Abstract
Background
To investigate the value of using contrast-enhanced transrectal ultrasound (CETRUS) to reduce unnecessary collection of biopsies during prostate cancer diagnosis and its utility in predicting biochemical recurrence in patients with localized prostate cancer.
Methods
This was a prospective study of suspected prostate cancer patients who were evaluated with CETRUS followed by a prostate biopsy. Prostate blood flow via CETRUS was graded using a 5-point scale. The relationship between CETRUS score and biopsy outcome was then analyzed for all patients; univariate and multi-variate analyses were used to determine the probable prognostic factors for biochemical recurrence in patients with localized prostate cancer that underwent a radical prostatectomy.
Results
A total of 347 patients were enrolled in the study. Prostate cancer was found in 164 patients. A significant positive correlation (r = 0.69, p < 0.001) was found between CETRUS scores and prostate cancer incidence. Using CETRUS scores ≥2 as the threshold for when to biopsy could have safely reduced the number of biopsies taken overall by 12.1% (42/347) and spared 23.0% (42/183) of patients from undergoing an unnecessary biopsy. 77 patients with localized prostate cancer underwent a radical prostatectomy. The median follow-up time was 30 months (range: 8–56 months) and 17 of these 77 patients exhibited biochemical recurrence during the follow-up period. 3-year biochemical recurrence-free survival rates were 86% for patients with low CETRUS scores (≤ 3) and 59% for patients with high scores (> 3; p = 0.015). Multivariate Cox regression analysis indicated that CETRUS score was an independent predictor of biochemical recurrence (HR: 7.02; 95% CI: 2.00–24.69; p = 0.002).
Conclusions
CETRUS scores may be a useful tool for reducing the collection unnecessary biopsy samples during prostate cancer diagnosis and are predictive of biochemical recurrence in patients with localized prostate cancer following a radical prostatectomy.
Similar content being viewed by others
Background
Prostate cancer is the most common solid neoplasm and the second leading cause of cancer death in men in the US [1]. Both incidence and mortality are increasing, with an estimated 60,300 new cases diagnosed and 26,600 prostate cancer-related deaths in China in 2015 [2]. Approximately one million prostate biopsies are conducted per year in the US. However, prostate-specific antigen (PSA) testing, the most widely used screening test for prostate cancer, leads to 750,000 unnecessary biopsies—and accompanying pain, inconvenience, financial burden, and risk of infection [3]. Therefore, one way to reduce the use of PSA testing and shift the ratio of benefit to harm in favor of patient benefit would be to improve alternate methods with predictive value and thereby reduce unnecessary biopsy collection [4].
About 15–40% of patients with localized prostate cancer will suffer recurrence following a radical prostatectomy [5]. Traditional clinicopathologic risk factors, such as serum PSA levels, pathological grading (Gleason score), and TNM staging are used to predict the probability of recurrence in prostate cancer patients, but these tools are not accurate for every patient [6]. Therefore, new tools are needed to complement the prognostic value of traditional risk factors. Such tools may help guide individual therapeutic management, improve patient counseling, and help optimize the design of clinical trials.
Previous studies have shown that contrast-enhanced transrectal ultrasound (CETRUS)-based blood flow grading is a reliable tool for predicting pathological outcomes of prostate-related diseases [7,8,9,10,11]. The diagnostic accuracy of CETRUS has continually improved with the development of new ultrasound contrast agents and equipment [12]. Although CETRUS is a very promising imaging tool, it is still not widely used to help diagnose or evaluate prostate cancer. In this study, we investigated whether CETRUS can reduce unnecessary biopsy collection during prostate cancer diagnosis. We also investigated whether CETRUS can predict biochemical recurrence following a radical prostatectomy in patients with localized prostate cancer.
