Abstract
Background
Loss of neural function is a critical but unsolved issue after cerebral ischemia insult. Neuronal plasticity and remodeling are crucial for recovery of neural functions after brain injury. Buyang Huanwu decoction, which is a classic formula in traditional Chinese medicine, can positively alter synaptic plasticity. This study assessed the effects of Buyang Huanwu decoction in combination with physical exercise on neuronal plasticity in cerebral ischemic rats.
Methods
Cerebral ischemic rats were administered Buyang Huanwu decoction and participated in physical exercise after the induction of a permanent middle cerebral artery occlusion. The neurobehavioral functions and infarct volumes were evaluated. The presynaptic (SYN), postsynaptic (GAP-43) and cytoskeletal (MAP-2) proteins in the coronal brain samples were evaluated by immunohistochemistry and western blot analyses. The ultrastructure of the neuronal synaptic junctions in the same region were analyzed using transmission electron microscopy.
Results
Combination treatment of Buyang Huanwu decoction and physical exercise ameliorated the neurobehavioral deficits (p < 0.05), significantly enhanced the expression levels of SYN, GAP-43 and MAP-2 (p < 0.05), and maintained the synaptic ultrastructure.
Conclusions
Buyang Huanwu decoction facilitated neurorehabilitation following a cerebral ischemia insult through an improvement in synaptic plasticity.

The Buyang Huanwu decoction (BYHWD) combined with physical exercise (PE) attenuates synaptic disruption and promotes synaptic plasticity following cerebral ischemia (stroke).
Similar content being viewed by others

Background
Cerebral ischemia is a major cause of adult mortality and morbidity in developed countries [1–3]. Although numerous drugs and therapies have been tested, there is no effective treatment that dramatically attenuates the neural deficits after stroke in the clinical setting. Because the central nervous system (CNS) of adult mammals has limited self-repair or regeneration, a CNS injury inevitably results in a loss of neural function [4, 5]. Neuronal plasticity and remodeling are critical processes that underlie normal CNS function [6]. The CNS can facilitate the neuronal plasticity induced by brain injury [7]. The decreased synaptic activity after brain injury leads to increased presynaptic release of neurotransmitters, as well as upregulated postsynaptic response to those neurotransmitters; this can, at least in part, restore neural function following an injury [8]. In addition to synaptic activity regulation, new synapse formation, which may compensate for the lost structural circuits, can be triggered by stroke [9–12]. This self-regulation of synaptic plasticity is activity- and experience-dependent [7]. Physical exercise improves the neurological impairments that follow brain insults [13–15]. In synaptic mechanism studies, the beneficial effects of physical exercise were correlated with the maintenance of pre- and postsynaptic components [16, 17].
Pharmacological compounds can assist the recovery from stroke and other CNS diseases [18–20]. Numerous pharmaceutical drugs have been studied, but few have dramatically positive results. The Buyang Huanwu decoction (BYHWD) has been utilized for hundreds of years to improve the recovery of neurological function in stroke-induced disabilities in China [21]. This formulation consists of: Radix Astragali, the root of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao; Radix Angelicae Sinensis, the root of Angelica sinensis (Oliv.) Diels; Radix Paeoniae Rubra, the root of Paeonia lactiflora Pall.; Chuanxiong Rhizoma, the root and rhizome of Ligusticum chuanxiong Hort.; Semen Persicae, the seed of Prunus persica (L) Batsch; Flos Carthami, the flower of Carthamus tinctorius L. and Pheretima, the dried body of Pheretima aspergillum (E. Perrier) [22, 23], The protective mechanisms underlying this classic traditional Chinese formula includes the stimulation of neural proliferation, the modulation of VEGF and Flk1 expression levels, alterations in glutamate levels, and a selective decrease of some amino acids in the cerebrospinal fluid [4, 22, 24]. BYHWD was also testified to be neuroprotective in the cerebral ischemic models in many other studies. A study reported oral administration of BYHWD inhibits caspase-3 and neuron apoptosis in the hippocampal CA1 region of Wistar rats following four-vessel occlusion (4-VO) [25]. Intragastric administration of BYHWD down-regulates inflammation, apoptosis and angiogenesis, as well as up-regulates neurogenesis and nervous system development in cerebral ischemia/reperfusion (CI/R) injured mice and rats [21, 22]. Despite its clinical and laboratory effectiveness, the functional recovery using BYHWD following cerebral ischemia has not been fully investigated. It is difficult to draw a definite conclusion on safety of BYHWD, admittedly, BYHWD seems well tolerable to all patients in clinically [26]. In addition, the safety of BYHWD on patients with acute cerebral ischemia has been testified in a Chinese clinical trial [27].
In this study, we evaluated the combination of BYHWD and physical exercise in cerebral ischemic rats, which had a permanent middle cerebral artery occlusion. We assessed the therapeutic effects of this combination therapy by measuring neurobehavioral functions and infarct volumes, analyzing the SYN, GAP-43 and MAP-2 expression levels, and evaluating the synaptic ultrastructure.
