Abstract
Background
To assess whether Sjogren’s Syndrome (SS) is associated with outcomes after total knee or hip arthroplasty (TKA/THA).
Methods
We used the 1998–2014 U.S. National Inpatient Sample data. We performed multivariable-adjusted logistic regression analyses to assess the association of SS with healthcare utilization (hospital charges, length of hospital stay, discharge to non-home setting), and in-hospital complications (implant infection, revision, transfusion, mortality), controlling for important covariates and confounders. In sensitivity analyses, we additionally adjusted the main models for hospital location/teaching status, bed size, and region.
Results
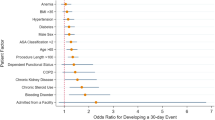
We examined 4,116,485 primary THAs and 8,127,282 primary TKAs performed from 1998 to 2014; 12,772 (0.2%) primary TKAs and 6222 (0.2%) primary THAs were done in people with SS. In multivariable-adjusted models, SS was associated with a statistically significant higher odds ratio (OR; 95% confidence interval (CI)) of discharge to a rehabilitation/inpatient facility post-THA, 1.13 (1.00, 1.28), but not post-TKA, 0.93 (0.86, 1.02). We noted no differences in the length of hospital stay or hospital charges. SS was associated with significantly higher adjusted odds of in-hospital transfusion post-THA, 1.37 (1.22, 1.55) and post-TKA, 1.21 (1.10, 1.34). No significant differences by SS diagnosis were seen in hospital stay, hospital charges implant infection, implant revision or mortality rates.
Conclusions
People with SS had higher transfusion rate post-TKA/THA, and higher rate of discharge to non-home setting post-THA. The lack of association of SS with post-arthroplasty complications should reassure patients, surgeons and policy-makers about the utility of TKA/THA in people with SS undergoing these procedures.
Similar content being viewed by others
Highlights
-
1.
Sjogren’s Syndrome was associated with a significantly higher odds of discharge to a rehabilitation/inpatient facility post-THA, but not post-TKA.
-
2.
Sjogren’s Syndrome was associated with significantly higher adjusted odds of in-hospital transfusion post-THA, and post-TKA.
-
3.
Sjogren’s Syndrome was not associated with implant infection or revision or mortality post-THA/TKA.
Background
Sjogren’s Syndrome (SS) is a systemic autoimmune disease, primarily of middle-aged women, characterized by dry eyes, dry mouth and systemic symptoms [1]. Its incidence and prevalence in the general population are 7/100,000 and 60/100,000 people, respectively [1]. SS can be primary, or secondary to diseases such as rheumatoid arthritis (RA), lupus, scleroderma, myositis etc. SS is associated with systemic inflammation with an over-expression of pro-inflammatory cytokines [2, 3].
With the aging of the population, an increasing number of people with SS and the general population are undergoing joint arthroplasty. Total knee arthroplasty (TKA) or hip arthroplasty (THA) are the two most common arthroplasty surgeries performed for end-stage arthritis [4]. Complications and healthcare utilization after arthroplasty are increased in other systemic inflammatory rheumatic disease such as RA, lupus and spondyloarthritis [5]. Whether SS, which is also a systemic autoimmune disease, has a similar impact on post-arthroplasty outcomes is unknown. Therefore, we assessed whether SS was associated with higher complication, mortality and healthcare utilization rates after primary TKA or THA.
Methods
Data source, study cohort and study outcomes
We used the 1998–2014 U.S. National Inpatient Sample (NIS) data, a 20% stratified sample of discharges from U.S. community hospitals [6]. NIS is the largest publicly available, de-identified all-payer inpatient health care database in the U.S. The University of Alabama at Birmingham’s Institutional Review Board approved this study and waived the need for informed consent (X120207004).
We identified a cohort of all hospitalizations with primary TKR or THA as the primary procedure by using validated International Classification of Disease, ninth revision, common modification (ICD-9-CM) codes for primary TKA (81.54) or primary THA (81.51) [7]. Among these, we identified those with and without Sjogren’s Syndrome (ICD-9-CM, 710.2), a valid approach with sensitivity of 95% and specificity of 96% [8], in non-primary position.
Our study outcomes of interest post-primary TKA/THA were: (1) three health care utilization measures: the discharge disposition to home vs. a rehabilitation/inpatient facility, the length of hospital stay above the median (> 3 days), and the hospital charges above the median for each calendar year; (2) three in-hospital complications identified by respective ICD-9-CM codes for transfusion, implant infection and implant revision; and (3) in-hospital mortality.
Statistical analysis
We performed separate multivariable-adjusted logistic regression analyses for each outcome controlling for clinically important variables, some of which were also potential and/or known confounders of TKA/THA outcomes [9,10,11,12,13], including age, race, sex, income, underlying diagnosis (listed in the primary diagnosis position), Deyo-Charlson comorbidity index, and insurance payer. Deyo-Charlson comorbidity index [14], is a validated measure of medical comorbidity that includes 17 comorbidities with score ranging 0–25, higher score indicating more comorbidity load. Sensitivity analyses additionally adjusted for hospital bed size, location/teaching status, and region [15, 16]. Sensitivity analyses examined the main multivariable-adjusted logistic regression analyses in two SS subgroups: (1) Primary SS: no concomitant rheumatic disease diagnoses; (2) Secondary SS: presence of one or more concomitant rheumatic disease diagnoses (systemic lupus erythematosus, 710.0; systemic sclerosis, 710.1; sicca syndrome, 710.2; dermatomyositis, 710.3; polymyositis, 710.4; mixed connective tissue disease, 710.9; antiphospholipid syndrome, 289.81; rheumatoid arthritis, 714).