Methods
Patient selection
Patients referred for a prostate biopsy based on elevated total serum PSA levels (> 4 ng/mL) or abnormal digital rectal examination (DRE) between Feb 2014 and Sep 2018 at our collaborator institutions (Yantai Yuhuangding Hospital, First Affiliated Hospital of Sun Yat-sen University and Cancer Center of Sun Yat-sen University) were included in the study. The exclusion criteria were as follows: (1) age > 80 years; (2) refusal of CETRUS screening; (3) patient had received a previous prostate biopsy; (4) patient had a severe cardiopulmonary disease. The study was approved by the ethics committee of the institutions involved. Written informed consent to participate in the study and for the publication of their individual data was obtained from each participant enrolled in the study.
Procedures
CETRUS was performed for each patient before prostate biopsy collection. The ultrasound equipment used for CETRUS was an IU 22 system (Philips, Holland), and the contrast agent used was SonoVue (Bracco, Milan, Italy). Conventional and contrast-enhanced transrectal ultrasound examinations were performed by sonographers that had more than 5 years of experience in conducting contrast-enhanced ultrasounds; these sonographers were not involved in the analysis of ultrasound images. All patients were examined in the left lateral position. A bolus of SonoVue (2.4 mL) was injected intravenously, followed by a 5 mL saline flush to ensure no residual contrast agent remained in the intravenous catheter. A series of axial sweeps through the gland were obtained from base to apex, with each sweep having a duration of 20–30 s [13]. This process was continuously observed for at least 3 min. Repeated injections (2.4 mL) were performed in 16 patients when initial results were unsatisfactory. All examinations at a given center were performed by the same sonographers. All CETRUS images were simultaneously examined by two sonographers. All sonographers involved were blinded to the patients’ clinical presentations and other imaging results.
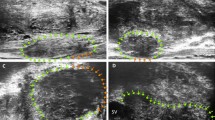
Diagnostic confidence was scored on a five-point scale based on blood flow, according to previously published reports: [8, 13] score 1, definitely benign, minimal enhancement (capsular and periurethral flow only); score 2, probably benign, mild enhancement (symmetric radial flow from capsular branches); score 3, indeterminate, mildly increased enhancement (asymmetric/increased flow in the prostate); score 4, probably malignant, moderately increased enhancement (asymmetric/increased flow in the prostate); score 5, definitely malignant, substantially increased enhancement (asymmetric/increased flow in the prostate) (Additional Fig. 1).
After CETRUS examination, a systematic 12-core prostate biopsy was performed. The systematic biopsy strategy consisted of collection of 6 standard cores and an additional 3 cores positioned more laterally on each side. Each biopsy sample was reviewed by a urological pathologist and reported individually. Tumors were classified according to the 2002 TNM staging system and graded according to the Gleason grading system. High-grade cancer was defined as a Gleason score 8 or higher.
Surgical management and follow-up
Patients with clinical stage T1 and T2 prostate cancer underwent a laparoscopic radical prostatectomy. Patients with pathologically diagnosed localized prostate cancer (T1/T2N0M0) were enrolled and followed up prospectively. Data collected from each patient included age at diagnosis, CETRUS score, tumor stage, Gleason score, body-mass index (BMI), Eastern Cooperative Oncology Group performance status (ECOG PS), and biochemical recurrence-free survival time. Postoperatively, patients were examined to obtain serum PSA measurements every 3 months for the first year following surgery and semi-annually from the second through the fifth years. Biochemical recurrence was defined as a PSA level ≥ 0.2 ng/mL and rising, and recurrence date was assigned to the first instance of a PSA value ≥0.2 ng/mL [6]. No patient received adjuvant therapy prior to development of biochemical recurrence. Biochemical recurrence-free survival was calculated from the date of surgery to the date of biochemical recurrence and was concluded at the date of death from other causes or the date of the last follow-up visit.
Statistical analysis
The correlation between CETRUS blood flow grades and biopsy outcomes were analyzed using a Spearman correlation test. Two groups were compared using the chi-square test for categorical variables. Receiver operating characteristic (ROC) curves and area under the curve (AUC) determinations were employed to assess the diagnostic accuracy of CETRUS. Survival curves were estimated using the Kaplan-Meier method. The univariate Cox proportional regression hazard model was used to analyze the correlation between variables and clinical outcomes. Multivariate survival analysis was performed on all parameters that were significant by univariate analysis using the Cox regression model. The predictive accuracy of prognostic factors was determined using time-dependent ROC analysis, and the AUC at 3 years post-surgery were used to measure predictive accuracy. R software version 2.7.1 (R Foundation for Statistical Computing, Vienna, Austria) was used for time-dependent ROC analysis. SPSS software (SPSS Standard version 16.0; SPSS Inc., Chicago, IL, USA) was used for all other calculations. Statistical significance was indicated by p < 0.05.
