Abstract
Background
Phyllanthus amarus (Schum & Thonn), a plant belonging to the family of Euphorbiaceae is used in Ivorian traditional medicine to treat cardiovascular disorders such as hypertension. However, although this plant has been described as a diuretic agent, the underlying mechanism remains unclear. Therefore, the aim of the present study was to investigate the mechanism action of diuretic effects of an ethanolic fraction of Phyllanthus amarus (EFPA) in rats.
Methods
Effects of EFPA on urinary excretion were carried out for doses ranging from 5 to 80 mg/kg given by intraperitoneal injection (i.p.) and compared with that induced by furosemide (5 mg/kg) after 8 h. Thereafter, the diuretic activity of EFPA was also evaluated in the presence of indomethacin (5 mg/kg, i.p.) in order to determine the involvement of prostaglandins, after 24 h.
Results
Between 5 and 80 mg/kg, EFPA induced a significant urinary excretion. The profile of urinary excretion showed that after 2 h, the highest dose of 80 mg/kg induced a urinary volumetric excretion (UVE), which was similar to that induced by furosemide. After 24 h, EFPA at 10 mg/kg increased significantly UVE, Na+ (43 mEq) and Cl¯ (97 mEq) urinary excretions without promoting kaliuresis. In rats pretreated with indomethacin, the urinary excretion and the natriuretic response of EFPA were significantly reduced.
Conclusion
Altogether, this study has shown that EFPA promotes a significant urinary excretion of water and Na+, confirming its diuretic activity. Moreover, the increased diuresis could be attributed, at least in part, to the involvement of prostaglandins.
Similar content being viewed by others
Background
The World Health Organization (WHO) encourages developing countries from the tropical area to develop therapeutic alternatives in the management of priority and emerging diseases such as high blood pressure and diabetes [1]. This strategy involves exploitation of the potential medicinal plants from the local pharmacopoeia. In this context, many studies on traditional medicine are undertaken in African countries against hypertension [2].
In Côte d’Ivoire, several traditional herbal preparations are used by the local population to treat hypertension, among them, Phyllanthus amarus (Schum & Thonn), a plant belonging to the family of Euphorbiaceae [3]. This plant has various applications in traditional medicine throughout the world [4, 5]. In the Ayurvedic medicine, decoctions of the whole plant are used to treat malaria [6], and skin disorders [7]. In traditional West African pharmacopoeia, particularly in Nigeria, the administration (per os) of decoctions of leaves and seeds extracts are used to treat diabetes [8].
Pharmacological studies in mice and rats have shown that Phyllanthus amarus has antiviral [5], hepatoprotective [9, 10] and hypoglycemic activities [11]. A previous study [12] has suggested that Phyllanthus amarus, described as diuretic agent, might be of interest in the treatment of hypertension. The antihypertensive activity of Phyllanthus amarus has been related to stimulation of muscarinic receptors leading to the formation of endothelium-derived nitric oxide (NO) and also to blocking of calcium channels [13, 14]. Moreover, Phyllanthus species have been shown to have several beneficial effects on the vascular function. Phyllanthin and hypophyllanthin, two major compounds from Phyllanthus amarus, decreased vascular tone by endothelium-independent mechanisms via the blockade of Ca2+ entry into the vascular smooth muscle and inhibition of phenylephrine-induced Ca2+ release from the endoplasmis reticulum [15]. Phyllanthus acidus also inhibited vascular tone in aortic rings by acting directly on the smooth muscle and/or by releasing NO from the endothelium [16]. Moreover, 6 weeks after oral administration of an n-butanol extract of the leaves of Phyllanthus acidus, a decreased contractile response to phenylephrine and an enhanced endothelium-dependent relaxation to acetylcholine were observed in aortic rings of middle-aged male rats [17].
Furthermore, several studies have shown that natural products such as plant extracts from Hibiscus sabdariffa and Vepris heterophylla have diuretic properties, which might contribute to regulate blood pressure [18, 19]. It is well known that diuretics such as thiazides play a key role in the management of hypertensive diseases. They are most often associated with classic antihypertensive drugs such as angiotensin II type 1 receptor (AT1R) blockers to optimize blood pressure control. In this case, the role of diuretics is to potentiate the therapeutic effect of AT1R blockers by inducing a significant renal elimination of sodium and water.
