Abstract
Background
GS-E3D is a newly developed pectin lyase-modified red ginseng extract. The purpose of this study was to investigate the therapeutic effects of GS-E3D on diabetes-related renal dysfunction in streptozotocin-induced diabetic rats.
Method
GS-E3D (25, 50, and 100 mg/kg body weight per day) was administered for 6 weeks. The levels of blood glucose and hemoglobin A1c, and of urinary albumin, 8-hydroxy-2′-deoxyguanosine (8-OHdG), and advanced glycation end-products (AGEs) were determined. Kidney histopathology, renal accumulation of AGEs, and expression of α-smooth muscle actin (α-SMA) were also examined.
Results
Administration of GS-E3D for 6 weeks reduced urinary levels of albumin, 8-OHdG, and AGEs in diabetic rats. Mesangial expansion, renal accumulation of AGEs, and enhanced α-SMA expression were significantly inhibited by GS-E3D treatment. Oral administration of GS-E3D dose-dependently improved all symptoms of diabetic nephropathy by inhibiting renal accumulation of AGEs and oxidative stress.
Conclusion
The results of this study indicate that the use of GS-E3D as a food supplement may provide effective treatment of diabetes-induced renal dysfunction.
Similar content being viewed by others
Background
Diabetic nephropathy is one of the most significant chronic complications of diabetes [1]. Hyperglycemia may enhance oxidative stress and inflammation in the renal tissues and lead to the development of kidney failure [2]. The current treatment strategy for patients with diabetic nephropathy is to prevent or delay disease progression by strict control of blood glucose [3]. Although various glucose-lowering drugs are clinically available, the prevalence of diabetic nephropathy has increased worldwide [4].
Reactive oxygen species (ROS) and advanced glycation end-products (AGEs) play major roles in the development and progression of diabetic kidney disease [5,6,7,8,9]. AGEs are heterogeneous sugar-derived irreversible protein modifications have been implicated in the pathogenesis of diabetes and other age-related diseases [6]. The formation of AGEs is accelerated under hyperglycemic conditions, as well as by oxidative stress. Oxidative stress, which is fueled by excessive ROS production during glucose autoxidation, drives the nonenzymatic, covalent attachment of glucose molecules to circulating proteins, thus forming AGEs [10]. Moreover, the receptor for AGEs (RAGE) has also been implicated in mediating AGE-induced renal damage [11]. The interaction between AGEs and RAGE can trigger ROS production and signaling pathways, leading to cell injury [12]. Oxidative stress arising from the interaction between AGEs and RAGE leads to apoptosis of renal glomerular cells [13] and podocytes [14].
AGE inhibitors such as aminoguanidine and LR-90 attenuate mesangial expansion and proteinuria in animal models of diabetes [15,16,17]. However, aminoguanidine has not been used clinically to treat diabetic nephropathy because of its adverse effects, which include pro-oxidant activities [18] and inhibition of nitric oxide synthase [19]. Some natural and synthetic compounds have been proposed to act as AGE inhibitors [20]. Generally, botanical products are often perceived to be safer than synthetic compounds. Therefore, interest in the use of herbal products has been increasing.
Panax ginseng is one of the most popular health foods and it has been used to increase vitality for centuries in Asian countries. Red ginseng, a product derived from P. ginseng, is manufactured by repetitive steaming and drying cycles [21]. This manufacturing process promotes the formation of additional beneficial compounds, known as ginsenosides. Red ginseng has shown potent pharmacological effects on the immune response, and on metabolic disease and cancer [22,23,24,25,26]. In addition, red ginseng has its long history of ethnopharmacological evidence for anti-diabetic use. Several investigations have revealed that red ginseng exerted anti-diabetic function in diabetic animals [27, 28] and diabetic patients [29]. Hong et al. showed that red ginseng lowered blood glucose levels and protected pancreatic ß-cell degeneration in streptozotocin (STZ)-induced diabetic mice [30]. Recently, our group showed that red ginseng decreased oxidative stress, AGEs accumulation and renal injury in D-galactose-induced aging rats [31]. More recently, Quan et al. showed that oral administration of red ginseng had a beneficial effect on AGEs-mediated renal injury in STZ-induced diabetic rats [32]. These previous in vitro and in vivo data suggest that red ginseng may exerts a number of beneficial activities, including anti-glycation, anti-oxidative and renoprotective effects under diabetic conditions.
