Abstract
Background
Uterine Fibroids (UFs) growth is ovarian steroid-dependent. Previous studies have shown that estrogen and progesterone play an important role in UF development. However, the mechanism underlying progesterone induced UF pathogenesis is largely unknown. In this study, we determined the expression of progesterone receptor and compared the expression level of progesterone-regulated genes (PRGs) in human myometrial cells from normal uteri (MyoN) versus uteri with UFs (MyoF) in response to progesterone.
Methods
Primary human myometrial cells were isolated from premenopausal patients with structurally normal uteri (PrMyoN). Primary human myometrial cells were also isolated from uterus with UFs (PrMyoF). Isolated tissues were excised at least 2 cm from the closest UFs lesion(s). Progesterone receptor (PR) expression was assessed using Western blot (WB). Expression levels of 15 PRGs were measured by qRT-PCR in PrMyoN and PrMyoF cells in the presence or absence of progesterone.
Results
WB analysis revealed higher expression levels of PR in PrMyoF cells as compared to PrMyoN cells. Furthermore, we compared the expression patterns of 15 UF-related PRGs in PrMyoN and PrMyoF primary cells in response to progesterone hormone treatment. Our studies demonstrated that five PRGs including Bcl2, FOXO1A, SCGB2A2, CYP26a1 and MMP11 exhibited significant progesterone-hyper-responsiveness in human PrMyoF cells as compared to PrMyoN cells (P < 0.05). Another seven PRGs, including CIDEC, CANP6, ADHL5, ALDHA1, MT1E, KIK6, HHI showed gain in repression in response to progesterone treatment (P > 0.05). Importantly, these genes play crucial roles in cell proliferation, apoptosis, cell cycle, tissue remodeling and tumorigenesis in the development of UFs.
Conclusion
These data support the idea that progesterone acts as contributing mechanism in the origin of UFs. Identification and analysis of these PRGs will help to further understand the role of progesterone in UF development.
Similar content being viewed by others
Background
Uterine fibroids (UFs) are smooth muscle cell tumors originating from the myometrium. Tumors occur in 70–80% of women overall and are clinically manifested in 25–50% by 50 years of age [1].
UFs are ovarian steroid hormones dependent [2]. While estrogen has been considered the major mitogenic factor in the uterus, progesterone (P4) also play a key role in UF growth and development [3]. There are conflicting results about the role of progesterone in UF development either it is stimulatory or inhibitory [4]. Several studies had proved the essential role of the progesterone in the pathogenesis of UFs. A xenograft animal model demonstrated that estradiol upregulated the PR levels, and volume maintenance and growth of UFs were P4 dependent (Hiroshi Ishikawa et al. Endocrinology 2010 and For human studies,pregnancy stimulateed UF development / growth were suppressed with antiprogestin therapy [5]. Several other studies demonstrated that GnRH agonists were capable of reducing UF size, and progestin add-back therapy prevented this reduction [6,7,8]. In addition, LNG (levo –norgestrine) (treatment in vitro decreased UF cell viability and induced apoptosis. Similarly, a number of antiprogestin drugs and SPRM (selective progesterone receptor modulator) have been developed and tested in clinical trials for the treatment of UFs, including Mifepristone, Asoprisnil and Ulipristal acetate. These studies provide strong evidence for the mitogenic effect of progesterone on UF pathogenesis [9,10,11].
The progesterone responses are mediated by two pathways, the rapid non-genomic signaling and slower genomic one. The genomic pathway can be mediated by binding of progesterone to PR result in binding to DNA and regulate the expression of target genes. There are two types of PR, PR-A and PR-B [12,13,14]. PR-A and -B actions are divergent from each other. PR-B differs from PR-A in that it contains an additional 164 amino acids at the amino-terminus [15, 16]. Another mode of action are non-genomic pathway, in which the progesterone activate a variety of rapid signaling events in the cells [17].
Eker rat model carrying a germ-line defect in the tuberous sclerosis complex-2 (Tsc-2) tumor suppressor has been used to determine the interaction between genetic susceptibility and early-life environmental exposure, which contributes to the pathogenesis of UFs [18, 19]. Developmental exposure to xenoestrogens such as diethylstilbestrol (DES) increased the tumor-suppressor-gene penetrance, tumor multiplicity and size in predisposed animals, and DES exposure caused reprogramming of estrogen-response genes expressed in UFs and resulted in alteration of these genes in UFs tumor genesis. Recently, we demonstrated that developmental exposure to DES expands the myometrial stem cells (MMSCs), which linked to the increases risk of UF development. In human studies, the correlation between number of MMSCs with risk of UFs was identified in women. Myometrium from Caucasian (CC) women with UFs exhibited increased numbers of MMSCs as compared to CC women without UFs, and myometrium from African-American (AA) women had the highest number of MMSCs: AA-with UFs > CC with UFs > AA-without UFs > CC-without UFs [20]. In addition, MMSC population expanded in African American women, is correlated with parity and UF number, and fluctuates with cyclic menstrual cycle hormone changes and age [21, 22]. These studies suggest that MyoF was primed and exhibited a distinct profiling at molecular and cellular levels as compared to MyoN, which become at risk for later tumorigenic transformation. However, how the P4 triggers the transformation is unknown.