Methods
Animals
The animal handling was approved by the Institutional Animal Care and Use Committee (IACUC) of the Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China. This experimental protocol is in agreement with the European Community guidelines (EEC Directive of 1986; 86/609/EEC). Adult male Sprague-Dawley rats were provided by the Medical Laboratory Animal Centre of Guangdong (Guangzhou, China). The animals (250–300 g) were housed in an animal room (12:12 h light/dark circle; 22–24 °C) with free access to food and water.
Induction of the permanent middle cerebral artery occlusion (MCAO)
The animals were anesthetized with ketamine (100 mg/kg; Gu-Tian Pharmaceutical Co., Ltd.; Fujian, China) and xylazine (10 mg/kg; Sigma-Aldrich; St. Louis, MI, USA). The animals’ temperature was maintained at 37.0 ± 0.5 °C using a heating pad (RWD Life Science; Shenzhen, China). The animals were placed in a supine position after anesthesia. Subsequently, under an operating microscope (Carl-Zeiss; Jena, Germany), a midline incision was made in the neck of the experimental subject. The right common carotid artery (CCA), external carotid artery (ECA) and internal carotid artery (ICA) were isolated. The ECA was cut approximately 5 mm above the common carotid artery bifurcation. We inserted a 4–0 suture (20 mm; Dermalon, 1744–31; Covidien; OH, USA) coated with silicone rubber (3 mm length, 0.4 mm diameter; Heraeus Kulzer; Hanau, Germany) into the ECA stump, where it was reversed into the ICA and finally to the ostium of the middle cerebral artery (MCA) (approximately 15 mm from the carotid bifurcation), as reported previously [28–30]. The 4–0 suture coated with silicone rubber is displayed in Fig. 1a.
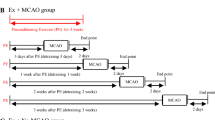
Laboratory techniques used in this study. A 4–0 suture coated with silicone rubber (a) was used for the MCAO induction. A programmable and motorized running wheel apparatus (b) was employed for the physical exercises post-cerebral ischemia. The design of this study (c). The intragastric administration of BYHWD began 2 h after the MCAO induction and was administered daily from Day 1 to 14 or until euthanasia. The physical exercises began on Day 3 post-ischemia and ended on Day 14 or euthanasia. The analytic modalities (neurobehavioral assessment, immunohistochemistry, and western blot) were performed on Day 3, 7 and 14 after the MCAO induction. Other techniques (TTC and TEM) were performed on Day 3 or 14 after the MCAO induction. BYHWD, Buyang Huanwu decoction; IHC, immunohistochemistry; MCAO, middle cerebral artery occlusion; NBA, neurobehavioral assessment; TEM, transmission electron microscope; TTC, triphenyltetrazolium chloride staining; WB, western-blot
The laser Doppler flowmetry (LDF) analysis for the measurement of the surface cerebral blood flow (CBF)
A successful occlusion was confirmed by monitoring the cerebral blood flow (CBF) in the ipsilateral cortex. The measurement of the surface CBF was conducted by a laser Doppler flowmetry (LDF) (Moor Instruments; Axminster, UK) during the MCAO induction, as previously described [31, 32]. In order to determine the surface CBF that was supplied by the bilateral MCA, 2 burr holes (ID =2 mm) were drilled 2.5 mm to the left and right, and 1.0 mm posterior to bregma, as previously described [31, 32]. When the value of the ipsilateral CBF decreased to 1/3 of the baseline value, the MCA was considered to be successfully occluded.
Buyang Huanwu decoction (BYHWD) preparation
The ingredients of BYHWD including Radix Astragali, Radix Angelicae Sinensis, Radix Paeoniae Rubra, Chuanxiong Rhizoma, Semen Persicae, Flos Carthami and Pheretima were mixed together at a ratio of 120:10:10:10:10:10:4.5 (dry weight). It was prepared as previously reported [4, 21]. All dried, crude drugs were ordered from Kang Mei Pharmaceutical Co., Ltd. (Puning, Guangdong, China) and were identified by the Department of Pharmacology at Guangdong Provincial Hospital of Chinese Medicine. To maintain the consistency of the herbal chemical ingredients, all herbs in this decoction were obtained from their original sources. The extraction procedure was performed according to the Chinese Pharmacopoeia. The details of the BYHWD ingredients were listed in Table 1, while the chemical fingerprints of the BYHWD ingredients were shown in the Additional file 1. The BYHWD was made by boiling the dried ingredients in distilled water at 100 °C for 30 min. A freeze-dried drug solution was subsequently made, and the BYHWD powder was produced under vacuum. The powder was dissolved in distilled water at a final concentration of 2.0 g/ml, which is equivalent to the dry weight of the raw materials.
Physical exercise
To quantify the physical exercise intensity, a programmable and motorized wheel apparatus (21 cm in diameter, 40 cm in length; Fig. 1b) was used. For pre-conditioning, the animals used the running wheel for exercise for 7 days prior to the MCAO induction. On the first day, the rats ran in the wheel at a speed of 5 revolutions (rev)/min (approximately 3 m/min). The speed of the wheel gradually increased to 10 rev/min (approximately 6 m/min) on Day 7, as reported previously [13, 33].