Recently, some transformation methods have been applied to red ginseng, including enzymatic conversion [33] and fermentation [34]. These biotransformation processes increased the pharmacological potency of red ginseng in several animal models of disease [35,36,37,38]. Wang et al. reported that ginsenosides were metabolized by microorganisms in a biotransformation product of red ginseng, which improved their intestinal absorption, increased bioactivity, and diminished the toxicity of the metabolite [39]. Indeed, Kim et al. reported that a fermented red ginseng extract by Lactobacillus fermentum had a more potent anti-oxidative activities than normal red ginseng in vitro and increased anti-oxidant enzyme activities in STZ-induced diabetic rats [40]. GS-E3D is a newly developed pectin lyase-modified red ginseng extract. This product showed anti-obesity activity in a mouse model [41] and had anti-inflammatory effects on macrophage cells in vitro [42]. To the best of our knowledge, no reports have described the therapeutic effects of GS-E3D on diabetes-related renal dysfunction. To address this, we studied the effect of GS-E3D on diabetes-induced renal dysfunction in a streptozotocin-induced diabetic rat model. In addition, we tested the hypothesis that GS-E3D would provide effective inhibition of renal AGE accumulation in this animal model.
Methods
GS-E3D preparation
Four-year-old dried P. ginseng was purchased from a local market (Wooshin Industrial Co., Ltd., Geumsan, Korea). The botanical identification was made by botanist Dr. M. K. Pyo (International Ginseng and Herb Research Institute, Korea). Voucher specimen (No. GS201104) is deposited in the herbarium of the International Ginseng and Herb Research Institute (Kumsan, Korea). GS-E3D was prepared according to our previous report [41]. Briefly, red ginseng extract, adjusted to 6 Brix, was incubated with 10% pectin lyase (EC 4.2.2.10; Novozyme, #33095, Denmark) at 50 °C for 5 d in a shaking incubator (150 rpm). To terminate the reaction, the processed extract was incubated at 95 °C for 10 min and then freeze-dried for storage prior to the subsequent experiments. The dried GS-E3D consisted of 120.2 mg/g crude saponin, which contained the following ginsenosides: 5.9 mg/g Rg1, 12.6 mg/g Re, 4.7 mg/g Rf, 30.2 mg/g Rb1, 14.0 mg/g Rc, 17.6 mg/gRb2, 2.5 mg/g Rb3, 27.7 mg/g Rd., 1.3 mg/g 20(S)-Rg3, 1.4 mg/g 20(R)-Rg3, 0.8 mg/g Rk1, and 1.5 mg/g Rg5.
Animals and experimental design
Seven-week-old male Sprague-Dawley with body weights of 200 ~ 225 g were purchased from Orient Bio (Seongnam, Korea) and acclimated for 1 week prior to the induction of diabetes by a single intraperitoneal injection of 60 mg/kg streptozotocin (STZ). Age-matched control rats received an equal volume of vehicle (0.01 M citrate buffer, pH 4.5). Rats were individually housed in plastic cages and maintained at 24 °C ± 2 °C with a 12 h light:dark cycle and received a standard pellet diet (Ralston Purina, St. Louis, MO, USA) and tap water ad libitum. One week after these injections, blood samples were obtained from the tail vein. Rats with a blood glucose level greater than 300 mg/dL were considered to be diabetic. The rats were then divided into 5 groups of 10 rats, as follows: (1) normal control rats (NOR); (2) STZ-induced diabetic rats (diabetes mellitus; DM); and (3, 4, and 5) STZ-induced diabetic rats treated with GS-E3D (25, 50, or 100 mg/kg body weight, respectively). GS-E3D was dissolved in distilled water and orally administered for 6 weeks, and the other groups were given the same amount of vehicle gavage for 6 weeks.
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Korea Institute of Oriental Medicine (Approval No. 15–100, Daejeon, Korea)
Blood glucose and hemoglobin A1c (HbA1c) levels
Blood samples were collected from the tail vein after a 16-h fast. Blood glucose and HbA1c levels were measured using an automated analyzer (Wako, Tokyo, Japan).
Quantification of urinary albumin, 8-Hydroxy-2′-Deoxyguanosine (8-OHdG), and advanced Glycation end-products (AGEs)
Individual rats were placed in metabolic cages for 24-h urine collection. Daily urinary albumin, 8-OHdG, and AGE levels were measured using a rat albumin enzyme-linked immunosorbent assay (ELISA) kit (Abcam, Cambridge, UK), an 8-OHdG Check ELISA kit (Cosmo Bio, Tokyo, Japan), and a rat AGEs ELISA kit (Cusabio, Wuhan, China), respectively.
Histopathology
Renal tissues were fixed in 10% neutralized formaldehyde and embedded in paraffin prior to preparing 4-μm sections. The sections were stained with periodic acid-Schiff reagent (Sigma, St. Louis, MO, USA) and counterstained with hematoxylin. A total of 20 glomeruli were randomly selected from each rat and the glomerular tuft and mesangial matrix areas were measured using Image J software (National Institutes of Health, Bethesda, MD, USA).