The object of this study is to identify progesterone responsive genes in cells from human MyoF verse MyoN tissues that will help to understand the role of progesterone in UF development.
Methods
Patients and myometrium specimens
The study was approved by Augusta University’s Institutional Review Board. Myometrium were obtained from Caucasian women who underwent abdominal hysterectomy for UFscause or any other causes. The ages of the patients are from 33 to 48 years old and none had received hormonal therapy for at least three cycles before surgery. The case interquartile range is 6. Informed consent was obtained from each patient before surgery for the use of extirpated uterine tissues for culture experiments.
Myometrial cell isolation and cell culture
Primary human myometrial cells were generated from the adjacent myometrial tissue of human uterus with UF after hysterectomy at least 2 cm away from the closest UFs lesion (PrMyoF). Also we isolated the primary human myometrial cells from uterus without UFs (PrMyoN). Isolation of the primary cell population from tissues was performed as described previously [23]. Briefly, a portion (0.5 cm3) of fresh myometrial tissue was washed in culture medium to remove blood and then chopped into small pieces under sterile conditions, transferred into a 15-ml screw cap tube, and suspended in Hanks Balanced Salt Solution containing 13 antibiotic-antimycotic (Thermo Fisher Scientific) and 300 U/ml collagenase type 4 (Worthington Biochemical Corp.). Suspended tissue pieces were incubated at 37 °C for at least 12 h to obtain individual cells and/or clumps of cells. The cell suspension was passed through a 100-μm pore-sized sterile nylon filter and the suspension of individual cells was plated out and incubated at 37 °C, allowing the cells to attach to the 100-mm sterile tissue culture-treated plate containing smooth muscle cell basal medium (SmBM; catalog no. CC- 3181; Lonza) containing 5% fetal bovine serum (FBS) and supplemented with SmBM singlequots (catalog no. CC-4149). This SmBM singlequot contains hEGF, insulin, hFGF-B, and gentamicin / amphotericin-B. These cell culture experiments were performed successfully with ten uterine tissue specimens collected from different patients, of which five were from the normal uterus and the other five were from uterus with UFs.
Protein extraction and Western blot analysis
Pellets were lysed in lysis buffer with protease and phosphatase inhibitor cocktail (Thermo Fisher scientific, Waltham, MA, USA), and protein was quantified using the Bradford method (Bio-Rad protein Assay kit, Hercules, CA, USA). Western blot was performed as described previously [24] Blots were done for two different isoforms of the progesterone receptors PR-A, PR-B. Both are polyclonal antibody used in dilution 1: 500 (Santa Cruz sc-7208, sc-538).
Cell treatment
Primary myometrial cells were cultured in 60-mm dishes at 30–40% confluence at an approximate density of 5 × 105 cells/dish at 370 C in a humidified atmosphere of 5% CO2 in the regular SmBM media. When the cells were reached at approximately 80% confluence, the cells were grown in serum-free medium for 24 h. Then the cells were treated with P4 (1.0 ng/mL) for 72 h.
Quantitative real-time PCR
RNA was isolated according to the protocol using RNeasy Mini Kit. Following RNA extraction, cDNA was made by Reverse-transcribing 1 μg of RNA using the (RNA to cDNA Eco Dry Premix (double Primed)). Aliquots of cDNA were made for each sample and stored at –20 °C until analyzed.
SYBR Green real-time PCR was performed as described previously [25]. Briefly, RNA expression of genes was detected using Sso Advanced Universal SYBR Green Supermix on a Bio-Rad CFX96 real-time PCR system. Data were analyzed using Bio-Rad CFX manager software. Each biological sample was run in triplicate for each individual experiment. All assays were carried out in 96-well format. Real-time fluorescent detection of PCR products was performed with the CF96X Real-Time PCR System (Bio-Rad) using the following thermocycling conditions: 1 cycle of 95 °C for 10 min; 40 cycles of 95 °C for 30 s, and 60 °C for 1 min. The primer sequences for qPCR were shown in Table 1 [26].
Statistical analysis
All values are expressed as means ± SE. Comparisons between two groups were done using the unpaired Student t-test. Differences between groups were examined by ANOV. Values of P< 0.05 were considered statistically significant.
Results
Subject characteristics
All the samples were taken from the Caucasian women with age ranges 33–48 years old (median 41.5). Subject characteristics is shown in Table 2 (n = 10).
Altered PR expression in MyoN and MyoF
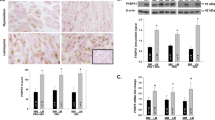
To determine if MyoN and MyoF exhibited differential gene expression pattern in response to P4 treatment, we first examined the expression of both PR-A, PR-B in MyoN and MyoF primary cells. Western blot (WB) analysis showed that the expression levels of PR-A were significantly higher in PrMyoF as compared to PrMyoN) (Fig. 1a). The similar result was achieved for the PR-B (Fig. 1b). We confirmed the result in other patients in our experiment (P < 0.05).
