Abstract
Background
Tuberculosis is a world-wide problem affecting humans and animals. There is increasing development of resistance of the pathogens to current antimycobacterial agents. Many authors have investigated activities of extracts and isolated compounds from plants. The traditional uses of plants have frequently been the criterion to select plants investigated. In this contribution, we investigate whether plant extracts with very good activity against Escherichia coli may also be active against mycobacteria.
Methods
The antimycobacterial activity of eight South African tree leaf extracts with high activity against Escherichia coli were determined in vitro against Mycobacterium smegmatis, M. fortuitum and M. aurum, using a serial microdilution method. The cellular cytotoxicity was also determined by the MTT assay using Vero monkey kidney cells. The selectivity index was determined by dividing the cytotoxicity of extracts by MIC.
Results
The antimycobacterial activity of the extracts ranged from 0.02 to 2.5 mg/ml. Mycobacterium smegmatis was more sensitive to the extracts (Average MIC = 0.96 mg/ml) and Mycobacterium aurum was comparatively resistant (Average MIC = 2.04 mg/ml). The extracts of Cremaspora triflora had strong antimycobacterial activity with a MIC of 0.05 mg/ml that compared reasonably well with that of streptomycin (0.01 mg/ml) and rifampicin (0.03 mg/ml), p > 0.05. Cremaspora triflora had the best selectivity index of 2.87 against Mycobacterium fortuitum.
Conclusion
The high activity of C. triflora extracts against the fast-growing mycobacteria and good cellular safety is promising. It may be interesting to investigate extracts against pathogenic M. tuberculosis, M. bovis and M. avium cultures and to isolate active antimycobacterial compounds.
Similar content being viewed by others
Background
Tuberculosis (TB) is a highly infectious disease, caused by Mycobacterium tuberculosis. TB typically affects the lungs but can also affect other organs in the body [1]. An estimated 5–15% of the 2–3 billion people exposed to M. tuberculosis will develop the disease in their lifetime. The probability is even higher among people infected with HIV [1, 2]. In 2014, about 9.6 million people were infected with TB and 1.5 million consequently died. A high proportion of the reported cases (28%) came from Africa [1]. The growing incidence of pathogenic mycobacterial multi-drug resistance to the best two first line antituberculosis drugs - streptomycin and isoniazid and extensively-drug resistance to both first and second line antituberculosis drugs that include fluoroquinolones and capreomycin highlights the critical need to search for newer anti-tuberculosis drugs. There are reports of the emergence of a ‘totally drug-resistant TB’ strain with a limited chance of successful treatment [1].
Natural products, either as a source of pure compounds or as standardised plant extracts, provide opportunities for new drug leads due to the high chemical diversity in plants [3]. It has been shown that extracts of Maerua edulis, Securidaca longipedunculata, Zanthoxylum capense and Tabernaemontana elegans have high activity against Mycobacteria spp. [4, 5].
To select plants for investigation, the traditional use can be considered. Because traditional healers have mainly water available and antimicrobial compounds are not readily soluble in water [6] this is not such a promising approach [7]. Random screening of tree leaf extracts indicated that many plant extracts have excellent activities [8]. The cell wall of Mycobacterium tuberculosis has similar characteristics to both Gram-positive and Gram-negative bacteria [9]. In addition, M. tuberculosis is closer related to Gram-negative bacteria than Gram-positive bacteria. Analysis of evolutionary distance between nearest ancestral units, suggest that M. tuberculosis is closely related to Escherichia coli and Pseudomonas aeruginosa [9]. Thus in this study we selected eight plant species with high antimicrobial activity against Escherichia coli to determine the potency, efficacy and safety of acetone tree leaf extracts against three fast growing Mycobacterium species. With one exception no previous studies were found on the antimycobacterial activity of extracts of these species.
Methods
Collection of plant material, drying and storage
Leaves of the selected tree species were collected and dried in the shade before grinding to a fine powder. The trees selected with family and voucher specimen numbers in brackets were Hypericum roeperianum G.W. Schimp.ex A. Rich. var. roeperianum, (Hypericaceae, PRU 120126), Cremaspora triflora (Thonn.) K. Schum (Rubiaceae, PRU 120129), Heteromorpha arborescens (Spreng.) Chan. & Schltdl (Apiaceae, PRU 120026), Bolusanthus speciosus (H. Bolus) Harms (Fabaceae, PRU 120027), Calpurnia aurea (Aiton) Benth ssp. aurea (Fabaceae, PRU 120125), Maesa lanceolata Forssk (Maesaceae, PRU120125), Elaeodendron croceum (Thunb.) DC (Celastraceae, PRU 120127) and Morus mesozygia Stapf ex A. Chev (Moraceae, PRU 120128) were collected in 2013, at the University of Pretoria, Botanical Garden, Pretoria National Botanical Garden and Lowveld National Botanical Garden in South Africa. The identity of the trees was confirmed from the tree labels. Voucher specimens were prepared and deposited in collaboration with the herbarium curator Mrs. Elsa van Wyk in the HGWJ Schweickerdt Herbarium of the University of Pretoria (PRU).