Results
Patient characteristics
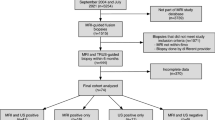
A total of 379 consecutive patients suspected of prostate cancer were referred for a prostate biopsy. Thirty-two patients were excluded. 347 patients were enrolled into the study and evaluated with CETRUS followed by collection of prostate biopsy samples. No adverse events related to the contrast agent were observed in any of the 347 patients. The mean patient age was 68.5 years (range: 53–79), mean prostate volume was 52.8 mL3 (range: 18–136), and median the PSA level was 8.9 ng/mL (range: 1.9–382). The number of men with a PSA level < 4, 4–10, 10–20 and > 20 were 28, 160, 116, and 43, respectively. Prostate cancer was found in a total of 164 of 347 (47.3%) patients. The proportion of patients with Gleason scores ≤6, =7 and ≥ 8 were 22.6% (37), 43.3% (76), and 31.1% (51), respectively. Among the 164 patients, 91 patients underwent a laparoscopic radical prostatectomy. Seventeen patients were excluded. Finally, 74 patients with localized prostate cancer were enrolled and followed up as shown in Fig. 1. Of the patients treated with a radical prostatectomy, the proportion of patients with Gleason scores ≤6, =7 and ≥ 8 were 24.7% (19), 41.6% (32), and 33.7% (26), respectively.
CETRUS is a useful tool for reducing the performance of unnecessary biopsies
The percentage of prostate cancer patients in CETRUS score groups 1–5 were 0% (0/42), 11.5% (6/52), 32.5% (40/123), 87.1% (61/70), and 95.0% (57/60), respectively. A significant positive correlation (r = 0.69, p < 0.001) was found between CETRUS scores and prostate cancer incidence. When cut off at 4 (≤ 3 as BPH and ≥ 4 as prostate cancer), the CETRUS score had an accuracy of 83.3% (289/347) in correctly diagnosing prostate cancer, with a sensitivity and specificity of 90.8% (118/130) and 78.8% (171/217), respectively. ROC analysis showed the AUC of the CETRUS score was 0.89 (95% CI: 0.85–0.92), indicating that patient CETRUS scores can be used to differentiate prostate cancer from benign prostatic hyperplasia (BPH) (Additional Table 1, Fig. 2). Moreover, when cut off at 2 (1 = BPH and ≥ 2 as prostate cancer), CETRUS scores demonstrated a specificity of 100% for differentiating prostate cancer from BPH, indicating that application of CETRUS using a cut-off score of 2 might be an effective tool for reducing the performance of unnecessary prostate biopsies. In this study, performing biopsy for cases with a CETRUS score ≥ 2 could have reduced the number of biopsies by 12.1% (42/347) without missing any cancer diagnoses and spared 23.0% (42/183) of men from undergoing an unnecessary biopsy (Table 1).
CETRUS can predict biochemical recurrence following a radical prostatectomy in patients with localized prostate cancer
The clinicopathologic characteristics of the 77 patients diagnosed with localized prostate cancer treated with a radical prostatectomy are summarized in Table 2. CETRUS score results were categorized in low score (≤ 3) and high score (> 3) groups. No significant correlation was found between CETRUS score and patient age, tumor stage, Gleason score, PSA level, BMI, or ECOG PS (p > 0.05, Table 2).