Western blot analysis of progesterone receptor A and B in MyoF and MyoN cells. Western blot analysis of proteins extracted from normal myometrium cells in normal uterus (MyoN) (n = 5) or myometrium of uterus with UFs (MyoF, n = 5) was performed. Total lysates from myometrial tissues were extracted and subjected to Western blot analysis using antibodies against Progesterone receptor A (a) and Progesterone receptor B respectively (b). β-actin was used as an endogenous control. A statistically significant increase in expression of PR-A was observed in PrMyoF cells as compared to PrMyoN cells (a, P < 0.05). b showed a statistically significant increase in expression of PR-B in PrMyoF compared to PrMyoN cells
Genes show gain of induction in response to progesterone (P4)
Previous studies have identified various progesterone target genes in endometriosis or during menstrual cycle [27,28,29]. In this study, we selected 15 UF-related genes and determined their differential expression between PrMyoF and PrMyoN cells in the presence or absence of the P4 (1.0 ng/ml) by qRT-PCR.
In MyoF primary cells, significant upregulation of five genes (Bcl2, FOXO1A, SCGB2A2, CYP26a1 and MMP11) was observed in response to P4 treatment (Fig. 2). As shown in Fig. 2a, although the FOXO1A gene showed no difference of RNA expression between MyoN and MyoF cells at basal levels, and no significant change was found in prMyoN cells in response to P4 treatment (P = 0.5), significant gain in induction was observed in prMyoF primary cells in response to P4 treatment (P < 0.05).
Gain of induction in response to P4. The expression levels of five genes including FOXO1A (a), Bcl2 (b), SCGB2A2 (c), MMP11 (d), CYP26a (e) were determined by qRT-PCR after treatment with P4 (1.0 ng/ml) for 3 days in PrMyoN and PrMyoF cells. (N = PrMyoN cells without treatment, Np = PrMyoN cells after treatment with P4, F = PrMyoF cells without treatment, Fp = PrMyoF cells after treatment with P4). These experiments were performed with 10 different cultured cell specimens. Five genes show either decrease or no change in response to P4 in MyoN cells. However, in PrMyoF cells, all the 5 genes including Foxo1A (a), Bcl2 (b), SCGB2A2 (c), MMP11 (d), CYP26a1(e), exhibited a significant upregulation in response to P4 treatment. * p < 0.05, **p < 0.01. ***p < 0.001
The basal levels of Bcl 2 gene expression between MyoN and MyoF primary cells did not reach significant difference (P = 0.7). However a significant increase of Bcl2 expression was observed in MyoF cells in response to P4 treatment (P < 0.01) (Fig. 2b), but not in MyoN cells. Similar finding was achieved for SCGB2A2 gene as MyoF cells exhibited gain in induction of SCGB2A2 gene expression (P < 0.05) but not MyoN cells in response to P4 treatment (Fig. 2c).
For MMP-11, a significant differential expression between MyoF and MyoN (p < 0.05) was observed. MyoN cells showed insignificant gain in induction in response to P4 treatment. However, MyoF cells exhibited a significant gain in induction after P4 treatment (P < 0.01) (Fig. 2d).
For CYP26a1 gene a significant gain in induction in MyoF was observed in response to P4(P = 0.05) (Fig. 2e). But gain in induction of CYP26a1 gene expression was not found in Pr MyoN cells .
Genes show gain of repression in response to progesterone (P4)
The other seven genes showed down regulation in MyoF cells in response to P4 treatment. Three of these genes are responsible for apoptosis and cell cycle. As shown in (Fig. 3a), the expression of CIDEC gene was significantly higher in PrMyoF as compared to PrMyoN cells (P < 0.05). In addition, P4 treatment resulted in a significant gain of repression in MyoF cell (P < 0.05), but not in MyoN cells. Also the gain of repression in respond to P4 was also found for CANP6 gene (Fig. 3b) (P < 0.05) and HHI gene (P < 0.05)(Fig. 3c) in MyoF cells, but not in MyoN cells. Comparing the basal levels of CANP6 and HHI expression between PrMyoN and PrMyoF, the expression of CANP6 gene exhibited no different but the expression of HHI gene exhibited statistically significant higher in PrMyoF cells as compared to PrMyoN cells (P < 0.001).
Gain of repression in response to P4. The expression of seven genes CIDEC (a), CANP6 (b), HHI (c), ADHL5 (d), ALDH1a1 (e), KIK6 (f), MT1E (g) was determined by q-RTPCR in Pr MyoN and PrMyoF cells after P4 treatment (1.0 ng/ml) for 3 days. N = PrMyoN cells without treatment, Np = PrMyoN cells after adding P4, F = PrMyoF cells without treatment, Fp = PrMyoF cells after adding P4. These experiments were performed with 10 different cultured cell specimens. * p < 0.05, ** p < 0.01. *** p < 0.001
The RNA expression of 2 genes (ADHL5, ALDH1A1) related to RA synthesis enzymes was also measured at basal levels as well as in P4-treated MyoN and MyoF cells. For ADHL5 gene (Fig. 3d), significant differential response to P4 was found between PrMyoN and PrMyoF cells. A significant gain in repression in PrMyoF cells after P4 treatment (P < 0.05), but not in PrMyoN cells. Similar finding was observed for ALDH1a1 expression in response to P4 in PrMyoF and PrMyoN cells (Fig. 3e).
The expression of gene KIK6 (Fig. 3f), which is the genet hat associated with regulation of inflammatory process, exhibited no difference PrMyoN and PrMyoF cells. And alteration of its expression was not observed after treatment with P4 in PrMyoN cells. However, a significant gain in repression after P4 treatment was seen in PrMyoF cells (P < 0.05).