Immunohistochemical staining
Deparaffinized sections were hydrated and treated with 1% H2O2 in methanol prior to incubation with antibodies raised against either AGEs (1:200; Transgenic Inc., Kobe, Japan) or α-smooth muscle actin (α-SMA) (1:250; Santa Cruz Biotechnology, Paso Robles, CA, USA) for 1 h at room temperature. Signal detection was achieved using the Envision kit (DAKO, Carpinteria, CA, USA) and visualized by 3,3′-diaminobenzidine tetrahydrochloride. As a negative control, tissue sections were incubated with serum from non-immunized animals, instead of the primary antibody. The immunohistochemical signal intensity was analyzed in 20 randomly selected glomeruli from each rat using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Group data were analyzed by one-way analysis of variance followed by Tukey’s multiple comparison test or by unpaired Student’s t-test, using GraphPad Prism 6.0 software (GraphPad, San Diego, CA, USA). Differences with a p value of <0.05 were considered statistically significant.
Results
Blood glucose control
Blood glucose levels are summarized in Table 1. The blood glucose and HbA1c levels were significantly increased in diabetic rats (p < 0.05). No differences in blood glucose or HbA1c levels were noted between GS-E3D-treated and vehicle-treated diabetic rats.
Renal histopathology and Albuminuria
Eight weeks after the induction of diabetes, DM rats showed focal mesangial matrix expansion and tubulointerstitial damage (Fig. 1a). A significant enlargement of the glomeruli was observed in DM rats, as compared with the NOR group. In the GS-E3D-treated diabetic rats, glomerular size was dose-dependently smaller than that observed in the vehicle-treated diabetic rats (Fig. 1b). In addition, urinary albumin levels were significantly higher in the DM rats than in the NOR rats (p < 0.05), and these were dose-dependently decreased by the administration of GS-E3D (Fig. 1c).
Renal histopathology and albuminuria. (a) Periodic acid-Schiff staining of glomeruli. Scale bar equals 50 μm. (b) Glomerular volume and (c) albuminuria in the indicated study groups. NOR, normal rat; DM, streptozotocin-induced diabetic rat; GS-E3D, DM treated with the indicated dose of GS-E3D. All data were expressed as mean ± the standard error of the mean (n = 8); *p < 0.05 vs. NOR group; #p < 0.05 vs. DM group
Oxidative status and renal tissue accumulation of AGEs
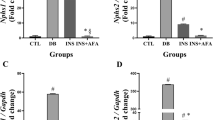
The formation of AGEs is accelerated in diseases associated with increased oxidative stress [43]. AGEs exert harmful biological effects by activating RAGE [44]. Oxidative stress induced by interactions between AGEs and RAGE is involved in renal injury [13]. Thus, we performed urinary ELISAs for 8-OHdG in order to evaluate the damage caused by oxidative stress. The mean urinary 8-OHdG level in DM rats was significantly higher than that in NOR rats (Fig. 2a). This increase was dose-dependently inhibited in diabetic rats that were treated with GS-E3D. In addition, to determine renal accumulation of AGEs in diabetic rats, urinary AGE levels were determined. At the end of the study, these levels were significantly higher in DM rats than in NOR rats (Fig. 2b). Treatment with GS-E3D dose-dependently decreased urinary excretion of AGEs. Immunohistochemical staining for AGEs clearly showed accumulation in renal tissues of DM rats. Significantly higher levels of AGEs were present in these animals than in NOR rats (Figs. 3a and b). However, renal accumulation of AGEs was dose-dependently reduced in diabetic rats that were treated with GS-E3D.
Urinary excretion of 8-hydroxy-2′-deoxyguanosine (8-OHdG) and advanced glycation end-products (AGEs). The urine levels of 8-OHdG (a) and AGEs (b) were determined by enzyme-linked immunosorbent assays. NOR, normal rat; DM, streptozotocin-induced diabetic rat; GS-E3D, DM treated with the indicated dose of GS-E3D. All data were expressed as mean ± the standard error of the mean (n = 8); *p < 0.05 vs. NOR group; #p < 0.05 vs. DM group
The effect of GS-E3D on renal accumulation of advanced glycation end-products (AGEs). (a) Immunohistochemical staining for AGEs. Scale bar equals 50 μm. (b) Quantification of the AGE signal intensity. NOR, normal rat; DM, streptozotocin-induced diabetic rat; GS-E3D, DM treated with the indicated dose of GS-E3D. All data were expressed as mean ± the standard error of the mean (n = 8); *p < 0.05 vs. NOR group; #p < 0.05 vs. DM group
GS-E3D inhibits Mesangial cell proliferation
Diabetic nephropathy is characterized by glomerular hypertrophy, caused by the proliferation of mesangial cells [45]. α-SMA is a marker of mesangial cell proliferation [46]. To determine whether GS-E3D prevented mesangial proliferation, we performed immunostaining for α-SMA. Immunohistochemical staining of α-SMA in glomeruli demonstrated a significant increase in DM rats, as compared with the NOR group. This increase was dose-dependently inhibited in rats treated with GS-E3D (Figs. 4a and b).