Animal groups and study design
The rats were randomly divided into 5 groups: 1) the sham group (n = 24), 2) the MCAO group (n = 38), 3) the MCAO + BYHWD group (n = 35), 4) the MCAO + physical exercise (PE) group (n = 36), and 5) the MCAO + BYHWD + PE group (n = 29). Before MCAO induction, the rats from the MCAO + PE and MCAO+ BYHWD + PE groups participated in physical exercise with the running wheel, as described above. The animals from the MCAO, MCAO + BYHWD, MCAO + PE and MCAO + BYHWD + PE groups underwent the permanent MCAO procedure. The sham group animals were given a sham surgery, which was identical to the MCAO induction procedure, without inserting the 4–0 silicone-coated nylon monofilament. The rats from the MCAO + BYHWD and MCAO + BYHWD + PE groups were administered BYHWD (20 g/kg) by intragastric administration. The medication began 2 h after the MCAO induction and was administered daily from 1 to 14 days or until euthanasia. The physical exercises were performed daily from 3 to 14 days post-ischemia in the MCAO+ BYHWD + PE group. The pattern of physical exercises were identical to the previous studies [13, 33].
According to previous study [22], we chose 10–40 g/kg as the dosage of BYHWD in the preliminary study (Cai J, Pan R, Xu D, Wang B, Zhou M, Chen H. unpublished data). We found that the neuroprotective effects (neurobehavioral assessments) in dosage of 20–40 g/kg were better than those in dosage of 10 g/kg. Therefore, the dosage of 20 g/kg was chosen in present study. In addition, we did not found any obvious side-effects in dosage of 10–40 g/kg in preliminary study (Cai J, Pan R, Xu D, Wang B, Zhou M, Chen H. unpublished data).
A subset of rats from all groups (n = 6 at each time point) underwent a neurobehavioral assessment on Day 3, 7 and 14 post-ischemia, and were then euthanized by decapitation for triphenyltetrazolium chloride (TTC) staining and infarct volume calculations (n = 5 on Day 3), immunohistochemical assays (n = 4 on Days 3, 7 and 14), western blot analyses (n = 6 on Days 3, 7 and 14) and transmission electron microscopic (TEM) examinations (n = 5 at Day 14). The experimental design was shown in Fig. 1c.
Neurobehavioral assessment
We adopted a scale of 0–18 (normal score, 0; maximal deficit score, 18) for the neurobehavioral measurement to assess the neurological deficits on Days 3, 7 and 14 after the MCAO induction [33–35]. The neurological severity score was a combination of motor, sensory, reflex and balance tests. The details of the neurobehavioral assessment were displayed in Additional file 2.
Infarct volume quantification
After euthanasia, the brains were extracted. The brain tissue was sliced into 2 mm cross-sections using a rat brain matrix (RWD, Life Science). The slices were incubated in a 2% solution of 2,3,5-TTC (Sigma-Aldrich; St Louis, MO, USA) at 37 °C for 20 min. The sample sections were photographed and analyzed by an observer blind to the group assignment using Image J software (NIH Program; Bethesda, MD, USA). The infarct volumes were calculated according to previously described methods [36].
Immunohistochemical assay
Animals were euthanized and transcardially perfused with saline and 4% paraformaldehyde. The brains were removed and stored in 4% paraformaldehyde for 24 h. The brain sections (3 μm) were fixed in 4% paraformaldehyde for 5 min, and then washed and blocked for 30 min in 10% (w/v) bovine serum albumin dissolved in 0.1 M phosphate buffer saline (PBS). The sections were incubated at 4 °C overnight with the primary antibodies against SYN (SYN-1; 1:200 dilution; Cell Signaling Technology; Beverly, MA, USA), GAP-43 (1:200 dilution; Abcam; Cambridge, MA, USA) and MAP-2 (1:200 dilution; Santa Cruz Biotechnology; Dallas, TEX, USA), followed by an incubation with the corresponding Alexa Fluor 568 secondary antibody (goat anti-rabbit; 1:200 dilution; Abcam) at room temperature for 1 h. After washing in PBS, the coverslips were mounted on glass slides with 4,6-diamidino-2-phenylindole (DAPI;1:1000 dilution; Sigma-Aldrich).
Western blot analysis
The frozen brain samples were lysed with a buffer containing 20 mM Tris (pH 7.6), 0.2% SDS, 1% TritonX-100, 1% deoxycholate, 1 mM phenylmethylsulfonyl fluoride, and 0.11 IU/mL aprotinin. All the ingredients were purchased from Sigma-Aldrich. The total protein was extracted from the brain samples and subjected to an 8% SDS-PAGE. The following antibodies were used: polyclonal antibodies against SYN (SYN-1; 1:1000 dilution; Cell Signalling Technology), GAP-43 (1:1000 dilution; Abcam), MAP-2 (1:1000 dilution; Santa Cruz Biotechnology) and β-actin (1:6000 dilution; Sigma-Aldrich). The densitometry analyses of the western blots were performed with Glyko Bandscan software (Glyko; Novato, CA, USA). The experiments were conducted at least 3 times.