The evaluation of the pharmacological effects of a Phyllanthus amarus aqueous extract administered intravenously in normotensive rabbits has shown that at doses ranging from 4.5 to 71.7 mg/kg, the extract induced a transient decrease in mean arterial blood pressure of about 4.8 ± 0.8 to 14.0 ± 0.6 mmHg [14]. The ethanolic fraction was the most potent fraction inducing about a 11.5 ± 0.5 mmHg reduction of mean arterial blood pressure at 15 mg/kg [14]. In addition, it seemed to be the most potent extract for diuresis, however the mechanism action of these diuretic effect remain unclear [20]. The present study has evaluated the possibility that an ethanolic fraction of Phyllanthus amarus has a diuretic effect, which might contribute to reduce blood pressure.
Methods
Plant material
The whole plant of Phyllanthus amarus (Euphorbiaceae) was collected in the district of Cocody (Abidjan, Côte d’Ivoire) in April 2014. This plant has been authenticated by an expert in Botany (Professor Ake-Assi Laurent) at the Centre National de Floristique (UFR-Biosciences, Félix Houphouët-Boigny University, Abidjan, Côte d’Ivoire) where the voucher specimen was recorded under No. 3, 141 and 248.
Extraction procedure
The ethanolic fraction of Phyllanthus amarus (EFPA) was prepared as previously described with minor modifications [14]. Briefly, the whole plant was harvested, washed, and extracted in boiling distilled water for 30 min at ratio of 500 g of plant for 1 l. The decoction was filtered and then lyophilized to obtain a powder of aqueous extract of Phyllanthus amarus (11.72 g from 1 kg of plant). 3 g of lyophilisate were then dissolved in 250 ml of a 70% ethanol solution and suspended into a separating funnel for 12 h. The upper phase was separated and dried using a rotary evaporator (Büchi) to obtain powder of ethanolic fraction of Phyllanthus amarus (EFPA). Extraction procedure was repeated 5 times and, the final yield of EFPA preparation was 0.26%.
Pharmacological studies
Animal
Male Wistar rats weighing between 180 and 250 g were used. Animals were randomly assigned 6 rats per group, including control, Furosemide (5 mg/kg), EFPA at different doses (5, 10, 20, 40 and 80 mg/kg), and EFPA (10 mg/kg) + indomethacin (5 mg/kg). They were bred in the animal house of the Ecole Normale Supérieure (Abidjan). Before starting the experiments, rats were isolated and acclimated for 14 days. They were maintained under standard laboratory conditions (25 ± 2 °C) with dark and light cycle (12/12 h) and had free access to a standard dry pellet diet and water ad libitum. Experiments were performed in accordance to the Guidelines for experiments involving animals [21, 22].
Evaluation of the diuretic activity
Diuretic activity was determined by the method described by Kau et al. (1984) with some modifications [23].
In the first step of experiments, the evaluation of the effects of different doses of the EFPA extracts on urinary excretion was carried out for doses ranging from 5 to 80 mg/kg body weight i.p. after an 8 h treatment period. The effect of Phyllanthus amarus extract was compared with that induced by Furosemide (5 mg/kg, i.p.), used as a reference loop diuretic drug. The doses evaluated were below the maximal tolerated doses of Phyllanthus amarus administrated i.p. [24].
Thereafter, the diuretic effect of EFPA (10 mg/kg) was evaluated after 24 h in the presence of indomethacin (5 mg/kg), a cyclooxygenase inhibitor, in order to evaluate the role of prostaglandins. Indomethacin was administered i.p. to rats 1 h prior to the administration of EFPA.
The administration of each test substance was preceded by fluid overloading with 50 ml/kg of water per os. Rats were placed individually in metabolic cages. Urine volumes were collected over either 8 or 24 h periods and were used to determine the urinary volumetric excretion (U.