Metallothionein (MT) family is responsible for binding to heavy metal ions and minimize reactive oxygen species. The response of several genes of MT family to P4 were examined (Figs. 3g and 4). MT1E (Fig. 3g) exhibited significant repression in its expression with P4 treatment in the PrMyoF cells (P < 0.05), but not in PrMyoN cells. However, no significant changes of other MT family genes including MT2A and MTG2 were observed between PrMyoN and PrMyoF cells at basal levels as well as in response to P4 treatment (Fig. 4a, b).
The expression of MT2A, MTG2 and Calcitonin genes in response to P4. The expression of three genes including MT2A (a), MTG2 (b) and calcitonin (c) in PrMyoN and PrMyoF cells was determined by real-time PCR after P4 treatment (1.0 ng/ml) for 3 days. N = PrMyoN cells without treatment, Np = PrMyoN cells after adding P4, F = PrMyoF cells without treatment, Fp = PrMyoF cells after adding P4. These experiments were performed with 10 different cultured cell specimens
The basal level of Calcitonin expression between PrMyoN and PrMyoF cells and their response to P4 was examined. There is no significant difference of RNA expression between MyoN verse MyoF cells. Furthermore, no significant difference of RNA expression in PrMyoN and PrMyoF cells in response to P4 treatment were found (Fig. 4c).
Discussion
P4 is a key hormone, which contributes to the UF pathogenesis. However, the molecular mechanism by which P4 promotes the UF development is largely unknown. In this study, we used PrMyoN and PrMyoN cell model system and characterized the expression pattern of P4 response genes in response to P4 treatment, which may contribute to increased risk of UF development.
Previous study showed that cultured UF cells had an increased response to P4 compared to cultured normal myometrial cells [30]. This study also showed that P4 receptor mRNA is highly expressed in UF cells as compared the cells from adjacent myometrium. In our study, we focused on P4 response in primary cells from normal myometrium (MyoN) and at-risk myometrium (MyoF). We demonstrated that the expression of PR was higher in PrMyoF as compared to PrMyoN cells. The differential response of PrMyoN and PrMyoF to P4 seems to be attractive. Among the genes we detected, we found two types of changes in response to P4 treatment, gain in induction and gain of repression respectively. These results suggested that the network of P4/PR signaling was varied between PrMyoN and PrMyoF and the primed PrMyoF turned out to be hyper-sensitive to P4, which might lead to increased risk of UF development.
In this study, the expression of 15 P4-responsive genes was examined in PrMyoF and PrMyoN cells using q-PCR analyses. Five of these genes including FOXO1A, CYP26a1, SCGB2A2, MMP11 and Bcl 2 showed significant up regulation in response to P4 treatment. The other seven genes exhibited a significant down regulation, these genes include CANP6, MT1E, ADHL5, Aldh1a1, KIK6, HHI, CIDEc. However, the expression of MT2A, MTG2 and calcitrone was not altered in response to P4 treatment.
Expression of genes control the apoptosis in response to progesterone
Apoptosis is a morphologic pattern of cell death [31]. There are multiple genes responsible for regulating this process. Korsmeye [32], reported that the Bcl-2 proto-oncogene has the ability to block apoptotic cell death in multiple contexts. Increase in expression of Bcl-2 in transgenic models will result in evasion of normal cell death mechanisms leading to accumulation of cells and tumor formation [33].
Previous studies showed that Bcl-2 protein expression was predominant in UF cells compared to that in normal myometrium cells [34]. The expression of Bcl-2 protein in normal myometrium cells was very low that raised the possibility that normal myometrium cells may be more susceptible to apoptotic cell death. In addition, UF cells exhibited increased expression of Bcl-2 protein in response to P4 treatment. But the expression of Bcl-2 protein in cultured normal myometrium cells was not affected by P4 treatment. Here in our study Bcl-2 gene expression in at-risk myometrium tissues from the uterus with UFs was remarkably augmented by P4 treatment and this change was not found in normal myometrial cells.
Another gene that responsible also for apoptosis is FOXO1A, it is a member of the FOXO subfamily of Forkhead transcription factors [35]. According to the previous study, activated FOXO proteins induced expression of genes that encode for proteins involved in cell cycle inhibition [36]. Our study showed that this gene exhibited hyper-response in PrMyoF cells after P4 treatment. Another study determined the progestin effect in FOXO1 expression and its activity in the endometrium during endometrium menstrual cycle. They showed that progestin enhanced FOXO1 mRNA levels in mid- and late-secretory endometrium [37]. In addition, FOXO1A was considered as a key transcription factor responsible for mediating apoptosis of decasualized human endometrial stromal cells (HESC) in response to progesterone withdrawal during the menstrual cycle by inducing the cell death. Moreover, this study explains the effect of admission of medroxyprogesterone acetate (MPA, a synthetic progestin) in enhancing the expression of FOXO1A in differentiating human endometrial cells. MP also simultaneously induced cytoplasmic retention and inactivation of this gene. Withdrawal of the MPA from decidualized HESCs resulted in rapid nuclear accumulation of FOXO1A gene, therefore leading to activation of apoptosis and cell death [37]. Similar finding was observed in PrMyoF cells, where the expression of FOXO1 was markedly increased in response to progesterone treatment, which provide a favorable condition for the pathogenesis of UFs.