The median age of the 77 patients was 65.1 years (range: 49–74 years), and the median follow-up time after surgery was 30 months (range: 8–56 months). Biochemical recurrence was observed in 22% (17/77) of patients during the follow-up period. The 3-year biochemical recurrence-free survival rates were 86% (95% CI: 73–93%) for patients with low CETRUS scores and 59% (95% CI: 53–67%) for patients with high CETRUS scores (Fig. 3). Univariate Cox regression analysis revealed that CETRUS score, clinical stage, Gleason score, PSA level, and ECOG PS were significantly correlated with biochemical recurrence-free survival (p = 0.015, 0.042, 0.011, 0.037 and 0.047, respectively, Table 3), while other clinicopathologic variables, including age and BMI, were not (p = 0.618, 0.205, respectively, Table 3). Using multivariate analysis to further examine the parameters identified as significant by univariate analysis, we determined that patient CETRUS score at the time of diagnosis was an independent predictor of biochemical recurrence (HR: 7.02; 95% CI: 2.00–24.69; p = 0.002; Table 3).
To develop a more accurate prognostic tool, we used Cox proportional hazards regression to construct a prognostic model combining CETRUS scores with other clinicopathologic risk factors. A time-dependent ROC curve was used to compare the predictive accuracy of the combined model with the predictive accuracy of CETRUS scores alone or individual clinicopathologic factors alone. As shown in Fig. 4, the model combining patient CETRUS scores, Gleason scores, tumor stage, and PSA levels (AUC at 3 years: 0.886; 95% CI: 0.754–1.000) had a better prognostic value than relying on CETRUS scores alone (AUC at 3 years: 0.696; 95% CI: 0.520–0.890; p = 0.006), Gleason scores alone (AUC at 3 years: 0.679; 95% CI: 0.554–0.811; p = 0.018), tumor stage alone (AUC at 3 years: 0.620; 95% CI: 0.500–0.748; p < 0.001), or PSA levels alone (AUC at 3 years: 0.611; 95% CI: 0.500–0.792; p = 0.004). The Memorial Sloan Kettering Cancer Center (MSKCC) nomogram is currently the most commonly used predictor of biochemical recurrence and survival for prostate cancer. Therefore, we compared pre-radical prostatectomy CETRUS scores (0.696) and MSKCC nomograms (0.709) for 3-year AUC and found there was no significant difference between the two methods (Fig. 5). Therefore, CETRUS scores may add prognostic value to the clinicopathologic risk factors currently used in the context of localized prostate cancer.
Discussion
Our study shows that using CETRUS as a supplement to PSA-based diagnosis can reduce the performance of unnecessary prostate biopsies. We also found that CETRUS scores can independently predict biochemical recurrence of localized prostate cancer following a radical prostatectomy.
PSA testing is not limited to diagnosing prostate cancer. Other conditions, such as benign prostatic hyperplasia and prostatitis, also present with increased PSA levels. Therefore, it is necessary to design screening programs that maximize benefits and minimize morbidity and costs [14]. Angiogenesis, or the process of new blood vessel formation, is necessary for cancer progression. When compared with healthy tissue, the site of prostate cancer is generally characterized by an increased number of newly created blood vessels [15, 16]. However, most of these vessels have small diameters and may not have sufficient flow to be detected by a conventional Doppler transrectal ultrasound. The development of ultrasound contrast agents and contrast-specific imaging techniques have facilitated introduction of CETRUS, a novel imaging modality for continuous visualization of neovascularity associated with prostate cancer [17]. Prostate cancer tissue is often characterized by increased microvessel density due to the proliferation of neovessels. In addition, the microvascular blood supply to prostate tissue is more uniform in malignant tissue than in benign tissue [18].