VE) from the ratio of the volume of urine excreted and the volume of fluid overloaded. The urine volumes expressed as mL/100 g were calculated based on body weight of rats and urinary volumetric excretion values are expressed as a percentage of the initial hydric overload (50 mL/kg). At the end of the 24 h period, urine samples were collected and aliquoted into eppendorf tubes. Animals were anaesthetized by intraperitoneal injections of pentobarbital (50 mg/kg). The thoracic cavity was opened by surgical operation and blood was taken directly by heart puncture, put into heparinized tubes and, thereafter the plasma was obtained by centrifugation. Finally, animals were sacrificed by exsanguination. Both urine and plasma aliquots were stored at − 80 °C for subsequent biochemical analysis.
Analytical procedures
In order to determine the electrolyte content in plasma and urine samples, several techniques were used. The determination of sodium (Na+) and potassium (K+) contents was done by photometry, chlorine (Cl−) and urea concentrations by a colorimetric technique, and the calcium concentration by quantitative CPC method (o-cresolphthalein complexone). The creatinine content was estimated by the kinetics method.
Drugs
Furosemide was from Tocris Bioscience (Abingdon, UK) and indomethacin from Sigma (Saint Quentin Fallavier, France).
Statistical analysis
Data are expressed as mean ± SEM. Statistical analysis of data was performed using GraphPad Instat software (Microsoft, San Diego, California, USA) and Graph Pad Prism software (Microsoft, San Diego, California, USA). Statistical analyses were assessed using one-way or two-way analysis of variance (ANOVA) followed by Bonferroni’s post- test when applicable. Statistical significance was considered at p < 0.05.
Results
Effect of EFPA on urinary excretion
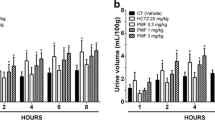
Intraperitoneal administration of EFPA at doses ranging from 5 to 80 mg/kg body weight induced significant urinary excretion in a dose-dependent manner compared to the control group (p < 0.001; Table 1; Additional file 1: Figure S1). After 8 h, urinary excretion ranged from 41.2 ± 3.3% to 63.3 ± 3.2%, respectively (Table 1; Additional file 1: Figure S1). Urinary volumes excreted by the highest dose of EFPA (80 mg/kg) and 5 mg/kg of Furosemide were time dependent and significant compared to that of the control group (p < 0.001; Fig. 1). After 2 h, Furosemide and EFPA caused similar urinary excretion, whereas at 8 h, the effect of Furosemide was significantly greater than that of EFPA (82.2% ± 3.0 versus 63.3 ± 3.2%, respectively at 8 h; Fig. 1).
Evolution of the urinary volumetric excretion induced by highest dose of EFPA (80 mg/kg) and Furosemide (5 mg/kg), in rats. The urinary volumetric excretion values are expressed as a percentage of the initial hydric overload (50 mL/kg). Data are given as means ± SEM of 6 different experiments. Statistical analyses were assessed using two-way analysis of variance (ANOVA) followed by Bonferroni’s post- test. *p < 0.001 versus control, and #p < 0.05 versus EFPA
Effect of indomethacin on EFPA-induced diuresis and excretion of electrolytes
After 24 h, EFPA at the dose of 10 mg/kg increased significantly the urinary volumetric excretion (58.1 ± 2.15%; p < 0.05; Fig. 2), compared with that of the control group (16.8 ± 2.29%; p < 0.05; Fig. 2). In presence of indomethacin, the urinary volumetric excretion of EFPA was significantly reduced, amounted to 33.1 ± 3.5% (p < 0.05; Fig. 2), however, it remained greater than that of the control group (p < 0.05; Fig. 2).