SCGB2A2 (Secretoglobin family 2A member 2) was considered as uteroglobin-related protein, which controls cell cycle and DNA replication. It was originally detected by differential RNA expression levels in Breast Cancer biopsies [38]. Previous studies demonstrated the effect of SCGB overexpression on cell proliferation in other human diseases and ovarian carcinoma. The role of SCGB2A2 in patho-physiology of the ovarian tumor was identified [39]. The overexpression of SCGB2A2 is positively correlated with the FIGO stage, the tumor grade and the mitotic index of the ovarian cancer [40]. In our study, although no significant expression of SCGB2A2 was observed in ProMyoF and PrMyoN cells, ProMyoF cells was remarkably augmented by P4, which was not the same with ProMyoN cells. This study suggests that P4 might promote the UF development by increased cell proliferation and enhancement of the DNA replication via SCGB2A2.
The cell death-inducing DFF45-like effector (CIDE) family includes CIDEa, CIDEb, and CIDEc. It has been reported that the (CIDE) family plays an important role in lipid and fat metabolism [40,41,42]. Previous studies have reported that CIDEa, CIDEc were highly expressed in adipose tissue, and in skeletal muscle. ICIDEc is capable of inducing apoptosis in mammalian cells [43]. DFF45 is a subunit of the DNA fragmentation factor which is cleaved by active caspase-3 during apoptosis. The main function of CIDEC is energy homeostasis, and its absence may result in insulin resistance, and resistance to diet-induced obesity [44]. Here in our study this gene showed gain of repression in response to P4 as a marker of decrease in the apoptosis that might be involved in UF development.
Another gene that showed gain in regression in our study was CANP6. It is calcium-activated cysteine proteinases. Calpains have been involved in many biological events including regulation of the cell cycle, apoptosis, cell adhesion and motility [45, 46]. So the regression of this gene will decrease the apoptosis as well as down regulation of cell cycle all together will favor the development of UFs.
Expression of genes control the retinoic acid in response to progesterone
RA, is the natural metabolite of vitamin A. Previous studies showed that RA signaling played an important role in the female reproductive trace function [47]. ADH5 and ALDH1a1 are RA synthesis enzymes and CYP26a1(cytochrome P450, family 26, subfamily a, polypeptide1) is a RA catabolizing enzyme. Previous studies demonstrated that the expression of these are altered during preganyc which may be related to progesterone signaling. ADH5 expression was increased by 2.5 folds during pregnancy. The expression of ALDH1a1 in the endometrial glandular compartment was increased on gestational early days until the implantation phase. The expression of CYP26a1 was strongly detected in the uterine epithelium. Moreover, these studies indicated that early pregnancy needed the synthesis and degradation of RA to be balanced to allow RA signaling to prepare for implantation without harmful effects on the embryo [48]. Our result has demonstrated that RA synthesis genes (ADH5, ALDH1a1) show gain in repression in response to P4, and RA catabolic enzyme (CYP26a1) were rapidly gain induction by the P4-PR axis. This might result in increasing retinoic acid catabolism and decrease in Vitamin A in the myometrium tissue. All this together will favorable the proliferation of the myometrium, which provide pro-fibroid condition to increase the risk of UF development.
Expression of other genes in response to progesterone
In human, over 20 functional Matrix metalloproteinases MMPs have been identified [48]. MMPs are zinc endopeptidases capable of releasing the growth factors that are bound to the extracellular matrix (ECM) [49] regulating cell-matrix and cell-cell interactions. Matrix metalloproteinase 11(MMP11) is responsible of serpins cleavage and so it stimulate the development of tumor [50, 51]. Our study showed that MMP-11 mRNA was significantly increased in myometrium cells from uterus with UFs compared with myometrium cells of normal one. Also gene expression of MMP11 showed significant gain in induction after P4 treatment in PrMyoF cells. Previous study demonstrated increased expression of MMP-11 in UFs as compared to myometrium.
KLK6 belonging to kallikreins gene family is a serine protease [52,53,54]. It is down-regulated by the P4-PR axis. KLk6 is responsible for regulation of the inflammatory process and vascular permeability, and edema [55]. Previous studies in mouse graved uterus showed that this gene was upregulated by the P4-PR axis signaling suggesting the important role of this gene in the implantation of the embryo in the uterus [27]. In our study, the repression of this gene in response to P4 may result in the formation of the UFs by losing the regulation of the inflammatory process.
Indian hedgehog (HHI), one of the Hedgehog family of ligands, is a P4-regulated gene in the uterus [56, 57]. It plays a role in down regulation of cellular division [58]. In the human endometrium, the role of Hedgehog signaling in UFs is largely unknown [59]. HHI gene shows a significant decrease in expression s between the early secretory to the mid secretory phase. It also plays a role in embryo implantation by regulation of stromal cell proliferation and inhibition of epithelial E2 signaling. In addition, Hedgehog signaling was involved in the women with endometriosis [60,61,62]. and in endometrial cancer [63]. Here in our study this gene showed a gain of repression in the PrMyoF cells in response to P4, suggesting that this change may be involved in the increased risk of UF pathogenesis.