TEM examination
The animals were euthanized and transcardially perfused with 50 ml of saline followed by 100 ml of 4% paraformaldehyde and 2.5% glutaraldehyde in 0.1 mol/l cacodylic acid buffer (pH 7.3). Following an ethanol series, the fixed brain tissues (right frontal lobe) were dehydrated and embedded in an epoxy resin. Next, the samples were cut into ultrathin sections, which were subsequently mounted on copper grids and stained in uranyl acetate and citric acid lead. The prepared samples were imaged using a H-7650 transmission electron microscope (Hitachi; Tokyo, Japan).
Statistical analyses
The data are shown as the means ± the standard error of the means (SEMs). A one-way analyses of variance (ANOVA) followed by a Student, Newman–Keuls or Dunnett’s post-hoc test, were utilized for the comparisons between more than 2 groups. SPSS 18.0 (SPSS; Chicago, IL, USA) was used for the statistical analyses, and the statistical significance was set at p < 0.05.
Results
Combination treatment of BYHWD and physical exercise decreased the mortality and ameliorated the neurological deficits
Seventy-two hours after MCAO induction, the mortality rates varied among the 4 groups: 23.7% (9/38) in the MCAO group, 17.1% (6/35) in the MCAO + BYHWD group, 22.2% (8/36) in the MCAO + PE group, and 13.8% (4/29) in the MCAO + BYHWD + PE group (Fig. 2a). The mortality of the MCAO + BYHWD + PE group was lower than the other 3 groups.
Comparisons of the mortalities and neurobehavioral assessment in all groups. Mortalities of all groups (a). Evaluations of the neurological deficits in all groups (n = 6 at each time point) (b). Scores of neurological deficits are expressed as the means ± standard error of the means; ns, not significant, p > 0.05; *, p < 0.05, compared to the MCAO group
On Days 3, 7, 14 post-ischemia, the neurological deficits of all groups were assessed using an 18-point system. A higher score indicated more deficits. We found that the MCAO group exhibited severe deficits (9.2 ± 0.6, 8.6 ± 0.7 and 7.2 ± 0.6 on Days 3, 7 and 14 post-ischemia, respectively). The MCAO + BYHWD group (9.4 ± 0.7, 7.6 ± 0.7 and 6.0 ± 0.5 on Days 3, 7 and 14 post-ischemia, respectively) and MCAO + PE group (9.3 ± 0.6, 7.4 ± 0.7 and 6.1 ± 0.5 on Days 3, 7 and 14 post-ischemia, respectively) exhibited moderate deficits. Noticeably, the MCAO + BYHWD + PE group exhibited milder neurological deficits at each time point relative to the other MCAO groups (9.2 ± 0.6, 6.9 ± 0.6 and 5.1 ± 0.5 on Days 3, 7 and 14 post-ischemia, respectively; Fig. 2b).
Combination treatment of BYHWD and physical exercise did not reduce infarct volumes
To determine whether combination treatment was neuroprotective, the infarct volumes of all groups were examined at each time point following the cerebral ischemia. The TTC-stained slices of the coronal brain sections were used to confirm the presence of successful cerebral infarctions in the MCAO induction animals. The representative examples were displayed in Fig. 3a. After TTC staining, the brain sections of sham group were uniformly stained a dark red and had no pale infarct area. On Day 3 post-ischemia, the infarct volumes in the MCAO, MCAO + BYHWD, MCAO + PE and MCAO + BYHWD + PE groups were 299.3 ± 8.5, 287.4 ± 7.6, 302.1 ± 11.5, 292.6 ± 7.2 mm3 respectively. There were no significant differences in the infarct volumes between these 4 groups (p > 0.05; Fig. 3b).
Representative TTC-stained slices and the infarct volumes in all groups. Representative TTC-stained slices of the coronal brain sections (a) from all groups exhibit the pale infarct area induced by a permanent MCAO. The infarct volumes in all groups on Day 3 after the MCAO induction (n = 5) (b). The bars indicate the means ± the standard errors of the means; ns, p > 0.05; *, p < 0.05, relative to the MCAO group
Combination treatment increased SYN, GAP-43 and MAP-2 protein levels after cerebral ischemia
Immunohistochemistry and western blots were employed to detect and quantify SYN (SYN-1), GAP-43 and MAP-2 protein levels. The immunohistochemical micrographs revealed that SYN, GAP-43 and MAP-2 proteins were normally expressed in the brain samples from sham group (Fig. 4A-a, B-a, C-a). However, in the micrographs of the peri-infarct zones from the MCAO group, the protein expression levels were dramatically reduced (Fig. 4A-b, B-b, C-b). The disruption of these proteins was mildly attenuated in the MCAO + BYHWD group (Fig. 4A-c, B-c, C-c) and the MCAO + PE group (Fig. 4A-d, B-d, C-d). The positive staining measurement of immunohistochemical micrographs were analyzed with Image J software (NIH Program, Bethesda, Maryland, USA). However, the positive staining measurement of SYN slices were employed relative intensity calculation technique as previously reported [37]. In the sections from the MCAO + BYHWD + PE group (Fig. 4A-e, B-e, C-e), the expression levels of these proteins were distinctly upregulated compared to the levels in the MCAO group (p < 0.05).