Inhibitory effect of indomethacin on urinary volumetric excretion induced by EFPA in rats. Rats were treated over 8 h period with a single dose of either vehicle (control group), EFPA (10 mg/kg) or EFPA (10 mg/kg) + indomethacin (5 mg/kg, 1 h pretreatment) administered i.p. Data are given as means ± SEM of 6 different experiments. Statistical analyses were assessed using one-way analysis of variance (ANOVA) followed by Bonferroni’s post- test *p < 0.05 versus control, and #p < 0.05 versus EFPA
After 24 h, EFPA (10 mg/kg) induced a significant urinary excretion of sodium (90.0 ± 4.7 mEq/L) compared to that of the control group (70.50 ± 6.1 mEq/L; Table 2; Additional file 2: Figure S2). In rats pretreated with indomethacin (5 mg/kg), EFPA-induced natriuresis was significantly reduced to about 43.5 ± 4.4 mEq/L (p < 0.05; Table 2; Additional file 2: Figure S2). In addition to sodium excretion, EFPA induced also a significant urinary excretion of Cl− from 81.2 ± 3.1 mEq/L to 97.3 ± 2.9 mEq/L, which however was not affected by indomethacin (96.7 ± 4.1 mEq/L; Table 2; Additional file 2: Figure S2). EAPA also caused a significant reduction in the urinary excretion of K+ compared with that of the control group (52.2 ± 2.0 mEq/L versus 36.7 ± 1.6 mEq/L, respectively in the control and EFPA group), but in the presence of indomethacin, excretion of K+ was increased (61.0 ± 2.4 mEq/L; Table 2; Additional file 2: Figure S2). The EFPA treatment did not significantly affect calciury in rats (1.8 ± 0.1 versus 1.7 ± 0.1 mEq/L in the control and EFPA group, respectively), also in presence of indomethacin (3.1 ± 0.2 mEq/L; Table 2; Additional file 2: Figure S2).
Effect of EFPA on plasma electrolyte levels
Control group and rats treated with EFPA (10 mg/kg for 24 h) had similar natremia (114.7 ± 5.5 and 125.5 ± 4.0 mEq/L, respectively; Table 3; Additional file 3: Figure S3), this effect was not affected in presence of indomethacin (119.3 ± 4.4 mEq/L; Table 3; Additional file 3: Figure S3). The plasma chlorine level observed in rats following the EFPA treatment was 102.0 ± 4.3 mEq/L, similar to that observed in the control group (104.3 ± 3.9 mEq/L), and not affected by indomethacin (103.3 ± 3.4 mEq/L; Table 3; Additional file 3: Figure S3). The potassium level was significantly reduced after EFPA treatment (70.5 ± 2.9 versus 54.7 ± 2.8 mEq/L; respectively in the control and EFPA group), this effect was not affected by indomethacin (Table 3; Additional file 3: Figure S3). The plasma calcium level following EFPA treatment was similar to that of the control group (5.2 ± 2.6 mEq/L and 5.4 ± 2.5 mEq/L, respectively) and this response was not affected by indomethacin (5.1 ± 2.5 mEq/L; Table 3; Additional file 3: Figure S3).
Effect of EFPA on renal function
After 24 h, the EFPA treatment (10 mg/kg for 24 h) affected slightly but not significantly the plasma concentration of urea and creatinine in rats, both responses where significantly increased in presence of indomethacin (Table 4).
Discussion
The present findings indicate that an ethanolic fraction of Phyllanthus amarus (EFPA) at doses ranging from 5 to 80 mg/kg, significantly induced diuresis during an 8 h period. The profile of urinary excretion showed that after 2 h, at a dose of 80 mg/kg, EFPA induced approximately the same UVE than that of 5 mg/kg of Furosemide. However, after 8 h, the urinary excretion of Furosemide was greater (82%) than that induced by the EFPA (63%). Thus, these observations indicate that the diuretic effect of EFPA is relatively strong and sustained [25], as indicated by about 58% diuretic effect of EFPA at 10 mg/kg after 24 h.
The effect of diuretic substances is commonly associated with the urinary excretion of electrolytes. The present findings indicate that EFPA induced important urinary excretion of sodium (43 mEq) in consistent with previous observations showing that a preparation of the whole plant of Phyllanthus amarus caused significant urinary sodium excretion in hypertensive humans [12]. Furthermore, the EFPA-induced natriuresis was associated with urinary excretion of chlorine (97 mEq) without promoting excretion of potassium. In addition, a leaf extract of Ficus exasperata (50 mg/kg, per os) also significantly decreased the plasma sodium level without promoting kaliuresis in rats [26].