Metallothioneins (MTs) comprise a family of genes clustered on chromosome 16q that bind to heavy metal ions and minimize reactive oxygen species. Previous studies demonstrated low MT expression in endometrium of women with endometriosis [29]. In our study MT1E is the only one we detected that showed significant repression with P4 treatment in the PrMyoF cells.
The location of the Calcitonin gene is in non-neuronal tissues. Its define function remains unclear, but previous studies identified their role in cardiovascular system as it exhibited a potent vasodilator effect [64]. There are many other researches done on calcitonin effect on the heart. These researchers show its role in prevention of ischemia as well as endotoxic shock [65]. These shock can be done by the suppressor effect of calcitonin on some pro-inflammatory cytokines production [e.g., macrophage inflammatory protein-2 (MIP-2) and keratinocyte chemoattractant (KC)] [66, 67]. Moreover, calcitonin has a protective effect against ischemia [68]. So the decrease in its expression may result in ischemia stimulation of the inflammatory reaction. However, in our study it showed gain in repression suggesting the complex role of this gene in response to P4 treatment.
Conclusion
Our studies demonstrate for the first time that PrMyo cells and PrMyoN from either at risk myometrium or normal myometrium exhibit a differential response to P4, the key hormone for UF development. P4-responsive genes in PrMyoF cells exhibit a P4-hyper-responsiveness, suggesting that myometrium from uterus with UFs is primed and become at risk for later tumorigenic transformation. However, due to sample size (n = 10) and race limitation (all from Caucasian), further investigating the role of P4 in alteration of normal myometrium in a large sample size as well as using tissues from at high risk populations such as African American are needed. Moreover, evaluating of P4 response in relevant animal model or 3D system is also highly needed for understanding the pathogenesis of UFs.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DFF:
-
DNA fragmentation factor
- ECM:
-
Extracellular matrix
- FBS:
-
Fetal bovine serum
- GnRH agonists:
-
Gonadotropin releasing hormone agonist
- HESC:
-
Human endometrial stromal cells
- KC:
-
Keratinocyte chemoattractant
- LNG IUS:
-
Levo –norgestrine intra-uterine device
- MMSC:
-
Myometrial stem cell
- MPA:
-
Medroxyprogesterone acetate
- MyoF:
-
Human myometrial cells from uteri with fibroid
- MyoN:
-
Human myometrial cells from normal uteri
- P4:
-
Progesterone
- PR:
-
Progesterone receptor
- PRGs:
-
Progesterone-regulated genes
- PrMyoF:
-
Primary human myometrial cells from uterus with fibroids
- PrMyoN:
-
Primary human myometrial cells from normal uterus
- qPCR:
-
Quantitative real-time pole maries chain reaction
- RA:
-
Retinoic acid
- SmBM:
-
Smooth muscle cell basal medium
- SPRM:
-
Selective progesterone receptor modulator
- UFs:
-
Uterine Fibroids
- WB:
-
Western blot
References
Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94:435–8.
Julie Kim J, Sefton EC. The role of progesterone signaling in the pathogenesis of uterine leiomyoma. Mol Cell Endocrinol. 2012;358:223–31.
Cermik D, Arici A, Taylor HS. Coordinated regulation of HOX gene expression in myometrium and uterine leiomyoma. Fertil Steril. 2002;78:979–84.
Marx J. Cell death studies yield cancer clues. Science. 1993;259:760–2.
Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151:2433–42.
Carr BR, Marshburn PB, Weatherall PT, Bradshaw KD, Breslau NA, Byrd W, Roark M, Steinkampf MP. An evaluation of the effect of gonadotropinreleasing hormone analogs and medroxyprogesterone acetate on uterine leiomyomata volume by magnetic resonance imaging: a prospective, randomized, double blind, placebo controlled, crossover trial. J Clin Endocrinol Metab. 1993;76:1217–23.
Friedman AJ, Daly M, Juneau-Norcross M, Rein MS, Fine C, Gleason R, Leboff M. A prospective, randomized trial of gonadotropin-releasing hormone agonist plus estrogen-progestin or progestin “add-back” regimens for women with leiomyomata uteri. J Clin Endocrinol Metab. 1993;76:1439–45.
Friedman AJ, Daly M, Juneau-Norcross M, Gleason R, Rein MS, LeBoff M. Long-term medical therapy for leiomyomata uteri: a prospective, randomized study of leuprolide acetate depot plus either oestrogen-progestin or progestin ‘add-back’ for 2 years. Hum Reprod. 1994;9:1618–25.
Bagaria M, Suneja A, Vaid NB, Guleria K, Mishra K. Low-dose mifepristone in treatment of uterine leiomyoma: a randomised double-blind placebo-controlled clinical trial. Aust N Z J Obstet Gynecol. 2009;49:77–83.
Engman M, Granberg S, Williams AR, Meng CX, Lalitkumar PG, Gemzell- Danielsson K. Mifepristone for treatment of uterine leiomyoma. A prospective randomized placebo controlled trial. Hum Reprod. 2009;24:1870–9.
Spitz IM. Clinical utility of progesterone receptor modulators and their effect on the endometrium. Curr Opin Obstet Gynecol. 2009;21:318–24.
