ± 0.49, 168.76 ± 0.61, 199.32 ± 1.88, 67.89 ± 1.02 and 79.01 ± 0.03 μM for compounds 1, 2, 3, 4, and 5, respectively. Interestingly, among all the compounds, compounds 4 and 5 showed strong cytotoxicity towards breast cancer cells (MCF7 and MDA-MB-231) than skin cancer cells (SK-MEL-5 and B16F10) with IC50 value of 15.75 ± 1.00 and 24.10 ± 1.06 for MCF7 as well as 14.65 ± 1.45 and 13.03 ± 0.33 for MDA-MB-231, respectively.
In addition, compound 4 and 5 were more cytotoxic to MDA-MB-231 cancer cell line (IC50 = 14.65 ± 1.45 and 13.03 ± 0.33 μM, respectively) than to normal cells (IC50 = 67.89 ± 1.02 and 79.01 ± 0.03 μM (Table 1), respectively with their respective selectivity indices of 4.63 and 6.06 (Table 2).
Table 2 shows the selectivity indices of the isolated compounds tested against the various cancer cell lines and the non-tumor cell line (MDCK). In the current study, treatments with compound 4 and 5 afforded the highest selectivity indices in breast cancer cell than skin cancer cells. Compound 4 showed the selectivity indices as 4.31 and 4.63 whereas compound 5 showed 3.28 and 6.06 in MCF7 and MDA-MB-231 cells, respectively (Table 2).
Discussion
A number of natural compounds have been isolated from different plant sources which have shown enormous biological potential [16,17,18,19]. In this study, five anthraquinone derivatives, such as 2-methyl-1,3,6-trihydroxy-9,10-anthraquinone 3-O-(6'-O-acetyl)-α-rhamnosyl(1 → 2)-β-glucoside (compound 1), 2-methyl-1,3,6-trihydroxy-9,10-anthraquinone (compound 2), alizarin (compound 3), xanthopurpurin (compound 4), and lucidin-ω-methyl ether (compound 5) were isolated from the root of R. philippinensis., and were characterized based on the spectral data analysis [16,17,18,19].
These anthraquinone derivatives showed significant anticancer potential as confirmed by their cytotoxicity effects against various cancer cell lines, such as cell lines SK-MEL-5, B16F10, MCF7, MDA-MB-231, including normal MDCK cell line. However, according to American National Center Institute, extract/compounds with IC50 values lower than 30 μM against experimental cancer cell lines constitute promising anticancer agents for drug development [20]. Therefore, compound 4 and 5 showed IC50 values greater than 30 μM against all cell lines tested, and were more cytotoxic to normal line to which the cancer cell lines. Moreover, among the testest compounds, anthraquinone derivatives xanthopurpurin (compound 4), and lucidin-ω-methyl ether (compound 5) showed highest selectivity indices in breast cancer cell than skin cancer cells.
Mounting evidences have considered that a value greater than or equals to 2.0 is an interesting selectivity index [21]. This value means that the compound is more than twice more cytotoxic to the cancer cell line as compared with the normal cell line [21]. These findings demonstrated that compound 4 and 5 can be considered promising lead molecules for the development of anticancer drugs, especially for breast cancer, because they provided indices value greater than 2.
Conclusions
It is very important to consider natural compounds as a chemotherapeutic agent for cancer which have minimum or no side effects on normal body cells of patients. To achieve this goal among various ways, one of the way is by employing lower doses of drug at which drug shows highly potent activity as well as exhibits high degree of selectivity. In this study, we presented the cytotoxicity potential of five anthraquinone derivatives isolated from the roots of Rubia philippinensis. The results of in vitro studies demonstrate the ability of the compounds 4 (xanthopurpurin) and 5 (lucidin-ω-methyl ether) for high selective toxicity at lower concentrations (Table 1) without showing toxicity towards normal cells, confirming that compounds 4 and 5 may have the potentiality to be developed as anticancer drugs, especially for breast cancer. Further research strategies should investigate cytotoxic potential of compound 4 and 5 against multifactorial drug-resistant cancers for their pharmaceutical formulations.
Abbreviations
- ANOVA:
-
Analysis of variance
- DMEM:
-
Dulbecco'’s Modified Eagle'’s Medium
- DMSO:
-
Dimethyl sulfoxide
- FAK:
-
Focal adhesion kinase
- FBS:
-
Foetal bovine serum
- HPLC:
-
High-performance liquid chromatography
- MAPK:
-
Mitogen-activated protein kinase
- MPLC:
-
Medium pressure liquid chromatography
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NMR:
-
Nuclear magnetic resonance
- SD:
-
Standard deviation
- UV:
-
Ultraviolet
- VLC:
-
Vacuum liquid chromatography
References
El-Kashak WA, Osman SM, Gaara AH, El-Toumy SA, Mohamed TK, Brouard I, et al. Phenolic metabolites, biological activities, and isolated compounds of Terminalia muelleri extract. Pharm Biol. 2017;55:2277–84.
Graham JG, Quinn ML, Fabricant DS, Farnsworth NR. Plants used against cancer - an extension of the work of Jonathan Hartwell. J Ethnopharmacol. 2000;73:347–77.
Solowey E, Lichtenstein M, Sallon S, Paavilainen H, Solowey E, Lorberboum-Galaski H, et al. Evaluating medicinal plants for anticancer activity. Sci World J. 2014;2014:e721402.