Preliminary phytochemical analysis of EFPA revealed the presence of alkaloids, polyphenols, terpenes and sterols [14]. The diuretic effect of EFPA could be most likely related to these secondary active metabolites. Indeed, polyphenolic compounds, triterpenoids and saponins have been described to contribute to the diuretic effect of several plant extracts including Musanga cecropioides, Viscum angulatum, Hibiscus sabdariffa and Nigella sativa [27,28,29,30].
Furosemide, a highly effective loop diuretic induced considerable loss of water associated with an urinary excretion of sodium, chlorine and potassium. However, previous reports have also indicated that Furosemide did not affect significantly the efflux of potassium in normal rats [31, 32]. Thus, the EFPA treatment seems to induce a similar diuretic effect as that of Furosemide, without a loss of potassium. The main mechanism associated with loop diuretics is linked to inhibition of the co-transport system Na+/K+/2Cl− of the ascending limb of the loop of Henle [33]. Thus, EFPA could possibly act upon this part of the nephron to exert an inhibition of sodium chloride reabsorption and, hence, inducing a significant urinary elimination of water. Moreover, the diuretic effect of EFPA could also be related to other mechanisms such as affecting prostaglandin E2 (PGE2) formation, which regulates kidney function in the distal nephron by stimulating excretion of sodium via inhibition of vasopressin-stimulated water and sodium absorption in the collecting duct [34, 35]. In addition, PGE2 has been shown to play a key role in reducing vascular tone, and, hence, increasing renal blood flow leading to an increase of water exchange and transmembrane electrolyte, and an increased glomerular filtration rate [36, 37]. PGE2 is synthesized by the cycloxygenase COX-1 and COX-2 located in the cortical zone of the renal tissue. Previous studies have indicated that inhibition of COX-1 and COX-2 with indomethacin resulted in retention of sodium and water associated with hypertension and edema in rats [34]. The present study indicates that treatment of rats with indomethacin significantly reduced the natriuretic and the diuretic responses of EFPA. These findings suggest that the diuretic properties of Phyllanthus amarus can be partially attributed, at least in part, to the involvement of diuretic prostaglandins. A similar mechanism has also been involved in the diuretic effect of several extracts of plant species used in traditional medicine such as Selaginella nothohybrida, Selaginella lepidophylla and Tropaeolum majus [31, 38].
The potassium-sparing effect of EFPA supposes also an action on the distal convoluted tubule and/or cortical collecting tube possibly by inhibiting the mineralocorticoid receptor, or with the exchanger Na+ / K+ by blocking sodium channels at the luminal membrane, respectively [25]. The present findings also indicate that Phyllanthus amarus could stimulate kidney function without causing an adverse effect on blood urea and creatinine [39].
Conclusion
The present findings indicate diuretic properties of an ethanolic fraction of Phyllanthus amarus aqueous extract associated with significant urinary excretion of Na+ and water, which involves diuretic prostaglandins, supporting its use in traditional medicine as a potential remedy against hypertension. The diuretic activity of the Phyllanthus amarus extract might contribute to explain the hypotensive action shown previously in normal rabbits [14]. Further studies are needed to evaluate the effectiveness of EFPA in experimental models of hypertension, and to identify the bioactive compounds responsible for the diuretic properties.
Abbreviations
- %:
-
Percentage
- Cl- :
-
Chlorine
- EFPA:
-
Ethanolic fraction of Phyllanthus amarus
- g:
-
Gram
- h:
-
Hours
- i.p:
-
Intraperitoneal
- K+ :
-
Potassium
- L:
-
Liter
- mEq:
-
Milliequivalents
- Na+ :
-
Sodium
- SEM:
-
Standard error mean
- UVE:
-
Urinary volumetric excretion
References
WHO. WHO traditional medicine strategy: 2002–2005 (2002). http://www.wpro.who.int. Accessed 15 Fev 2016.