Misrahi M, Atger M, d’Auriol L, Loosfelt H, Meriel C, Fridlansky F, Guiochon-Mantel A, Galibert F, Milgrom E. Complete amino acid sequence of the human progesterone receptor deduced from cloned cDNA. Biochem Biophys Res Commun. 1987;143:740–8.
Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9.
Robinson-Rechavi M, Escriva Garcia H, Laudet V. The nuclear receptor superfamily. J Cell Sci. 2003;116:585–6.
Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms a and B. EMBO J. 1990;9:1603–14.
Samalecos A, Gellersen B. Systematic expression analysis and antibody screening do not support the existence of naturally occurring progesterone receptor (PR)-C, PR-M, or other truncated PR isoforms. Endocrinology. 2008;149:5872–87.
Gellersen B, Fernandes MS, Brosens JJ. Non-genomic progesterone actions in female reproduction. Hum Reprod Update. 2009;15:119–38.
Cook JD, Davis BJ, Cai SL, Barrett JC, Conti CJ, Walker CL. Interaction between genetic susceptibility and early-life environmental exposure determines tumor-suppressor-gene penetrance. Proc Natl Acad Sci U S A. 2005;102(24):8644–9.
Greathouse KL1, Cook JD, Lin K, Davis BJ, Berry TD, Bredfeldt TG, Walker CL. Identification of uterine leiomyoma genes developmentally reprogrammed by neonatal exposure to diethylstilbestrol. Reprod Sci 2008;15(8):765–778. https://doi.org/10.1177/1933719108322440.
Mas A, Stone L, O'Connor PM, Yang Q, Kleven D, Simon C, Walker CL, Al-Hendy A. Developmental exposure to endocrine disruptors expands murine myometrial stem cell compartment as a prerequisite to leiomyoma tumorigenesis. Stem Cells. 2017;35(3):666–78. https://doi.org/10.1002/stem.2519 Epub 2016 Nov 11.
Lauren E, Fernung P, Kimya Jones Y, Aymara Mas Z, Daniel Kleven Y, Jennifer L, Waller X, Al-Hendy A. Expanding upon the Human Myometrial Stem Cell Hypothesis and the Role of Race, Hormones., Age, and Parity in a Profibroid Environment. Am J Pathol. 2018;188:10.
Halder SK, Osteen KG, Al-Hendy A. Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and -9 in human uterine fibroid cells. Hum Reprod 2013; 28(9):2407–2416.
Ali M, Shahin SM, Sabri NA, Al-Hendy A, Yang Q. Hypovitaminosis D exacerbates the DNA damage load in human uterine fibroids, which is ameliorated by vitamin D3 treatment. Acta Pharmacol Sin. 2018. https://doi.org/10.1038/s41401-018-0184-6.
Oatley MJ, Kaucher AV, Yang QE, Waqas MS, Oatley JM. Conditions for Long-Term Culture of Cattle Undifferentiated Spermatogonia. Biol Reprod. 2016;95(1):14. https://doi.org/10.1095/biolreprod.116.139832.
Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36.
Jeong JW, et al. Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Genes Endocrinology, August. 2005;146(8):3490–505.
shimomura Y, et al. Up-regulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. J Clin Endocrinol Metab. 1998;83:6.
Burney et al. Progesterone resistance in endometriosis endocrinology, August 2007, 148(8):3814–3826.
Brandon DD, Bethea CL, Strawn EY, et al. Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am J Obstet Gynecol. 1993;169:78–85.
Kerr JER, Wylie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57.
Korsmyer SJ. Bcl-2 initiates a new categoly of oncogenes: regulators of cell death. Blood. 1992;80:879–86.
Mcdonnell TJ, Deane N, Platt FM, et al. BCL-2 immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88.
Matsuo M, Maruo T, Samoto T. Increased expression of Bcl-2 protein in human uterine leiomyoma and its up-regulation by progesterone. J Clin Endocrinol Metab. 1997;82:293–9.
Christian M, Zhang X, Schneider-Merck T, Unterman TG, Gellersen B, White JO, Brosens JJ. Cyclic AMPinduced forkhead transcription factor, FKHR, cooperates with CCAAT/enhancer-binding protein in differentiating human endometrial stromal cells. J Biol Chem. 2002;277:20825–32.
Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cyclecycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–7.
Labied et al. • Progestins regulate the expression and activity of the Forkhead transcription factor FOXO1 inmDifferentiating human endometrium. Mol Endocrinol, January. 2006;20(1):35–44.
Watson MA, Fleming TP. Isolation of differentially expressed sequence tags from human breast cancer. Cancer Res. 1994;54:4598–602.
Fischer K, von Brünneck AC, Hornung D, et al. Differential expression of secretoglobins in normal ovary and in ovarian carcinoma--overexpression of mammaglobin-1 is linked to tumor progression. Arch Biochem Biophys. 2014;547:27–36.
Gong J, Sun Z, Li P. CIDE proteins and metabolic disorders. Curr Opin Lipidol. 2009;20(2):121–6.
Yonezawa T, Kurata R, Kimura M, et al. Which CIDE are you on? Apoptosis and energy metabolism. Mol BioSyst. 2011;7(1):91–100.
Zhou Z, Yon Toh S, Chen Z, et al. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat Genet. 2003;35(1):49–56.