High magnification micrographs of the immunohistochemical fluorescent labeling of SYN (SYN-1), GAP-43 and MAP-2. The immunohistochemistry micrographs display the locations of the SYN (a), GAP-43 (b) and MAP-2 (c) proteins in sham (a), MCAO (b), MCAO + BYHWD (c), MCAO + PE (d) and MCAO + BYHWD + PE (e) groups. The scale bars =20 μm. The bars indicate the means ± the standard errors of the means; *, p < 0.05, relative to the MCAO group
Western blot analyses revealed that the protein expression levels of SYN (SYN-1), GAP-43 and MAP-2 in the sham group were detectable. These protein expression levels were remarkably reduced in the MCAO group post-ischemia compared to the levels of the sham group (p < 0.05). However, the decreased levels of these proteins were ameliorated to a certain extent after the MCAO induction in the MCAO + BYHWD and MCAO + PE group, although not statistically significant (p > 0.05). In the MCAO + BYHWD + PE group, these proteins were distinctly up-regulated compared to the expression levels in the MCAO group (p < 0.05). The representative western blot micrographs were displayed in Fig. 5a, and the relative expression levels of SYN, GAP-43 and MAP-2 are disclosed in Fig. 5b.
Expression levels of SYN (SYN-1), GAP-43 and MAP-2 in all groups at each time point. The proteins expression levels at each time point following the MCAO are presented in representative western-blotting autoradiograms (a). We detected SYN at 77 kDa, GAP-43 at 48 kDa, MAP-2 at 70 kDa and the β-actin loading control at 43 kDa. Lane 1, sham group; Lane 2, MCAO group; Lane 3, MCAO + BYHWD group; Lane 4, MCAO + PE group; Lane 5, MCAO + BYHWD + PE group. The quantitative analyses (n = 6) of the western blot results for the expression levels of SYN, GAP-43 and MAP-2 are presented in the bar graphs (b). The bars indicate the means ± standard errors of the means. *, p < 0.05, compared to the MCAO group
Combination treatment maintained the ultrastructural integrity of the synapse
TEM technique was used to analyze the ultrastructural changes of the synapse post-ischemia. The ultrastructure of the synapse in the sham group remained intact and clear. In the MCAO group, the axons and dendrites were chaotic, and there were no proper synapses in the peri-infarct region. In the MCAO + BYHWD and MCAO + PE groups, the axonal and dendritic membranes and the synaptic structures were healthier and more intact compared to the morphology found in the MCAO group. However, in the MCAO + BYHWD + PE group, the synaptic ultrastructure looked similar to the sham group’s ultrastructure, although there were some indistinct axons and dendritic membranes. The representative TEM micrographs were shown in Fig. 6.
TEM micrograms. Representative synapse ultrastructures of all groups. An intact synaptic ultrastructure is shown in panel sham, and a disorderly synaptic ultrastructure is illustrated in panels MCAO, MCAO + BYHWD, MCAO + PE and MCAO + BYHWD + PE. The arrowheads presented in panels MCAO, MCAO + BYHWD, MCAO + PE and MCAO + BYHWD + PE indicate the synaptic disruptions. Scale bars =200 nm
Discussion
The recovery after a CNS injury requires cortical plasticity, including synaptic plasticity [6, 7, 38]. Cerebral plasticity is influenced by many factors. Activity- and experience-dependent plasticity is very important for cerebral plasticity in both normal and injured CNS [10]. One treatment protocol that persistently induces a post-stroke behavioral recovery is the forced use of the disabled limbs in a patterned or task-specific behavioral activity task [7, 39–42]. This treatment strategy was developed using the theory of activity- and experience-dependent plasticity for the rehabilitation of the affected limbs. However, one treatment modality is unable to meet the extensive rehabilitative needs after stroke. Thus, pharmacological neuromodulation may lead to a significant improvement in rehabilitative outcomes [18, 43–45]. However, few drugs have been effective in facilitating physical rehabilitation. Buyang Huanwu decoction (BYHWD) is believed to be an important promoter in neurorehabilitation after stoke [27]. In this study, BYHWD was evaluated in synaptic plasticity.
According to Fig. 2a, the survival rates varied among the 4 MCAO groups. The mortalities of the 2 groups that were administered BYHWD were lower than the other MCAO groups. This finding agrees with a previous study [21]. However, the mortality rates of the MCAO mice reported by that study were approximately 50–83%, leading to a discrepancy in the total mortality between that study and the results presented here. This may be due to the anatomical variations of the posterior communicating artery in the MCAO mice, which can be optimized in future studies [46]. In this study, the physical exercise treatment was added after 3 days of cerebral ischemia. A neurobehavioral assessment scale was employed to evaluate the neurological deficits after MCAO induction [34, 35]. We found that the neurological deficits of the combination treatment group was similar to that of the other MCAO groups on Day 3 after the MCAO induction. It was remarkably alleviated on Day 7 and 14 post-ischemia, compared to the MCAO rats without treatment. This result indicates that BYHWD effectively facilitated the effects of the physical exercise after a cerebral ischemia insult.