Liwa CA, Smart LR, Frumkin A, Epstein HAB, Fitzgerald DW, Peck RN. Traditional herbal medicine use among hypertensive patients in sub-Saharan Africa: a systematic review. Curr Hypertens Rep. 2014;16:437–9.
N’guessan K, Tiébré MS, Aké-Assi E, Zirihi GN. Ethnobotanical study of plants used to treat arterial hypertension, in traditional medicine, by Abbey and Krobou populations of Agboville (Côte d’Ivoire). Eur J Sci Res. 2009;35:85–98.
Kuttan R, Harikumar KB. Phyllanthus species: scientific evaluation and medicinal applications. 1st ed. Boca Raton: CRC Press; 2012. p. 149–267.
Patel JR, Tripathi P, Sharma V, Chauhan NS, Dixit VK. Phyllanthus amarus: Ethnomedicinal uses, phytochemistry and pharmacology: a review. J Ethnopharmacol. 2011;138:286–313.
Keluskar P, Ingle S. Ethnopharmacology guided screening of traditional Indian herbs for selective inhibition of Plasmodium specific lactate dehydrogenase. J Ethnopharmacol. 2012;144:201–7.
Upadhyay B, Parveen AK, Dhaker AK, Kumar A. Ethnomedicinal and ethnopharmaco-statistical studies of eastern Rajasthan, India. J Ethnopharmacol. 2010;129:64–86.
Adeneye AA. The leaf and seed aqueous extract of Phyllanthus amarus improves insulin resistance diabetes in experimental animal studies. J Ethnopharmacol. 2012;144:705–11.
Faremi TY, Suru SM, Fafunso MA, Obioha UE. Hepatoprotective potentials of Phyllanthus amarus against ethanol-induced oxidative stress in rats. Food Chem Toxicol. 2008;46:2658–64.
Naaz F, Javed S, Abdin MZ. Hepatoprotective effect of ethanolic extract of Phyllanthus amarus Schum. Et Thonn. On aflatoxin B1 induced liver damage in mice. J Ethnopharmacol. 2007;113:503–9.
Adeneye AA, Amole OO, Adeneye AK. Hypoglycemic and hypocholesterolemic activities of the aqueous leaf and seed extract of Phyllanthus amarus in mice. Fitoterapia. 2006;77:511–4.
Srividya N, Periwal S. Diuretic, hypotensive and hypoglycemia effect of Phyllanthus amarus. Indian J Exp Biol. 1995;33:861–4.
Amaechina FC, Omogbai EK. Hypotensive effect of aqueous extract of the leaves of Phyllanthus amarus Schum and Thonn (Euphorbiaceae). Acta Pol Pharm. 2007;64:547–52.
Amonkan AK, Kamagaté M, Yao ANR, Konan AB, Kouamé MN, Koffi C, Kati-Coulibaly S, Die-Kakou H. Comparative effects of two fractions of Phyllanthus amarus (Euphorbiaceae) on the blood pressure in rabbit. Greener. J Med Sci. 2013;3:129–34.
Inchoo M, Chirdchupunseree H, Pramyothin P, Jianmongkol S. Endothelium-independent effects of phyllanthin and hypophyllanthin on vascular tension. Fitoterapia. 2011;82:1231–6.
Leeya Y, Mulvany MJ, Queiroz EF, Marston A, Hostettmann K, Jansakul C. Hypotensive activity of an n-butanol extract and their purified compounds from leaves of Phyllanthus acidus (L.) Skeels in rats. Eur J Pharmacol. 2010;649:301–13.
Chongsa W, Kanokwiroon K, Jansakul C. Effects of 6 weeks oral administration of Phyllanthus acidus leaf water extract on the vascular functions of middle-aged male rats. J Ethnopharmacol. 2015;176:79–89.
Ntchapda F, Bonabe C, Kemeta Azambou DR, Talla E, Dimo T. Diuretic and antioxidant activities of the aqueous extract of leaves of Vepris heterophylla (Engl.) R. Let (Rutaceae) in rats. BMC Complement Altern Med. 2016;16:516.
Wright CI, Van-Buren L, Kroner CI, Koning MM. Herbal medicines as diuretics: a review of the scientific evidence. J Ethnopharmacol. 2007;114:1–31.