Preparation of extracts
Ground dry leaf powder (1.0 g) of each plant was poured into 50 ml polyester centrifuge tubes and extracted with 30 ml acetone for one hour [10]. After this they were centrifuged at 4000 x g for 10 min. The supernatants were decanted into preweighed glass vials through Whatman No. 1 filter paper and concentrated to dryness under a stream of cold air. The dried extracts were made up to a concentration of 10 mg/ml (stock solution) in acetone to be used in subsequent assays and stored at 5 °C in tightly stoppered glass tubes.
Antimycobacterial activity assay
Mycobacterial cultures
Mycobacterium smegmatis (ATCC 1441), Mycobacterium aurum (NCTC 10437) and Mycobacterium fortuitum (ATCC 6841) were cultured as described by McGaw et al. [3], and maintained on Löwenstein–Jensen agar slants, supplemented with glycerol. Inocula suspensions were prepared by mixing a few microbial colonies with sterile distilled water to render a concentration of cells equal to standard 1 McFarland solution (approximately 4 × 107 cfu/ml). The suspension was then diluted with freshly prepared Middlebrook 7H9 broth supplemented with 10% oleic acid, albumin, dextrose, and catalase (OADC) to obtain a final inoculum density of approximately 4 × 105 cfu/ml. A serial microplate broth microdilution technique [11] was used to obtain the MIC values of the various extracts.
Cytotoxic activity
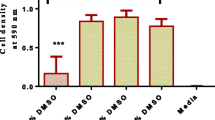
The 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) reduction assay [12] was used to determine the cytotoxicity of the extracts against Vero cells.
Statistical analysis
All experiments were conducted in triplicate and values expressed as the mean ± standard deviation. Variations in mean were analysed using one-way analysis of variance (ANOVA), and means were statistically significant if p < 0.05.
Results and discussion
There are several reasons given to justify the constant search for new anti-TB drugs to improve the current treatment regimen by reducing therapy time and addressing drug resistance especially against multi-drug resistant (MDR) and extreme drug resistant (XDR) mycobacterial strains. Development of natural anti-TB drugs with minimal hepatotoxic and nephrotoxic effects could add to the collection of available drugs used in TB treatment. In many other human diseases very useful drugs have been discovered from plants. Not any of the drugs (isoniazid, rifampicin, capreomycin, fluoroquinolones, kanamycin and amikacin) used as first or second line drugs in the chemotherapy of TB has its origin from plant derived natural products [13].
The choice of using fast growing and non-pathogenic Mycobacterium spp. in antimycobacterial assays was based on their avirulent nature and similarity in sensitivity to pathogenic Mycobacterium strains [14, 15]. In addition, there are published reports on the use of M. fortuitum as an alternative screening model to Mycobacterium tuberculosis for potential antitubercular drug development [14]. Subsequently, Aro et al. [16] found that M. aurum is the best predictor of the activity against pathogenic M. tuberculosis with a correlation coefficient of 0.9 In addition, Mycobacterium smegmatis was the best predictor strain to substitute pathogenic M. bovis and M. tuberculosis, MIC values obtained using M. fortuitum correlated well with those of M. bovis BCG [3].
In addition to the antimycobacterial activity, the total activity was also calculated by dividing the mass in mg extracted from 1 g of dried material with the MIC in mg/ml (7). The total activity indicates the volume to which the extract from one g of plant material can be diluted and still inhibit the growth of the microorganism.
The MIC of activity of the extracts against M. smegmatis ranged from 0.04 to 2.5 mg/ml. C. triflora extracts had the best activity (MIC 0.04 mg/ml), while M. lanceolata had moderate activity (MIC 0.16 mg/ml); H. roeperianum and M. mesozygia had weak activity with MIC = 0.63 mg/ml. The other three species had very low activity. C. triflora had the second best total antimycobacterial activity (TAA) value of 504 ml/g and an SI value of 1.44. TAA value of 504 ml/g infers that if 1 g of the dry acetone crude extract is diluted with 504 ml of water it will still inhibit the growth of the targeted microorganism [10]. Other extracts had relatively poor activity against M. smegmatis and SI less than 1.0 (Table 1).
The MIC values of the extracts against M. fortuitum ranged from 0.02 mg/ml to 2.5 mg/ml. Cremaspora triflora again had the best activity (MIC = 0.02 mg/ml), while M. lanceolata and M. mesozygia had moderate activity with MICs of 0.31 mg/ml. C. triflora extracts had the best total antimycobacterial activity against M. fortuitum with a value of 1008 ml/g, and a good selectivity index value of 2.87 (Table 1).