Multiparametric magnetic resonance imaging (mpMRI) is still the best method for diagnosing prostate cancer. The sensitivity of an MRI guided direct biopsy is as high as 89% of prostate cancer in clinical significance [19]. However, especially in developing countries, this method cannot be widely utilized in many clinics, so the relatively portable B-ultrasound has become another popular tool for diagnosis. Microbubble ultrasound contrast agents allow for the detection of microvessels below the resolution of a conventional transrectal ultrasound. Sedelaar et al. concluded in 2001 that CETRUS has the potential to reveal malignant prostate lesions with increased microvessel density, after finding that enhanced areas on CETRUS had a microvessel density 1.93 times higher than non-enhanced areas [20]. Ultrasound is a real-time, easily accessible, cost-effective, and non-invasive imaging modality. In addition, because ultrasound contrast agents have a low incidence of side effects and are not nephrotoxic, CETRUS can be used in patients with iodine allergies, impaired renal function, or other contraindications that may make them unsuitable for contrast-enhanced computed tomography or MRI [21]. Several studies have shown that the CETRUS-based blood flow grades significantly correlate with the histopathological outcome of biopsy samples. Thus, CETRUS can improve prostate cancer diagnosis, and performance of prostate biopsies based on CETRUS scores represents an innovative approach to detecting significant disease with fewer biopsy cores [8, 9, 22, 23]. With the development of new ultrasound contrast agents and equipment, the diagnostic accuracy of CETRUS is increasing, which will be helpful in eliminating unnecessary biopsy collection in patients without cancer [24]. However, at present, there is limited knowledge of the impact of CETRUS in reducing unnecessary biopsy collection in patients without prostate cancer. In the present study, a second generation contrast agent and low mechanical index were used to improve the survival of microbubbles in the circulation and facilitate real-time depiction of both macrocirculation and microcirculation. In addition, a low mechanical index was used to prolong the time of parenchymal enhancement [25]. The present study showed a strong correlation between CETRUS blood flow grade scores and histopathological findings. Asymmetrical, substantially increased enhancement of the prostate observed during CETRUS was more likely indicate the presence of malignant growth. On the contrary, minimal enhancement with only capsular and periurethral flow was more likely to be benign growth. We found that combining CETRUS scores with additional information besides PSA levels can help predict the result of biopsies in men with elevated PSA. Deciding to perform biopsies on the basis of a CETRUS score ≥ 2 could have reduced the number of biopsies by 12% without missing cancer and spared 23% of men from undergoing an unnecessary biopsy.
Traditional clinicopathologic risk factors are inadequate for accurately predicting the prognosis of patients with localized prostate cancer following a radical prostatectomy. Therefore, additional tools for predicting prostate cancer recurrence may help to identify high-risk patients who might benefit from early intervention. Previous studies have shown that angiogenesis correlates with tumor recurrence following a radical prostatectomy [26,27,28]. Contrast-enhanced ultrasonography, which is useful for assessing microvessel density, has also been reported to have potential value in predicting the outcome of patients with prostate cancer [29, 30]. Our study demonstrated that CETRUS scores may be a reliable predictor of biochemical recurrence following a radical prostatectomy in patients with localized prostate cancer. CETRUS score successfully categorized patients into high-risk and low-risk subgroups with a significant difference in 3-year biochemical recurrence-free survival. Furthermore, the combination of CETRUS score with others clinicopathologic risk factors had a better prognostic value than CETRUS scores alone or any of the clinicopathologic risk factors alone, suggesting that CETRUS scores complement the prognostic ability of traditional clinicopathologic features. Ultimately, patients with the same stage or grade of localized prostate cancer could potentially be stratified into different CETRUS-score-defined risk groups for disease recurrence, and such stratification may lead to more effective personalized management post-diagnosis and initial treatment.
This study has several noteworthy limitations. First, a 12-core systematic biopsy scheme was used as the reference standard, and this approach may have missed significant tumors. Second, 46% of the men with PCa who underwent a radical prostatectomy had a CETRUS score of ≤3. These patients can easily be mistaken for benign or indeterminate diagnoses. Third, since our study was conducted across only three institutions, the results may not be universally generalizable. We acknowledge that prospective, large-scale, multicenter studies are necessary to confirm our results. With further clinical research confirming the reliability of our research results and improvements to the accuracy of B-ultrasound equipment, performance of B-ultrasounds can not only reduce the puncture rate of patients with localized prostate cancer following a radical surgery, but also reduce unnecessary prostate puncture and biopsy collection by improving the accuracy of prostate cancer screening.