The effect of GS-E3D on α-smooth muscle actin (α-SMA) expression in glomeruli. (a) Immunohistochemical staining for α-SMA. Scale bar equals 50 μm. (b) Quantification of the α-SMA signal intensity. NOR, normal rat; DM, streptozotocin-induced diabetic rat; GS-E3D, DM treated with the indicated dose of GS-E3D. All data were expressed as mean ± the standard error of the mean (n = 8); *p < 0.05 vs. NOR group; #p < 0.05 vs. DM group
Discussion
GS-E3D is commercial pectin lyase-modified red ginseng extract with an enhanced level of the ginsenoside Rd. The results of this study showed that the administration of GS-E3D might ameliorate diabetic nephropathy in a rat STZ-induced model of diabetes. GS-E3D-treated diabetic rats showed significant improvements in markers of renal function, such as urinary albumin levels. In addition, GS-E3D reduced urinary 8-OHdG excretion and AGE accumulation in renal tissues, as well as inhibiting mesangial proliferation.
Hyperglycemia is present in STZ-induced diabetic rats, and diabetic nephropathy and mesangial expansion progress rapidly in this model [47]. Mesangial cells are smooth muscle-like contractile cells that are interspersed with the glomerular capillaries. Mesangial expansion by means of proliferation, hypertrophy, and matrix deposition is an early characteristic sign of diabetic nephropathy [48, 49]. Microalbuminuria is often the first clinical sign of renal dysfunction in patients with DM. Although albuminuria may partially result from defective reabsorption of proteins in the proximal tubule [50], it is associated with mesangial expansion in the majority of patients with DM [51]. Based on these findings, we hypothesized that GS-E3D might ameliorate mesangial expansion and thus reduce albuminuria. This protective effect of GS-E3D was partly attributed to its antioxidant and anti-AGE activities.
Hyperglycemia is an important causal factor that underlies the development of diabetic nephropathy. Recently, a type 1 diabetes mellitus (T1DM) animal model has often been used to study the mechanisms involved in diabetic complications behind the actions of anti-diabetic drugs independent of their glucose-lowering effects. For example, metformin and dipeptidyl peptidase IV (DPP4) inhibitors, such as vildagliptin and alogliptin, which is a well-known anti-diabetic drug for type 2 diabetes mellitus (T2DM), had no effect on blood glucose in the T1DM animal model [52,53,54]. Therefore, the purpose of this study was also to evaluate the effect of GS-E3D on diabetic nephropathy in a model without a blood glucose reduction. In our study, GS-E3D appeared protective effect on the diabetes-induced renal dysfunction independent of glycemic control. Additionally, Hong et al. showed that red ginseng lowered blood glucose levels in streptozotocin (STZ)-induced diabetic mice [30]. However, its oral dosage in mouse was 25 mg/mouse (1025 mg/kg; correspondence in a six-week old C57BL/6 mouse body weight of 20 g). In the present study, the highest dosage of GS-E3D (100 mg/kg) is about 10-fold less than that reported by Hong et al. Although, GS-E3D failed to decrease blood glucose in STZ-induced diabetic rat, GS-E3D has significant effects on the parameters of renal structure and function through inhibition of renal AGEs accumulation without the strong reduction of blood glucose.
Although various initiators of diabetic nephropathy have been proposed including glycation, the polyol pathway, and oxidative stress, one of the major consequences of hyperglycemia is the formation of AGEs. The formation of AGEs in renal tissue is closely correlated with the development of diabetic nephropathy [55, 56]. AGEs were previously reported to induce mesangial expansion and proteinuria [57]. The irreversible formation of AGEs damages the renal tissues and blood vessels [58]. Mesangial cells may be sensitive AGE targets because they express RAGE [59]. Consistent with this interpretation, our results identified mesangial expansion in the glomeruli that showed AGE accumulation in STZ-induced diabetic rats with albuminuria; treatment with GS-E3D ameliorated diabetes-associated renal dysfunction and AGE accumulation in this rat model.
ROS play an important role in the pathogenesis of diabetic nephropathy. Inhibition of ROS generation has been demonstrated to be effective in preventing the development and progression of diabetic nephropathy [60]. Moreover, Li et al. reported that antioxidants decreased high-glucose-induced ROS-related mouse mesangial cell dysfunction [61]. Enhanced generation of ROS was also induced by the interaction of AGEs with RAGE [62]. It was previously reported that red ginseng had antioxidant activity [63, 64]. Therefore, the reduction of urinary 8-OHdG levels by GS-E3D treatment may be due to its antioxidant and anti-AGE activities.