SYN (SYN-1), GAP-43 and MAP-2 are associated with presynaptic and postsynaptic plasticity as well as the neuronal cytoskeleton [6, 38, 47, 48]. The synaptic plasticity markers SYN (SYN-1), GAP-43 and MAP-2 are up-regulated in the post-ischemic milieu, thus, they were postulated to play pivotal roles in neurorehabilitation [49–53]. We qualitatively analyzed these 3 proteins to determine the effects of the combination therapy of BYHWD and physical exercise on synaptic plasticity. The immunohistochemical data suggested that BYHWD or physical exercise alone can ameliorate the disruption of these 3 synaptic proteins that was induced by the MCAO induction. However, the effects of the individual treatments were not significant, while the combination treatment was. Furthermore, we quantitatively analyzed the changes of these 3 proteins with various treatment modalities using western blot. The combination of the BYHWD and physical exercise treatment alleviated the decreases in the SYN, GAP-43 and MAP-2 proteins after the cerebral ischemia. These changes were consistent with the neurological deficits in the various groups after the MCAO induction. It was reported that there were differences in synaptic change over time [54]. Otherwise, we did not identify significant differences in SYN (SYN-1), GAP-43 and MAP-2 protein levels on each time point (p > 0.05). Similarly, the time-dependent synaptic change did not relate to each kind of synaptic junctions [55].
In this study, we assessed the synaptic ultrastructure of all groups on Day 14 post-cerebral ischemia using TEM. We found a clear and intact synaptic ultrastructure in the high magnification micrographs from sham group, and a disorganized synaptic ultrastructure in all the MCAO groups (Fig. 6). The synaptic disorganization was markedly ameliorated in the group that received the combination treatment of BYHWD and physical exercise.
Conclusions
In summary, we conclude that BYHWD could be a potential therapeutic pharmaceutical approach, which can effectively facilitate neurorehabilitation following cerebral ischemia insult through improved synaptic plasticity.
Abbreviations
- BYHWD:
-
Buyang Huanwu decoction
- CBF:
-
Cerebral blood flow
- CCA:
-
Common carotid artery
- CNS:
-
Central nervous system
- ECA:
-
External carotid artery
- ICA:
-
Internal carotid artery
- LDF:
-
Laser Doppler flowmetry
- MCA:
-
Middle cerebral artery
- MCAO:
-
Middle cerebral artery occlusion
- TEM:
-
Transmission electron microscope;
References
Bajaj A, Schernhammer ES, Haidinger G, Waldhor T. Trends in mortality from stroke in Austria, 1980-2008. Wien Klin Wochenschr. 2010;122(11–12):346–53.
Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2(1):43–53.
Rothwell PM. The high cost of not funding stroke research: a comparison with heart disease and cancer. Lancet. 2001;357(9268):1612–6.
Cai G, Liu B, Liu W, Tan X, Rong J, Chen X, Tong L, Shen J. Buyang Huanwu decoction can improve recovery of neurological function, reduce infarction volume, stimulate neural proliferation and modulate VEGF and Flk1 expressions in transient focal cerebral ischaemic rat brains. J Ethnopharmacol. 2007;113(2):292–9.
Bjorklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000;3(6):537–44.
Reines A, Cereseto M, Ferrero A, Sifonios L, Podesta MF, Wikinski S. Maintenance treatment with fluoxetine is necessary to sustain normal levels of synaptic markers in an experimental model of depression: correlation with behavioral response. Neuropsychopharmacol: Off Pub Am Coll Neuropsychopharmacol. 2008;33(8):1896–908.
Overman JJ, Carmichael ST. Plasticity in the injured brain: more than molecules matter. Neuroscientist : Rev J Neurobiol Neurol Psychiatry. 2014;20(1):15–28.
Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5(2):97–107.
Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24(27):6209–17.
Carmichael ST. Plasticity of cortical projections after stroke. Neuroscientist : Rev J Neurobiol Neurol Psychiatry. 2003;9(1):64–75.
Carmichael ST, Wei L, Rovainen CM, Woolsey TA. New patterns of intracortical projections after focal cortical stroke. Neurobiol Dis. 2001;8(5):910–22.
Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26(11):2135–44.
Hu X, Zheng H, Yan T, Pan S, Fang J, Jiang R, Ma S. Physical exercise induces expression of CD31 and facilitates neural function recovery in rats with focal cerebral infarction. Neurol Res. 2010;32(4):397–402.
Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke. 2003;34(10):2475–81.
Hu FB, Stampfer MJ, Colditz GA, Ascherio A, Rexrode KM, Willett WC, Manson JE. Physical activity and risk of stroke in women. JAMA. 2000;283(22):2961–7.
Middleton LE, Corbett D, Brooks D, Sage MD, Macintosh BJ, McIlroy WE, Black SE. Physical activity in the prevention of ischemic stroke and improvement of outcomes: a narrative review. Neurosci Biobehav Rev. 2013;37(2):133–7.
Rudolf R, Khan MM, Labeit S, Deschenes MR. Degeneration of neuromuscular junction in age and dystrophy. Front Aging Neurosci. 2014;6:99.