Shaikh R, Pund M, Dawane A, Iliyas S. Evolution of anticancer, antioxidant, and possible anti-inflammatory properties of selected medicinal plants used in Indian traditional medication. J Tradit Complement Med. 2014;4:253–7.
Quan KT, Park HS, Oh J, Park HB, Ferreira D, Myung CS, Na M, et al. Arborinane triterpenoids from Rubia philippinensis inhibit proliferation and migration of vascular smooth muscle cells induced by the platelet-derived growth factor. J Nat Prod. 2016;79:2559–69.
Park HS, Quan KT, Han JH, Jung SH, Lee DH, Jo E, et al. Rubiarbonone C inhibits platelet-derived growth factor-induced proliferation and migration of vascular smooth muscle cells through the focal adhesion kinase, MAPK and STAT3 Tyr705 signaling pathways. Br J Pharmacol. 2017;174:4140–54.
Sakulpanich A, Gritsanapan W. Determination of anthraquinone glycoside content in Cassia fistula leaf extracts for alternative source of laxative drug. Int J Biomed Pharm Sci. 2009;3:42–5.
Zhou XM, Chen QH. Biochemical study of Chinese rhubarb. XXII. Inhibitory effect of anthraquinone derivatives on Na+-K+-ATPase of the rabbit renal medulla and their diuretic action. Acta Pharm Sin. 1988;23:17–20.
Matsuda H, Shimoda H, Morikawa T, Yoshikawa M. Phytoestrogens from the roots of Polygonum cuspidatum (Polygonaceae): structure-requirement of hydroxyanthraquinones for estrogenic activity. Bioorg Med Lett. 2001;11:1839–42.
Aburjai TA. Anti-platelet stilbenes from aerial parts of Rheum palaestinum. Phytochemistry. 2000;55(5):407–10.
Agarwal SK, Singh SS, Verma S, Kumar S. Antifungal activity of anthraquinone derivatives from Rheum emodi. J Ethnopharmacol. 2000;72:43–6.
Semple SJ, Pyke SM, Reynolds GD, Flower RL. In vitro antiviral activity of the anthraquinone chrysophanic acid against poliovirus. Antivir Res. 2001;49:169–78.
Nemeikaite-Ceniene A, Sergediene E, Nivinskas H, Cenas N. Cytotoxicity of natural hydroxyanthraquinones: role of oxidative stress. J Biosci. 2002;57:822–7.
Alam MB, Bajpai VK, Lee J, Zhao P, Byeon JH, Ra JS, et al. Inhibition of melanogenesis by jineol from Scolopendra subspinipes mutilans via MAP-kinase mediated MITF downregulation and the proteasomal degradation of tyrosinase. Sci Rep. 2017;7:e45858.
Bajpai VK, Alam MB, Quan KT, Kwon KR, Ju MK, Choi HJ, et al. Antioxidant efficacy and the upregulation of Nrf2-mediated HO-1 expression by (+)-lariciresinol, a lignan isolated from Rubia philippinensis, through the activation of p38. Sci Rep. 2017;7:e46035.
Itokawa H, Mihara K, Takeya K. Studies on a novel anthraquinone and its glycosides isolated from Rubia cordifolia and R. akane. Chem Pharm Bull. 1983;31:2353–8.
Berger Y, Castonguay A, Brassard P. Carbon-13 nuclear magnetic resonance studies of anthraquinones. Org Mag Reson. 1980;14:103–8.
Chung MI, Jou SJ, Cheng TH, Lin CN. Antiplatelet constituents of Formosan Rubia akane. J Nat Prod. 1994;57:313–6.
Banthorpe DV, White JJ. Novel anthraquinones from undifferentiated cell cultures of Galium verum. Phytochemistry. 1995;38:107–11.
Badisa RB, Darling-Reed SF, Joseph P, Cooperwood JS, Latinwo LM, Goodman CB, et al. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009;29(8):2993–6.
de Oliveira PF, Alves JM, Damasceno JL, Machado Oliveira RA, Dias HJ, Miller Crotti AE, Tavares DC. Cytotoxicity screening of essential oils in cancer cell lines. Rev Bras Farmacogn. 2015;25(2):183–88.
Acknowledgements
This study was supported by the research fund of Chungnam National University.
Availability of data and materials
All data in combined within the manuscript and additional files.
Author information
Authors and Affiliations
Contributions
Designed the experiments: VKB, MBA, KTQ, HJC. Performed the experiments: MBA, KTQ, HA, MKJ. Analyzed the data: VKB, MBA, SHL, YKH, MN. Conception and design, analysis and interpretation of data, and contribution of reagents/materials/analysis tools: MKN, SHL. Manuscript preparation and revision: VKB, MBA, YKH, MN. All authors have approved the final draft of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable. This study did not involve use of animal or human subjects. The cell line was purchased from American type tissue culture (ATCC).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Supplementary data contain ten supplementary figures. Among them Figures S1-S5 represent proton and carbon NMR data of the isolated compounds, whereas Figures S6-S10 represent cytotoxic effects of isolated compounds (1–5) against MDCK, SK-MEL-5, B16F10, MCF7, and MDA-MB-231 cell lines. (DOC 899 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bajpai, V.K., Alam, M.B., Quan, K.T. et al. Cytotoxic properties of the anthraquinone derivatives isolated from the roots of Rubia philippinensis. BMC Complement Altern Med 18, 200 (2018). https://doi.org/10.1186/s12906-018-2253-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-018-2253-2