M. aurum was much more resistant to the plant extracts and only C. triflora had good activity with an MIC value of 0.08 mg/ml, and total antimycobacterial activity value of 255 ml/g and a low SI value of 0.72 (Table 1).
These results were more or less in line with the results of McGaw et al. [3] who investigated the activity of compounds isolated from Euclea species against different Mycobacterium species. It is possible that the fast growing Mycobacterium species are more susceptible than the slow growing species. It also appeared that M. smegmatis would be the best predictor of activity against pathogenic mycobacteria [3].
Palgrave (1997) in Erastus et al. [17] reported the traditional use of the dried bark of Bolusanthus speciosus in the treatment of tuberculosis. Our results investigating the leaves did not support this claim. The acetone leaf extracts of Bolusanthus speciosus had low antimycobacterial activity with a mean MIC value of 1.67 mg/ml against the three tested microorganisms. It is not strange that in vitro studies do not confirm traditional use of plant extracts. This could be due to several reasons. Different extractants could be used, microbial infections could influence activity of extracts left without refrigeration for some time. Clinical identification of the disease and recovery as well as the placebo effect could also have an influence. The activity of plant extracts may also change during different seasons and there could be synergistic activities if extracts of more than one plant species is used.
Maesa lanceolata, traditionally used to treat sore throat and influenza [18, 19], had moderate activity against M. smegmatis and M. fortuitum with MIC values of 0.16 mg/ml and 0.31 mg/ml respectively (Table 1). This may support its use traditionally in the treatment of respiratory infections.
Cremaspora triflora had the best activity against all the Mycobacterium spp. with a mean MIC value of 0.05 ± 0.01 mg/ml and E. croceum had the weakest activity against the microbes with MIC value of 2.5 ± 0.41 mg/ml (Fig. 1). Mycobacterium smegmatis was more sensitive to the extracts than M. fortuitum and M. aurum (Fig. 2), however the difference in the mean between M. smegmatis and M. fortuitum was not statistically significant p > 0.05 (Fig. 2). Means of M. smegmatis and M. fortuitum differed significantly from that of M. aurum, p < 0.05. Our findings agree with a previous report [20] that M. smegmatis was more sensitive to the essential oil of the gall of Pistacia atlantica followed by M. fortuitum, and M. aurum.
The mean MIC values (mg/ml) of acetone leaf extracts of the different medicinal plants and the positive controls (Streptomycin and Rifampicin) against M. smegmatis, M. fortuitum and M. aurum. Legend: a, b and c = no statistical significant difference in the mean MIC values, p < 0.05. HR = Hypericum roeperianum, CT = Cremaspora triflora, HA = Heteromorpha arborescens, BS = Bolusanthus speciosus, CA = Calpurnia aurea, ML = Maesa lanceolata, EC = Elaeodendron croceum, MM = Morus mesozygia, Strept. =Streptomycin, Rifam. = Rifampicin
The extracts of C. triflora have potential as a good candidate for elaborate antimycobacterial investigation. Results from this study on the good activity of the extracts of C. triflora (Table 1) against the different mycobacterial strains differs with the report that the extracts of C. triflora had moderate activity against M. smegmatis and M. aurum, with MIC values of 0.23 mg/ml and 0.10 mg/ml respectively [16]. The discrepancies on the potency report of C. triflora extracts in our study and that of Aro et al. [16], may be attributed to differences in the season and time of plant collection and storage conditions. It is however agreed that the plant has potential for development as an alternative anti-TB drug.
Conclusions
Approximately 88% of all the test acetone leaf extracts had weak activities against the non-pathogenic mycobacterial species. This indicates that high activity against Escherichia coli is not well correlated with antimycobacterial activity. Cremaspora triflora extracts were exceptional, with significant activity against the three fast-growing mycobacterial organisms. Mycobacterium aurum was more resistant to the different extracts. The excellent antibacterial activities and selectivity index of the extracts of C. triflora makes it the preferred species for in depth pharmacological and biological investigations.
Abbreviations
- HIV:
-
Human Immunodeficiency Virus
- MDR:
-
Multidrug resistant
- MIC:
-
Minimum inhibitory concentration
- MTT 3-(4,5-dimethylthiazol-2-yl)-2:
-
5-diphenyltetrazolium bromide
- TAA:
-
Total antimycobacterial activity
- TB:
-
Tuberculosis
- XDR:
-
Extreme drug resistant
References
WHO: Global Tuberculosis Report 2015. 20th edition. France: WHO Press; 2015.
Campion EW, Getahun H, Matteelli A, Chaisson RE, Raviglione M. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372:2127–35.