Several agents such as aminoguanidine and LR-90 have been proposed as AGE inhibitors, which attenuate mesangial expansion and proteinuria in diabetic animal models [15,16,17]. Our previous studies showed that some natural herbal products derived from Polygonum cuspidatum [65], Cassiae semen [66], or Litsea japonica [67] prevented diabetes-induced renal injury via inhibition of AGE accumulation in experimental animal models of diabetes. P. ginseng has been proven effective for anti-diabetes and anti-oxidation in many model systems [68]. In previous study, Korean red ginseng inhibited cyclosporine-induced renal injury [69]. Ginsenoside Rd attenuated renal dysfunction by preventing oxidative stress in cisplatin or cephaloridine-induced acute renal failure and ischemic-reperfused rats [70,71,72]. Although the efficacy of Korean red ginseng on diabetic nephropathy are poorly understood, Quan et al. reported that Korean red ginseng reduced the formation and secretion of AGEs from the kidneys of diabetic rats [32]. The ginsenoside Rd is one of the bioactive compounds present in red ginseng and this ameliorated astrocyte damage induced by methylglyoxal, an AGE precursor [73]. Yokozawa et al. showed that ginsenoside Rd also inhibited mesangial cell proliferation [74]. Unfortunately, we did not compare the effects of GS-E3D with those of an unmodified red ginseng extract. However, because GS-E3D had an enhanced level of Rd., as compared with an unmodified red ginseng extract [42], GS-E3D may have the more potent preventive effect on diabetes-induced renal dysfunction.
Conclusion
Our data indicate that GS-E3D ameliorated diabetes-induced renal dysfunction in STZ-induced diabetic rats. We also demonstrated that GS-E3D protected these animals from ROS- and AGE-related renal injury. These effects could be attributed, at least in part, to reduced AGE deposition. However, the pharmacological mechanisms underlying the effects of GS-E3D need further investigation.
Abbreviations
- 8-OHdG:
-
8-Hydroxy-2′-deoxyguanosine
- AGEs:
-
Advanced glycation end products
- DM:
-
Diabetes mellitus
- ELISA:
-
Enzyme-linked immunosorbent assay
- NOR:
-
Normal control
- RAGE:
-
Receptor for advanced glycation end products
- ROS:
-
Reactive oxygen species
- STZ:
-
Streptozotocin
- α-SMA:
-
α-smooth muscle actin
References
White KE, Bilous RW. Type 2 diabetic patients with nephropathy show structural-functional relationships that are similar to type 1 disease. J Am Soc Nephrol. 2000;11(9):1667–73.
Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med. 2008;233(1):4–11.
Kang AY, Park SK, Park SY, Lee HJ, Han Y, Lee SR, Suh SH, Kim DK, Park MK. Therapeutic target achievement in type 2 diabetic patients after hyperglycemia, hypertension, dyslipidemia management. Diabetes Metab J. 2011;35(3):264–72.
Satirapoj B, Adler SG. Prevalence and Management of Diabetic Nephropathy in western countries. Kidney Dis. 2015;1(1):61–70.
Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40(4):405–12.
Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–34.
Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57(6):1446–54.
Nistala R, Whaley-Connell A, Sowers JR. Redox control of renal function and hypertension. Antioxid Redox Signal. 2008;10(12):2047–89.
Yan SD, Yan SF, Chen X, Fu J, Chen M, Kuppusamy P, Smith MA, Perry G, Godman GC, Nawroth P, et al. Non-enzymatically glycated tau in Alzheimer's disease induces neuronal oxidant stress resulting in cytokine gene expression and release of amyloid beta-peptide. Nat Med. 1995;1(7):693–9.
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–70.
Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res. 2003;93(12):1159–69.
Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26(8):1702–11.
Yamamoto Y, Yamamoto H. Interaction of receptor for advanced glycation end products with advanced oxidation protein products induces podocyte injury. Kidney Int. 2012;82(7):733–5.
Chuang PY, Yu Q, Fang W, Uribarri J, He JC. Advanced glycation endproducts induce podocyte apoptosis by activation of the FOXO4 transcription factor. Kidney Int. 2007;72(8):965–76.
Ahmed N. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67(1):3–21.
Rahbar S, Figarola JL. Novel inhibitors of advanced glycation endproducts. Arch Biochem Biophys. 2003;419(1):63–79.
Peppa M, Brem H, Cai W, Zhang JG, Basgen J, Li Z, Vlassara H, Uribarri J. Prevention and reversal of diabetic nephropathy in db/db mice treated with alagebrium (ALT-711). Am J Nephrol. 2006;26(5):430–6.
Suji G, Sivakami S. DNA damage by free radical production by aminoguanidine. Ann N Y Acad Sci. 2006;1067:191–9.
Tilton RG, Chang K, Hasan KS, Smith SR, Petrash JM, Misko TP, Moore WM, Currie MG, Corbett JA, McDaniel ML, et al. Prevention of diabetic vascular dysfunction by guanidines. Inhibition of nitric oxide synthase versus advanced glycation end-product formation. Diabetes. 1993;42(2):221–32.