Floel A, Cohen LG. Recovery of function in humans: cortical stimulation and pharmacological treatments after stroke. Neurobiol Dis. 2010;37(2):243–51.
Liepert J. Pharmacotherapy in restorative neurology. Curr Opin Neurol. 2008;21(6):639–43.
Rosser N, Floel A. Pharmacological enhancement of motor recovery in subacute and chronic stroke. NeuroRehabilitation. 2008;23(1):95–103.
Wang HW, Liou KT, Wang YH, Lu CK, Lin YL, Lee IJ, Huang ST, Tsai YH, Cheng YC, Lin HJ, et al. Deciphering the neuroprotective mechanisms of Bu-yang Huan-wu decoction by an integrative neurofunctional and genomic approach in ischemic stroke mice. J Ethnopharmacol. 2011;138(1):22–33.
Zhao LD, Wang JH, Jin GR, Zhao Y, Zhang HJ. Neuroprotective effect of Buyang Huanwu decoction against focal cerebral ischemia/reperfusion injury in rats--time window and mechanism. J Ethnopharmacol. 2012;140(2):339–44.
Wei RL, Teng HJ, Yin B, Xu Y, Du Y, He FP, Chu KT, Luo BY, Zheng GQ. A systematic review and meta-analysis of buyang huanwu decoction in animal model of focal cerebral ischemia. Evid Based Complement Alternat Med. 2013;2013:138484.
Wang L, Huang Y, Wu J, Lv G, Zhou L, Jia J. Effect of Buyang Huanwu decoction on amino acid content in cerebrospinal fluid of rats during ischemic/reperfusion injury. J Pharm Biomed Anal. 2013;86:143–50.
Li XM, Bai XC, Qin LN, Huang H, Xiao ZJ, Gao TM. Neuroprotective effects of Buyang Huanwu decoction on neuronal injury in hippocampus after transient forebrain ischemia in rats. Neurosci Lett. 2003;346(1–2):29–32.
Li JH, Liu AJ, Li HQ, Wang Y, Shang HC, Zheng GQ. Buyang huanwu decoction for healthcare: evidence-based theoretical interpretations of treating different diseases with the same method and target of vascularity. Evid Based Complement Alternat Med. 2014;2014:506783.
Hao CZ, Wu F, Shen J, Lu L, Fu DL, Liao WJ, Zheng GQ. Clinical efficacy and safety of buyang huanwu decoction for acute ischemic stroke: a systematic review and meta-analysis of 19 randomized controlled trials. Evid Based Complement Alternat Med. 2012;2012:630124.
Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91.
Greco R, Tassorelli C, Stefania Mangione A, Levandis G, Certo M, Nappi G, Bagetta G, Blandini F, Amantea D. Neuroprotection by the PARP inhibitor PJ34 modulates cerebral and circulating RAGE levels in rats exposed to focal brain ischemia. Eur J Pharmacol. 2014;744:91–7.
Lin X, Miao P, Mu Z, Jiang Z, Lu Y, Guan Y, Chen X, Xiao T, Wang Y, Yang GY. Development of functional in vivo imaging of cerebral lenticulostriate artery using novel synchrotron radiation angiography. Phys Med Biol. 2015;60(4):1655–65.
Cai J, Pan R, Jia X, Li Y, Hou Z, Huang RY, Chen X, Huang S, Yang GY, Sun J, et al. The combination of astragalus membranaceus and ligustrazine ameliorates micro-haemorrhage by maintaining blood-brain barrier integrity in cerebrally ischaemic rats. J Ethnopharmacol. 2014;158PA:301–9.
Cai J, Sun Y, Yuan F, Chen L, He C, Bao Y, Chen Z, Lou M, Xia W, Yang GY, et al. A novel intravital method to evaluate cerebral vasospasm in rat models of subarachnoid hemorrhage: a study with synchrotron radiation angiography. Plos One. 2012;7(3):e33366.
Zhang L, Hu X, Luo J, Li L, Chen X, Huang R, Pei Z. Physical exercise improves functional recovery through mitigation of autophagy, attenuation of apoptosis and enhancement of neurogenesis after MCAO in rats. BMC Neurosci. 2013;14:46.
Borlongan CV, Randall TS, Cahill DW, Sanberg PR. Asymmetrical motor behavior in rats with unilateral striatal excitotoxic lesions as revealed by the elevated body swing test. Brain Res. 1995;676(1):231–4.
Schallert T, Kozlowski DA, Humm JL, Cocke RR. Use-dependent structural events in recovery of function. Adv Neurol. 1997;73:229–38.
Yang G, Chan PH, Chen J, Carlson E, Chen SF, Weinstein P, Epstein CJ, Kamii H. Human copper-zinc superoxide dismutase transgenic mice are highly resistant to reperfusion injury after focal cerebral ischemia. Stroke. 1994;25(1):165–70.
Jensen EC. Quantitative analysis of histological staining and fluorescence using Image J. Anat Rec (Hoboken). 2013;296(3):378–81.
Nahmani M, Turrigiano GG. Adult cortical plasticity following injury: recapitulation of critical period mechanisms? Neuroscience. 2014;283:4–16.