Yao NG, Kamagaté M, Amonkan AK, Koffi C, Kpahe F, Kouamé MN, Dié-Kacou H. Comparative effects of aqueuos extract of Phyllanthus amarus and its fractions on urinary excretion in rat. J Phytopharmacol. 2016;5:182–4.
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412.
McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–6.
Kau ST, Keddi JR, Andrews D. A method for screening diuretic agents in the rat. J Pharmacol Methods. 1984;11:67–75.
Coulibaly FA, Djyh BN, Doumbia I, Yapi HF, Djaman AJ. Évaluation des marqueurs sériques enzymatiques du cœur chez les lapins traités par Phyllanthus amarus (Euphorbiacées). Phythothérapie. 2010;8:348–52.
Ernst ME, Gordon JA. Diuretic therapy: key aspects in hypertension and renal disease. J Nephrol. 2010;23:487–93.
Amonkan AK, Konan AB, Ahui BML, Bleyéré MN, Kouakou LK, Bouaffou GMK. Diuretic effects of extract of Ficus exasperata Vahl. Leaves in rat. Pak J Biol Sci. 2013;16:1383–7.
Dongmo AB, Kamanyi A, Boplet M. Saponins from the leaves of Musanga cecropioides (Cecropiaceae) constitute a possible source of potent hypotensive principles. Phytother Res. 1996;10:23–7.
Jadhav RB, Bhatnagar SP, Surana SJ. Diuretic activity of squamate mistletoe, Viscum angulatum. Pharm Biol. 2010;48:417–21.
Jiménez-Ferrer E, Alarcón-Alonso J, Aguilar-Rojas A, Zamilpa A, Jiménez-Ferrer CI, Tortoriello J, Herrera-Ruiz M. Diuretic effect of compounds from Hibiscus sabdariffa by modulation of the aldosterone activity. Planta Med. 2012;78:1893–8.
Toma C, Olah N, Vlase L, Mogoșan C, Mocan A. Comparative studies on polyphenolic composition, antioxidant and diuretic effects of Nigella sativa L. (black cumin) and Nigella damascena L. (lady-in-a-mist) seeds. Molecules. 2015;20:9560–74.
Gasparotto A Jr, Boffo MA, Lourenço EL, Stefanello ME, Kassuya CA, Marques MC. Natriuretic and diuretic effects of Tropaeolum majus (Tropaeolaceae) in rats. J Ethnopharmacol. 2009;122:517–22.
Lahlou S, Tahraoui A, Israili Z, Lyoussi B. Diuretic activity of the aqueous extracts of Carum carvi and Tanacetum vulgare in normal rats. J Ethnopharmacol. 2006;110:458–63.
Jaggi AS, Kaur A, Bali A, Singh N. Expanding spectrum of sodium potassium chloride co-transporters in the pathophysiology of diseases. Curr Neuropharmacol. 2015;13:369–88.
Breyer MD, Breyer RM. Prostaglandin E receptors and the kidney. Am J Physiol Renal Physiol. 2000;279:F12–23.
Madeddu P, Emanueli C, El-Dahr S. Mechanisms of disease: the tissue kallikrein-kinin system in hypertension and vascular remodeling. Nat Clin Pract Nephrol. 2007;3:208–21.
Lamas S, Rodríguez-Puyol D. Endothelial control of vasomotor tone: the kidney perspective. Semin Nephrol. 2012;32:156–66.
Sato M, Nakayama T, Soma M, Aoi N, Kosuge K, Haketa A, Izumi Y, Matsumoto K, Sato N, Kokubun S. Association between prostaglandin E2 receptor gene and essential hypertension. Prostaglandins Leukot Essent Fatty Acids. 2007;77:15–20.
Aguilar MI, Benítez WV, Colín A, Bye R, Ríos-Gómez R, Calzada F. Evaluation of the diuretic activity in two Mexican medicinal species: Selaginella nothohybrida and Selaginella lepidophylla and its effects with ciclooxigenases inhibitors. J Ethnopharmacol. 2015;163:167–72.