Osawa T, Kato Y. Protective role of antioxidative food factors in oxidative stress caused by hyperglycemia. Ann N Y Acad Sci. 2005;1043:440–51.
Hong SY, Oh JH, Lee I. Simultaneous enrichment of deglycosylated ginsenosides and monacolin K in red ginseng by fermentation with Monascus Pilosus. Biosci Biotechnol Biochem. 2011;75(8):1490–5.
Kim P, Park JH, Kwon KJ, Kim KC, Kim HJ, Lee JM, Kim HY, Han SH, Shin CY. Effects of Korean red ginseng extracts on neural tube defects and impairment of social interaction induced by prenatal exposure to valproic acid. Food Chem Toxicol. 2013;51:288–96.
Park HM, Kim SJ, Mun AR, Go HK, Kim GB, Kim SZ, Jang SI, Lee SJ, Kim JS, Kang HS. Korean red ginseng and its primary ginsenosides inhibit ethanol-induced oxidative injury by suppression of the MAPK pathway in TIB-73 cells. J Ethnopharmacol. 2012;141(3):1071–6.
Paul S, Shin HS, Kang SC. Inhibition of inflammations and macrophage activation by ginsenoside-re isolated from Korean ginseng (Panax Ginseng C.A. Meyer). Food Chem Toxicol. 2012;50(5):1354–61.
Wang CZ, Anderson S, Du W, He TC, Yuan CS. Red ginseng and cancer treatment. Chin J Nat Med. 2016;14(1):7–16.
Hosseini A, Ghorbani A. Cancer therapy with phytochemicals: evidence from clinical studies. Avicenna J Phytomedicine. 2015;5(2):84–97.
Kim JH, Hahm DH, Yang DC, Kim JH, Lee HJ, Shim I. Effect of crude saponin of Korean red ginseng on high-fat diet-induced obesity in the rat. J Pharmacol Sci. 2005;97(1):124–31.
Liu TP, Liu IM, Cheng JT. Improvement of insulin resistance by panax ginseng in fructose-rich chow-fed rats. Horm Metab Res. 2005;37(3):146–51.
Vuksan V, Sung MK, Sievenpiper JL, Stavro PM, Jenkins AL, Di Buono M, Lee KS, Leiter LA, Nam KY, Arnason JT, et al. Korean red ginseng (Panax Ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis. 2008;18(1):46–56.
Hong YJ, Kim N, Lee K, Hee Sonn C, Eun Lee J, Tae Kim S, Ho Baeg I, Lee KM. Korean red ginseng (Panax Ginseng) ameliorates type 1 diabetes and restores immune cell compartments. J Ethnopharmacol. 2012;144(2):225–33.
Park S, Kim CS, Min J, Lee SH, Jung YS. A high-fat diet increases oxidative renal injury and protein glycation in D-galactose-induced aging rats and its prevention by Korea red ginseng. J Nutr Sci Vitaminol. 2014;60(3):159–66.
Quan HY, Kim DY, Chung SH. Korean red ginseng extract alleviates advanced glycation end product-mediated renal injury. J Ginseng Res. 2013;37(2):187–93.
Ko SR, Choi KJ, Uchida K, Suzuki Y. Enzymatic preparation of ginsenosides Rg2, Rh1, and F1 from protopanaxatriol-type ginseng saponin mixture. Planta Med. 2003;69(3):285–6.
Park CS, Yoo MH, Noh KH, Oh DK. Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl Microbiol Biotechnol. 2010;87(1):9–19.
Cheon JM, Kim DI, Kim KS. Insulin sensitivity improvement of fermented Korean red ginseng (Panax Ginseng) mediated by insulin resistance hallmarks in old-aged ob/ob mice. J Ginseng Res. 2015;39(4):331–7.
Park SY, Kim HB, Kim JH, Lee JM, Kim SR, Shin HS, Yi TH. Immunostimulatory effect of fermented red ginseng in the mouse model. Prev Nutr Food Sci. 2014;19(1):10–8.
Oh J, Lee SR, Hwang KT, Ji GE. The anti-obesity effects of the dietary combination of fermented red ginseng with levan in high fat diet mouse model. Phytother Res. 2014;28(4):617–22.
Lee EJ, Song MJ, Kwon HS, Ji GE, Sung MK. Oral administration of fermented red ginseng suppressed ovalbumin-induced allergic responses in female BALB/c mice. Phytomedicine. 2012;19(10):896–903.
Wang HY, Qi LW, Wang CZ, Li P. Bioactivity enhancement of herbal supplements by intestinal microbiota focusing on ginsenosides. Am J Chin Med. 2011;39(6):1103–15.