Conclusions
In summary, CETRUS-based blood flow grading is a reliable tool for reducing unnecessary collection of prostate biopsy samples for prostate cancer diagnosis. This CETRUS-based approach should also be considered to be a novel independent predictor for biochemical recurrence following radical prostatectomy in patients with localized prostate cancer. Our findings indicate that inclusion of CETRUS scores could in the current staging system could add significant prognostic value.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CETRUS:
-
contrast-enhanced transrectal ultrasound
- PSA:
-
prostate-specific antigen
- DRE:
-
digital rectal examination
- BMI:
-
body-mass index
- ECOG PS:
-
Eastern Cooperative Oncology Group performance status
- ROC:
-
Receiver operating characteristic
- AUC:
-
area under the curve
- BPH:
-
benign prostatic hyperplasia
- mpMRI:
-
Multiparametric magnetic resonance imaging
- MSKCC:
-
Memorial Sloan Kettering Cancer Center.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32.
Vickers AJ, Roobol MJ, Lilja H. Screening for prostate cancer: early detection or overdetection? Annu Rev Med. 2012;63:161–70.
Hayes JH, Barry MJ. Screening for prostate cancer with the prostate-specific antigen test: a review of current evidence. JAMA. 2014;311:1143–9.
Kotb AF, Elabbady AA. Prognostic factors for the development of biochemical recurrence after radical prostatectomy. Prostate Cancer. 2011;2011:485189. https://doi.org/10.1155/2011/485189.
Blum DL, Koyama T, M'Koma AE, et al. Chemokine markers predict biochemical recurrence of prostate cancer following prostatectomy. Clin Cancer Res. 2008:7790–7.
Postema AW, Frinking PJ, Smeenge M, et al. Dynamic contrast-enhanced ultrasound. parametric imaging for the detection of prostate cancer. BJU Int. 2016;117:598–603.
Uemura H, Sano F, Nomiya A, et al. Usefulness of perflubutane microbubble-enhanced ultrasound in imaging and detection of prostate cancer: phase II multicenter clinical trial. World J Urol. 2013;31:1123–8.
Sano F, Uemura H. The utility and limitations of contrast-enhanced ultrasound for the diagnosis and treatment of prostate cancer. Sensors (Basel). 2015;15:4947–57.
Gao Y, Liao XH, Ma Y, et al. Prostate ultrasound imaging: evaluation of a two-step scoring system in the diagnosis of prostate cancer. Discov Med. 2017;24:295–303.
Trabulsi EJ, Sackett D, Gomella LG, et al. Enhanced transrectal ultrasound modalities in the diagnosis of prostate cancer. Urology. 2010;76:1025–33.
Halpern EJ. Contrast-enhanced ultrasound imaging of prostate cancer. Rev Urol. 2006;8(Suppl 1):29–37.
Li X, Pan Y, Huang Y, et al. Developing a model for forecasting Gleason score ≥7 in potential prostate cancer patients to reduce unnecessary prostate biopsies. Int Urol Nephrol. 2016;48:535–40.
Adesunloye BA, Karzai FH, Dahut WL. Angiogenesis inhibitors in the treatment of prostate cancer. Chem Immunol Allergy. 2014;99:197–215.
Grivas N, Goussia A, Stefanou D, et al. Microvascular density and immunohistochemical expression of VEGF, VEGFR-1 and VEGFR-2 in benign prostatic hyperplasia, high-grade prostate intraepithelial neoplasia and prostate cancer. Cent European J Urol. 2016;69:63–71.
Schalk SG, Demi L, Bouhouch N, et al. Contrast-enhanced ultrasound angiogenesis imaging by mutual information analysis for prostate Cancer localization. IEEE Trans Biomed Eng. 2017;64:661–70.
Kay PA, Robb RA, Bostwick DG. Prostate cancer microvessels: a novel method for three-dimensional reconstruction and analysis. Prostate. 1998;37:270–7.
Wulphert Venderink, Tim M Govers, Maarten de Rooij, Jurgen J Fütterer, J P Michiel Sedelaar (2015) Cost-Effectiveness Comparison of Imaging-Guided Prostate Biopsy Techniques: Systematic Transrectal Ultrasound, Direct In-Bore MRI, and Image Fusion. AJR Am J Roentgenol :208 (5), 1058–1063.