Inohara N, Koseki T, Chen S, Wu X, Nunez G. CIDE, a novel family of cell death activators with homology to the 45 kDa subunit of the DNA fragmentation factor. EMBO J. 1998;17:2526–33.
Joseph et al. Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor b-3 TGF-b3 . Fertility and Sterility_2009 march; Vol. 93, No. 5.
Burgess HA, Reiner O. Cleavage of doublecortin-like kinase by calpain releases an active kinase fragment from a microtubule anchorage domain. J Biol Chem. 2001;276:36397–403.
Carafoli E, Molinari M. Calpain: a protease in search of a function? Biochem Biophys Res Commun. 1998;247:193–203.
Ponnamperuma RM, Kirchhof SM, Trifiletti L, De Luca LM. Ovariectomy increases squamous metaplasia of the uterine horns and survival of SENCAR mice fed a vitamin A-deficient diet. Am J Clin Nutr. 1999;70:502–8.
Murphy G, Nagase H. Progress in matrix metalloproteinase research. Mol Asp Med. 2008;29:290–308.
Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73.
Pei D, Majmudar G, Weiss SJ. Hydrolytic inactivation of a breast carcinoma cell–derived serpin by human stromelysin-3. J Biol Chem. 1994;269:25849–55.
Matziari M, Dive V, Yiotakis A. Matrix metalloproteinase 11 (MMP- 11; stromelysin-3) and synthetic inhibitors. Med Res Rev. 2007;27:528–52.
Yousef GM, Diamandis EP. An overview of the kallikrein gene families in humans and other species: emerging candidate tumour markerssmall star, filled. Clin Biochem. 2003;36:443–52.
Yousef GM, Diamandis EP. Expanded human tissue kallikrein family–a novel panel of cancer biomarkers. Tumour Biol. 2002;23:185–92.
Yousef GM, Diamandis EP. Human tissue kallikreins: a new enzymatic cascade pathway? Biol Chem. 2002;383:1045–57.
Bhoola KD, Figueroa CD, Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992;44:1–80.
Takamoto N, Zhao B, Tsai SY, DeMayo FJ. Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol. 2002;16:2338–48.
Matsumoto H, Zhao X, Das SK, Hogan BL, Dey SK. Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev Biol. 2002;245:280–90.
Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–121.
Garcia N, Bozzini N, et al. May sonic hedgehog proteins be markers for malignancy in uterine smooth muscle tumors? Hum Pathol. 2016;50:43–50. https://doi.org/10.1016/j.humpath.2015.08.026.
Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Julie Kim J. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248:94–103.
Ito K, Utsunomiya H, Yaegashi N, Sasano H. Biological roles of estrogen and progesterone in human endometrial carcinoma–new developments in potential endocrine therapy for endometrial cancer. Endocr J. 2007;54:667–79.
Rose PG. Endometrial carcinoma. N Engl J Med. 1996;335:640–9.
Feng YZ, Shiozawa T, Miyamoto T, Kashima H, Kurai M, Suzuki A, Ying-Song J, Konishi I. Overexpression of hedgehog signaling molecules and its involvement in the proliferation of endometrial carcinoma cells. Clin Cancer Res. 2007;13:1389–98.
Russell FA, King R, Smillie S-J, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94(4):1099–142.
Zhao FP, Guo Z, Wang PF. Calcitonin gene related peptide (CGRP) inhibits norepinephrine induced apoptosis in cultured rat cardiomyocytes not via PKA or PKC pathways. Neurosci Lett. 2010;482:163–6.
Gomes RN, Castro-Faria-Neto HC, Bozza PT, Soares MB, Shoemaker CB, David JR, Bozza MT. Calcitonin gene-related peptide inhibits local acute inflammation and protects mice against lethal endotoxemia. Shock. 2005;24:590–4.
Wang X, Ebong SJ, Call DR, Newcomb DE, Bolgos GR, Remick DG. Calcitonin gene-related peptide partially reverses decreased production of chemokines KC and MIP-2 following murine sepsis. Inflammation. 2002;26:167–74.
Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–34.
Brzozowski T, Konturek PC, Konturek SJ, et al. Ischemic preconditioning of remote organs attenuates gastric ischemia-reperfusion injury through involvement of prostaglandins and sensory nerves. Eur J Pharmacol. 2004;499:201–13.
Acknowledgements
We would like to thank Dr. Lauren Prusinski Fernung for her help in collecting human tissues and primary cell isolation.
Funding
National Institutes of Health grants: U54 MD007602 (AA) and R01 ES 028615 (AA), as well as the Augusta University Intramural Grants Program (QY).
Author information
Authors and Affiliations
Contributions
AA and QY designed the research. MO, AK performed the experiments and analyzed the data. MO wrote the manuscript. QY edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by Augusta University’s Institutional Review Board. Myometrium were obtained from Caucasian women who underwent abdominal hysterectomy. Informed written consent was obtained from each patient before surgery for the use of extirpated uterine tissues for myometrial cell isolation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Omar, M., Laknaur, A., Al-Hendy, A. et al. Myometrial progesterone hyper-responsiveness associated with increased risk of human uterine fibroids. BMC Women's Health 19, 92 (2019). https://doi.org/10.1186/s12905-019-0795-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-019-0795-1