McGaw LJ, Lall N, Hlokwe TM, Michel A, Meyer JJM, Eloff JN. Purified compounds and extracts from Euclea species with antimycobacterial activity against Mycobacterium bovis and fast-growing mycobacteria. Biol Pharm Bull. 2008;31:1429–33.
Madikizela B. Pharmacological evaluation of South African medicinal plants used for treating tuberculosis and related symptoms. Pietermaritzburg: University of KwaZulu-Natal; 2014.
Kuete V. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Med. 2010;76:1479–91.
Kotze M, Eloff JN. Extraction of antibacterial compounds from Combretum microphyllum (Combretaceae). South African J Bot. 2002;68:62–7.
Eloff JN, McGaw LJ: Using African Plant Biodiversity to Combat Microbial Infections. In Novel Plant Bioresources: Applications in Food, Medicine and Cosmetics. Edited by Gurib-Fakim A. John Wiley; 2014:163–173.
Pauw E, Eloff JN. Which tree orders in southern Africa have the highest antimicrobial activity and selectivity against bacterial and fungal pathogens of animals? BMC Complement Altern Med. 2014;14:317.
Fu LM. Is Mycobacterium tuberculosis a closer relative to Gram –positive or Gram-negative bacterial pathogens? Tuberculosis. 2002;82:85–90.
Eloff JN. On expressing the antibacterial activity of plant extracts - a small first step in applying scientific knowledge to rural primary health care. S Afr J Sci. 2000;96:116–8.
Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711–3.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Nneka NI, Sunday JA. Plant natural products research in tuberculosis drug discovery and development: a situation report with focus on Nigerian biodiversity. African J Biotechnol. 2014;13:2307–20.
Okunade AL, Elvin-Lewis MPF, Lewis WH. Natural antimycobacterial metabolites: current status. Phytochemistry. 2004;65:1017–32.
Chung GAC, Aktar Z, Jackson S, Duncan K. High-throughput screen for detecting antimycobacterial agents. Antimicrob Agents Chemother. 1995;39:2235–8.
Aro AO, Dzoyem JP, Hlokwe TM, Madoroba E, Eloff JN, McGaw LJ: Some South African Rubiaceae tree leaf extracts have Antimycobacterial activity against pathogenic and non-pathogenic Mycobacterium species. Phytother Res 2015, 1010(April):1004–1010.
Erasto P, Bojase-Moleta G, Majinda RRT. Antimicrobial and antioxidant flavonoids from the root wood of Bolusanthus speciosus. Phytochemistry. 2004;65:875–80.
Sindambiwe JB, Calomme M, Geerts S, Pieters L, Vlietinck AJ, Vanden Berghe DA. Evaluation of biological activities of triterpenoid saponins from Maesa lanceolata. J Nat Prod. 1998;61:585–90.
Manguro LOA, Midiwo JO, Tietze LF, Hao P. Triterpene saponins of Maesa lanceolata leaves. ARKIVOC. 2011;2011:172–98.
Sifi I, Dzoyem JP, Quinten M, Yousfi M, McGaw LJ, Eloff JN. Antimycobacterial, antioxidant and cytotoxic activities of essential oil of gall of Pisticia atlantica Desf. From Algeria. African J Tradit Complement Altern Med. 2015;12:150–5.
Elisha IL, Dzoyem JP, Botha FS, Eloff JN. The efficacy and safety of nine South African medicinal plants in controlling Bacillus anthracis Sterne vaccine strain. BMC Complement Altern Med. 2016;16:5.
Acknowledgements
The Curators of the Pretoria National Botanical Gardens, the Lowveld National Botanical Gardens in Nelspruit and the University of Pretoria Botanical Garden allowed the collection of plant material.
Funding
This manuscript is part of the output of a Ph.D. research project supported by the National Research Foundation of South Africa (Eloff IPPR 953991) the Medical Research Council of South Africa (SIR McGaw) and the University of Pretoria.
Availability of data and materials
All data supporting our findings are adequately contained within the manuscript.
Authors’ contributions
ILE carried out the research and wrote the first draft of the manuscript. FSB assisted in the research work and revised the manuscript. BM assisted in the antimycobacterial assay and revised the manuscript. LJM revised the manuscript. JNE identified the project, guided the research, revised and submitted the manuscript. All authors have read and approved the final version of the manuscript.
Competing interests
We declare that we have no financial or competing interests, which may have inappropriately influenced us in writing this article.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Elisha, I.L., Botha, F.S., Madikizela, B. et al. Acetone leaf extracts of some South African trees with high activity against Escherichia coli also have good antimycobacterial activity and selectivity index. BMC Complement Altern Med 17, 327 (2017). https://doi.org/10.1186/s12906-017-1831-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-017-1831-z