Sedelaar JP, van Leenders GJ, Hulsbergen-van de Kaa CA, et al. Microvessel density: correlation between contrast ultrasonography and histology of prostate cancer. Eur Urol. 2001;40:285–93.
Claudon M, Cosgrove D, Albrecht T, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28–44.
de Zordo T, Ladurner M, Horninger W, et al. New ultrasound technologies for the diagnostics of prostate cancer. Radiologe. 2011;51:940–6.
Sano F, Terao H, Kawahara T, et al. Contrast-enhanced ultrasonography of the prostate: various imaging findings that indicate prostate cancer. BJU Int. 2011;107:1404–10.
Aigner F, Mitterberger M, Rehder P, et al. Status of transrectal ultrasound imaging of the prostate. J Endourol. 2010;24:685–91.
Frauscher F, Klauser A, Halpern EJ, et al. Detection of prostate cancer with a microbubble ultrasound contrast agent. Lancet. 2001;357:1849–50.
Erbersdobler A, Isbarn H, Dix K, et al. Prognostic value of microvessel density in prostate cancer: a tissue microarray study. World J Urol. 2010;28:687–92.
Talagas M, Uguen A, Garlantezec R, et al. VEGFR1 and NRP1 endothelial expressions predict distant relapse after radical prostatectomy in clinically localized prostate cancer. Anticancer Res. 2013;33:2065–75.
Nordby Y, Andersen S, Richardsen E, et al. Stromal expression of VEGF-A and VEGFR-2 in prostate tissue is associated with biochemical and clinical recurrence after radical prostatectomy. Prostate. 2015;75:1682–93.
Xu G, Wu J, Yao MH, et al. Parameters of prostate Cancer at contrast-enhanced ultrasound: correlation with prostate cancer risk. Int J Clin Exp Med. 2015;8:2562–9.
Huang H, Zhu ZQ, Zhou ZG, et al. Contrast-enhanced transrectal ultrasound for prediction of prostate cancer aggressiveness: the role of normal peripheral zone time-intensity curves. Sci Rep. 2016. https://doi.org/10.1038/srep38643.
Mitterberger M, Aigner F, Pinggera GM, et al. Contrast-enhanced colour Doppler-targeted prostate biopsy: correlation of a subjective blood-flow rating scale with the histopathological outcome of the biopsy. BJU Int. 2010;106:1315–8.
Acknowledgments
None.
Funding
The study was supported by the Natural Science Foundation of Shandong province (ZR2013HL070), the Projects of Medical and Health Technology Development Program of Shandong province (2016WS0712), the National Natural Science Foundation of China (81972376) and the National Key R&D Program of China (2016YFC0902602). The funders of the projects conceived and designed the experiments.
Author information
Authors and Affiliations
Contributions
HW and YF: project development, data collection, study design, manuscript editing; JL, JZ, and JLin: manuscript writing, study design, data collection, statistical analysis; ZW, JY, and JH: manuscript editing, data collection; JH and ZH: data collection, statistical analysis; HH, YH, ZL, JH, WC, and LS: data collection, data interpretation. HW, JH, and JLperformed 12-core systematic prostate biopsies. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study has been approved by the Institutional Ethical Committee of Yantai Yuhuangding Hospital, First Affiliated Hospital of Sun Yat-sen University and Cancer Center of Sun Yat-sen University. Written informed consent was obtained from each patient prior to data collection and experiments.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hong-wei Zhao, Jian Li, Jia-Zheng Cao, and Juan Lin are co-first authors
Supplementary information
Additional File 1: Table 1
: Diagnostic efficacy of CETRUS scores in differentiating prostate cancer from benign disease at different cut-off points.
Additional File 2: Figure 1
: Baseline and CETRUS scores 1–5 are depicted in images a-e, respectively.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, Hw., Li, J., Cao, JZ. et al. Contrast-enhanced transrectal ultrasound can reduce collection of unnecessary biopsies when diagnosing prostate cancer and is predictive of biochemical recurrence following a radical prostatectomy in patients with localized prostate cancer. BMC Urol 20, 100 (2020). https://doi.org/10.1186/s12894-020-00659-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-020-00659-6