Grotta JC, Noser EA, Ro T, Boake C, Levin H, Aronowski J, Schallert T. Constraint-induced movement therapy. Stroke. 2004;35(11 Suppl 1):2699–701.
Sawaki L, Butler AJ, Leng X, Wassenaar PA, Mohammad YM, Blanton S, Sathian K, Nichols-Larsen DS, Wolf SL, Good DC, et al. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil Neural Repair. 2008;22(5):505–13.
Wolf SL, Blanton S, Baer H, Breshears J, Butler AJ. Repetitive task practice: a critical review of constraint-induced movement therapy in stroke. Neurol. 2002;8(6):325–38.
Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296(17):2095–104.
Benowitz LI, Carmichael ST. Promoting axonal rewiring to improve outcome after stroke. Neurobiol Dis. 2010;37(2):259–66.
Carmichael ST. Translating the frontiers of brain repair to treatments: starting not to break the rules. Neurobiol Dis. 2010;37(2):237–42.
Wittenberg GF. Experience, cortical remapping, and recovery in brain disease. Neurobiol Dis. 2010;37(2):252–8.
Yuan F, Tang Y, Lin X, Xi Y, Guan Y, Xiao T, Chen J, Zhang Z, Yang GY, Wang Y. Optimizing suture middle cerebral artery occlusion model in C57BL/6 mice circumvents posterior communicating artery dysplasia. J Neurotrauma. 2012;29(7):1499–505.
Bogen IL, Jensen V, Hvalby O, Walaas SI. Glutamatergic neurotransmission in the synapsin I and II double knock-out mouse. Semin Cell Dev Biol. 2011;22(4):400–7.
Ng SC, de la Monte SM, Conboy GL, Karns LR, Fishman MC. Cloning of human GAP-43: growth association and ischemic resurgence. Neuron. 1988;1(2):133–9.
Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–72.
Pendharkar AV, Levy SL, Ho AL, Sussman ES, Cheng MY, Steinberg GK. Optogenetic modulation in stroke recovery. Neurosurg Focus. 2016;40(5):E6.
Gorup D, Bohacek I, Milicevic T, Pochet R, Mitrecic D, Kriz J, Gajovic S. Increased expression and colocalization of GAP43 and CASP3 after brain ischemic lesion in mouse. Neurosci Lett. 2015;597:176–82.
Kim T, Mehta SL, Kaimal B, Lyons K, Dempsey RJ, Vemuganti R. Poststroke induction of alpha-Synuclein mediates ischemic brain damage. J Neurosci. 2016;36(26):7055–65.
Boulos S, Meloni BP, Arthur PG, Bojarski C, Knuckey NW. Assessment of CMV, RSV and SYN1 promoters and the woodchuck post-transcriptional regulatory element in adenovirus vectors for transgene expression in cortical neuronal cultures. Brain Res. 2006;1102(1):27–38.
Kolb B, Muhammad A, Gibb R. Searching for factors underlying cerebral plasticity in the normal and injured brain. J Commun Disord. 2011;44(5):503–14.
Comeau WL, McDonald RJ, Kolb BE. Learning-induced alterations in prefrontal cortical dendritic morphology. Behav Brain Res. 2010;214(1):91–101.
Acknowledgements
The authors thank Dr. Shanshan Wang for data collection.
Funding
This study was supported by National Natural Science Foundation of China (NSFC No. 81301013) to JC, Guangdong Natural Science Foundation of China (No. S2013040013591 to JC; No. 2014A030310457 to RP) and Postdoctoral Science Foundation of China (No. 2015 M572298) to RP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Authors’ contributions
Conceived and designed the experiments: JC, RP, HC. Performed the experiments: RP, JC, MZ, DX, JZ. Analyzed the data: LZ, YG. Conducted chemical fingerprint of BYHWD ingredients: XL. Contributed reagents/materials/analysis tools: HC, JC. Wrote the paper: JC. Revised the manuscript: R-Y H, JC. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
In this study, animal procedures were carried out according to a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China. This protocol was in accordance with the European Community guidelines (EEC Directive of 1986; 86/609/EEC).
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Additional files
Additional file 1:
Chemical fingerprints of the BYHWD ingredients. UPLC-PAD/ESI-MS chromatogram of Buyang Huanwu decoction is shown in panel a: U-HPLC-PAD chromatography (upper) and ESI-MS (positive) total ion current (bottom). In other panels, UPLC-ESI-MS chromatogram of Buyang Huanwu decoction (uppers in panels b, c, d, e, f, g, h) and red peony root (bottom in panel b), Astragalus membranaceus (bottom in panel c), Chinese angelica root (bottom in panel d), Ligusticum wallichii (bottom in panel e), earthworm (bottom in panel f), safflower (bottom in panel g), peach seed (bottom in panel h) are displayed. (TIFF 8833 kb)
Additional file 2:
Modified neurobehavioral assessment score. (PDF 15 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Pan, R., Cai, J., Zhan, L. et al. Buyang Huanwu decoction facilitates neurorehabilitation through an improvement of synaptic plasticity in cerebral ischemic rats. BMC Complement Altern Med 17, 173 (2017). https://doi.org/10.1186/s12906-017-1680-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-017-1680-9