Kim HJ, Lee SG, Chae IG, Kim MJ, Im NK, Yu MH, Lee EJ, Lee IS. Antioxidant effects of fermented red ginseng extracts in streptozotocin- induced diabetic rats. J Ginseng Res. 2011;35(2):129–37.
Lee HY, Park KH, Park YM, Moon DI, Oh HG, Kwon DY, Yang HJ, Kim O, Kim DW, Yoo JH, et al. Effects of pectin lyase-modified red ginseng extracts in high-fat diet-fed obese mice. Lab Anim Res. 2014;30(4):151–60.
Hong SC, Oh MH, Lee H, Park YS, Kim NY, Park SH, Park JD, Jang JD, Kim SH, Kim EJ, et al. Pectinase-modified red ginseng (GS-E3D) inhibit NF-ΚB translocation and nitric oxide production in lipoplysaccharide-stimulated RAW 264.7 cells. Int J Pharm Pharm Sci. 2015;7(9):322–5.
Witko-Sarsat V, Friedlander M, Nguyen Khoa T, Capeillere-Blandin C, Nguyen AT, Canteloup S, Dayer JM, Jungers P, Drueke T, Descamps-Latscha B. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161(5):2524–32.
Guo ZJ, Niu HX, Hou FF, Zhang L, Fu N, Nagai R, Lu X, Chen BH, Shan YX, Tian JW, et al. Advanced oxidation protein products activate vascular endothelial cells via a RAGE-mediated signaling pathway. Antioxid Redox Signal. 2008;10(10):1699–712.
Brosius FC 3rd. New insights into the mechanisms of fibrosis and sclerosis in diabetic nephropathy. Rev Endocr Metab Disord. 2008;9(4):245–54.
Johnson RJ, Iida H, Alpers CE, Majesky MW, Schwartz SM, Pritzi P, Gordon K, Gown AM. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991;87(3):847–58.
Siu B, Saha J, Smoyer WE, Sullivan KA, Brosius FC 3rd. Reduction in podocyte density as a pathologic feature in early diabetic nephropathy in rodents: prevention by lipoic acid treatment. BMC Nephrol. 2006;7:6.
Young BA, Johnson RJ, Alpers CE, Eng E, Gordon K, Floege J, Couser WG, Seidel K. Cellular events in the evolution of experimental diabetic nephropathy. Kidney Int. 1995;47(3):935–44.
Wolf G, Sharma K, Chen Y, Ericksen M, Ziyadeh FN. High glucose-induced proliferation in mesangial cells is reversed by autocrine TGF-beta. Kidney Int. 1992;42(3):647–56.
Tojo A, Onozato ML, Kurihara H, Sakai T, Goto A, Fujita T. Angiotensin II blockade restores albumin reabsorption in the proximal tubules of diabetic rats. Hypertens Res. 2003;26(5):413–9.
Fujii M, Inoguchi T, Maeda Y, Sasaki S, Sawada F, Saito R, Kobayashi K, Sumimoto H, Takayanagi R. Pitavastatin ameliorates albuminuria and renal mesangial expansion by downregulating NOX4 in db/db mice. Kidney Int. 2007;72(4):473–80.
Tan Z, Xu Z, Gui Q, Wu W, Yang Y. Gliquidone versus metformin: differential effects on aorta in streptozotocin induced diabetic rats. Chin Med J. 2014;127(7):1298–303.
Liu WJ, Xie SH, Liu YN, Kim W, Jin HY, Park SK, Shao YM, Park TS. Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats. J Pharmacol Exp Ther. 2012;340(2):248–55.
Davidson EP, Coppey LJ, Dake B, Yorek MA. Treatment of streptozotocin-induced diabetic rats with alogliptin: effect on vascular and neural complications. Exp Diabetes Res. 2011;2011:810469.
Coughlan MT, Mibus AL, Forbes JM. Oxidative stress and advanced glycation in diabetic nephropathy. Ann N Y Acad Sci. 2008;1126:190–3.
Fukami K, Yamagishi S, Ueda S, Okuda S. Role of AGEs in diabetic nephropathy. Curr Pharm Des. 2008;14(10):946–52.
Soler MJ, Riera M, Batlle D. New experimental models of diabetic nephropathy in mice models of type 2 diabetes: efforts to replicate human nephropathy. Exp Diabetes Res. 2012;2012:616313.
Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–25.
Berrou J, Tostivint I, Verrecchia F, Berthier C, Boulanger E, Mauviel A, Marti HP, Wautier MP, Wautier JL, Rondeau E, et al. Advanced glycation end products regulate extracellular matrix protein and protease expression by human glomerular mesangial cells. Int J Mol Med. 2009;23(4):513–20.