Lawson-Evi P, Eklu-Gadegbeku K, Agbonon A, Moukha KS, Creppy EE, Gbésassor G. Toxicological assessment on extracts of Phyllanthus amarus Schum and Thonn. Sci Res Essays. 2008;3:410–5.
Acknowledgements
Authors are thankful to the authorities of Ecole Normale Supérieure (Abidjan) and those of UFR-Biosciences (Félix Houphouët-Boigny University, Abidjan, Côte d’Ivoire) for providing support to the work. We are also grateful to Professor Ake-Assi Laurent team at the Centre National de Floristique (UFR-Biosciences, Félix Houphouët-Boigny University, Abidjan, Côte d’Ivoire).
Funding
This study was supported by a grant of the Service de Coopération et d’Action Culturelle of French Embassy of Côte d’Ivoire (Campus France N° 767294B and 796998H) and by a scholarship from the Ministère de l’Enseignement Supérieur et de la Recherche Scientifique of Côte d’Ivoire (Décision N° 436/MERS/DBE/SD-BHCI du 11/09/2013).
Availability of data and materials
All data generated or analysed during this study are included in this published article. The raw used and/or analysed during the current study can be available from the corresponding author on reasonable request. Plant material used in this study is registered under No. 3, 141 and 248 at the Centre National de Floristique (CNF) of Félix Houphouët-Boigny University, Abidjan, Côte d’Ivoire.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: AKA, MK, ANY, SKC and HDK. Perfomed experiments: ANY, FK, MNK and KC. supervised the study: SKC and HDK. Analyzed the data: AKA, MK, ANY, KC, CA, VSK and HDK. Contributed reagents/materials/analysis tool: AKA and PC. Wrote the first draft of paper and contributed to manuscript preparation: ANY, MK, PC, CA, VSK, SKC and HDK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Animal experiments were performed with the authorization of Scientific Council of UFR-Biosciences of Félix Houphouët-Boigny University (2013/14), Abidjan, Côte d’Ivoire. Study was carried out following the Guidelines for experiments involving animals: the ARRIVE guidelines (published by Kilkenny et al., 2010 and McGrath et al., 2010).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Figure S1. Urinary volumetric excretion induced by increasing doses of EFPA in rats. The urinary volumetric excretion values are expressed as a percentage of the initial hydric overload (50 mL/kg). Data are given as means ± SEM of 6 different experiments. Statistical analyses were assessed using two-way analysis of variance (ANOVA) followed by Bonferroni’s post- test. *p ˂ 0.001 versus control. (TIFF 216 kb)
Additional file 2:
Figure S2. Inhibitory effect of indomethacin on urinary electrolyte excretion induced by EFPA in rats. Rats were treated over a 24 h period with a single dose of vehicle (control group), EFPA (10 mg/kg) or EFPA (10 mg/kg) + indomethacin (5 mg/kg, 1 h pretreatment) administered i.p. Data are given as means ± SEM of 6 different experiments. Statistical analyses were assessed using two-way analysis of variance (ANOVA) followed by Bonferroni’s post- test. *p < 0.05 versus control, and #p < 0.05 versus EFPA. (TIFF 140 kb)
Additional file 3:
Figure S3. Inhibitory effect of indomethacin on plasma electrolyte level induced by EFPA in rats. Rats were treated over a 24 h period with a single dose of either vehicle (control group), EFPA (10 mg/kg) or EFPA (10 mg/kg) + indomethacin (5 mg/kg, 1 h pretreatment) administered i.p. Data are given as means ± SEM of 6 different experiments. Statistical analyses were assessed using two-way analysis of variance (ANOVA) followed by Bonferroni’s post- test. *p < 0.05 versus control, and #p < 0.05 versus EFPA. (TIFF 174 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Yao, A.N., Kamagaté, M., Amonkan, A.K. et al. The acute diuretic effect of an ethanolic fraction of Phyllanthus amarus (Euphorbiaceae) in rats involves prostaglandins. BMC Complement Altern Med 18, 94 (2018). https://doi.org/10.1186/s12906-018-2158-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-018-2158-0