Luo ZF, Feng B, Mu J, Qi W, Zeng W, Guo YH, Pang Q, Ye ZL, Liu L, Yuan FH. Effects of 4-phenylbutyric acid on the process and development of diabetic nephropathy induced in rats by streptozotocin: regulation of endoplasmic reticulum stress-oxidative activation. Toxicol Appl Pharmacol. 2010;246(1–2):49–57.
Li H, Wang F, Zhang L, Cao Y, Liu W, Hao J, Liu Q, Duan H. Modulation of Nrf2 expression alters high glucose-induced oxidative stress and antioxidant gene expression in mouse mesangial cells. Cell Signal. 2011;23(10):1625–32.
Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, Sourris KC, Penfold SA, Bach LA, Cooper ME, Forbes JM. Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes. 2008;57(2):460–9.
Hong SH, Suk KT, Choi SH, Lee JW, Sung HT, Kim CH, Kim EJ, Kim MJ, Han SH, Kim MY, et al. Anti-oxidant and natural killer cell activity of Korean red ginseng (Panax Ginseng) and urushiol (Rhus Vernicifera Stokes) on non-alcoholic fatty liver disease of rat. Food Chem Toxicol. 2013;55:586–91.
Yang H, Lee SE, Jeong SI, Park CS, Jin YH, Park YS. Up-regulation of Heme Oxygenase-1 by Korean red ginseng water extract as a Cytoprotective effect in human endothelial cells. J Ginseng Res. 2011;35(3):352–9.
Sohn E, Kim J, Kim CS, Jo K, Kim JS. Extract of Rhizoma Polygonum Cuspidatum reduces early renal podocyte injury in streptozotocininduced diabetic rats and its active compound emodin inhibits methylglyoxalmediated glycation of proteins. Mol Med Rep. 2015;12(4):5837–45.
Jung DH, Kim YS, Kim NH, Lee J, Jang DS, Kim JS. Extract of Cassiae semen and its major compound inhibit S100b-induced TGF-beta1 and fibronectin expression in mouse glomerular mesangial cells. Eur J Pharmacol. 2010;641(1):7–14.
Sohn E, Kim J, Kim CS, Lee YM, Jo K, Shin SD, Kim JH, Kim JS. The extract of Litsea Japonica reduced the development of diabetic nephropathy via the inhibition of advanced Glycation end products accumulation in db/db mice. Evid Based Complement Alternat Med. 2013;2013:769416.
Yin J, Zhang H, Ye J. Traditional chinese medicine in treatment of metabolic syndrome. Endocr Metab Immune Disord Drug Targets. 2008;8(2):99–111.
Doh KC, Lim SW, Piao SG, Jin L, Heo SB, Zheng YF, Bae SK, Hwang GH, Min KI, Chung BH, et al. Ginseng treatment attenuates chronic cyclosporine nephropathy via reducing oxidative stress in an experimental mouse model. Am J Nephrol. 2013;37(5):421–33.
Yokozawa T, Liu ZW, Dong E. A study of ginsenoside-rd in a renal ischemia-reperfusion model. Nephron. 1998;78(2):201–6.
Yokozawa T, Owada S. Effect of ginsenoside-rd in cephaloridine-induced renal disorder. Nephron. 1999;81(2):200–7.
Yokozawa T, Liu ZW. The role of ginsenoside-rd in cisplatin-induced acute renal failure. Ren Fail. 2000;22(2):115–27.
Chu JM, Lee DK, Wong DP, Wong RN, Yung KK, Cheng CH, Yue KK. Ginsenosides attenuate methylglyoxal-induced impairment of insulin signaling and subsequent apoptosis in primary astrocytes. Neuropharmacology. 2014;85:215–23.
Yokozawa T, Iwano M, Dohi K, Hattori M, Oura H. Inhibitory effects of ginseng on proliferation of cultured mouse mesangial cells. Japanese journal of nephrology. 1994;36(1):13–8.
Acknowledgments
The authors would like to thank all of the colleagues who contributed to this study.
Funding
This research was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Export Promotion Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (315049–05-2-CG000) and the Korea Institute of Oriental Medicine (K17810).
Availability of data and materials
Data are all contained within the paper.
Author information
Authors and Affiliations
Contributions
This work was carried out in collaboration between all authors. Chan-Sik Kim performed the experiments and wrote the manuscript. Kyuhyung Jo and Jin Soo Kim performed the experiments and analyzed the data. Mi Kyung Pyo prepared the herbal extracts. Junghyun Kim designed and supervised the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experiments that used animals were approved by the Institutional Animal Care and Use Committee of the Korea Institute of Oriental Medicine (Approval No. 15–100, Daejeon, Korea).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kim, CS., Jo, K., Kim, J.S. et al. GS-E3D, a new pectin lyase-modified red ginseng extract, inhibited diabetes-related renal dysfunction in streptozotocin-induced diabetic rats. BMC Complement Altern Med 17, 430 (2017). https://doi.org/10.1186/s12906-017-1925-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-017-1925-7