Results
Of the 4,116,485 primary THAs and 8,127,282 primary TKAs performed from 1998 to 2014, 12,772 (0.2%) primary TKAs and 6222 (0.2%) primary THAs were done in people with SS. Compared to no SS, a higher proportion of those with a diagnosis of SS was female, had an underlying diagnosis of rheumatoid arthritis or a Deyo-Charlson index score of ≥2, for both primary THA and primary TKA cohorts (Table 1). Of these, the number with primary versus secondary SS were as follows: (1) Primary TKA: Primary SS, 8477 (66%); Secondary SS, 4295 (34%); (2) Primary THA: Primary SS, 4240 (68%); Secondary SS, 1982 (32%).
SS was associated with a statistically significant higher odds ratio (OR; 95% confidence interval (CI)) of discharge to a rehabilitation/inpatient facility post-THA, 1.13 (1.00, 1.28), but not post-TKA, 0.93 (0.86, 1.02) after multivariable-adjustment (Table 2). We noted no differences in the length of hospital stay or hospital charges. We found significantly higher adjusted odds of in-hospital transfusion post-THA, 1.37 (1.22, 1.55) and post-TKA, 1.21 (1.10, 1.34) associated with SS (Table 2). No differences by SS diagnosis were seen in implant infection, implant revision or mortality rates. Sensitivity analyses adjusting for additional hospital characteristics confirmed the findings from the main analyses with minimal attenuation of ORs (Table 2).
Sensitivity analyses were performed for primary vs. secondary SS (Additional file 1). Main study findings were reproduced for the higher odds of transfusion for both primary and secondary SS. Association of SS with discharge to a rehabilitation/inpatient facility post-THA were no longer significant, when subgroups of primary and secondary SS were examined. Additionally, primary SS was associated with higher odds of revision post-TKA; and secondary SS with higher odds of length of hospital stay > 3 days and lower odds of death post-THA (Additional file 1).
Discussion
In a nationally representative U.S. sample, we found that SS, a multi-system autoimmune disease, was associated with a significantly higher odds of discharge to a rehabilitation/inpatient facility post-THA with 1.13-fold higher odds, but not post-TKA. Fatigue [17] and reduced functional ability [18], frequent in SS, may interfere with optimal in-hospital rehabilitation and recovery. This can potentially increase the risk of discharge to a rehabilitation or an inpatient facility. The reason for increased risk of discharge to a rehabilitation facility only after THA, but not TKA, may warrant further study. The strength of association was not very high, therefore the absolute impact of this significant association in people with SS may be small.
SS was associated with a higher risk of transfusion after THA and TKA, at 1.2–1.4 fold higher. This association was reproduced in subgroups of people with primary or secondary SS. Systemic inflammation [2, 3] in SS with associated anemia [19] and cytopenia [17] can lead to a higher transfusion risk post-THA/TKA.
We found that SS was not associated with complications including implant infection, revision or mortality after primary THA or TKA. Our finding contrasts with previously noted higher post-TKA/THA complication rates in people with other systemic inflammatory conditions, such as RA, SpA or lupus [5]. Limited joint or organ involvement in SS compared to RA, SpA or lupus [20] and/or the assessment of in-hospital complications in our study (vs. all post-operative complications) may explain these differences. An absence of an association of SS with post-arthroplasty infection, revision or mortality should reassure patients with SS and surgeons that these risks are not increased post-arthroplasty. The implications of possibly increased infection in primary SS post-TKA and decreased mortality in secondary SS post-THA are unclear since these were sub-group analyses; we believe that these findings need further study and replication.
Study limitations include residual confounding bias (direction of bias unclear), the lack of data on SS severity and laboratory measures, misclassification bias which likely biased findings towards the null, and the lack of longitudinal data. Bilateral simultaneous THA/TKA can not be distinguished from unilateral procedures in the NIS; however, these constitute < 1% THA and < 3% TKA, and therefore the bias is likely small. We were unable to separately examine the associations for primary versus secondary Sjogren’s syndrome due to the lack of a separate ICD-9 code for these. However, since the analyses are adjusted for underlying reason for THA/TKA which includes all rheumatic conditions associated with THA/TKA, the associations are for the presence versus absence of Sjogren’s Syndrome. These findings should alert the clinician to a higher transfusion risk in SS patients undergoing THA or TKA, and reassure the policy-makers that early THA/TKA outcomes are only minimally impacted by SS.
Conclusions
In conclusion, SS was associated with a higher risk of transfusion after THA and TKA, higher odds of discharge to a rehabilitation/inpatient facility post-THA, but not post-TKA. SS was not associated with post-arthroplasty infection, revision or mortality. These findings can inform patients, providers and policy-makers regarding the minimal impact of SS on post-primary TKA/THA outcomes.
Availability of data and materials
These data are easily available from the Agency for Healthcare Research and Quality (AHRQ’s) “Healthcare Cost and Utilization Project (HCUP)” and can be obtained after completing an on-line Data Use Agreement training session and signing a Data Use Agreement. The contact information for requesting the data is as follows:
HCUP Central Distributor.
Phone: (866) 556–4287 (toll-free).
Fax: (866) 792–5313.
E-mail: HCUPDistributor@ahrq.gov
Abbreviations
- SS:
-
Sjogren’s Syndrome
- NIS:
-
National Inpatient Sample
- TKA:
-
Total knee arthroplasty
- THA:
-
Total hip arthroplasty
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- SE:
-
Standard error
- ICD-9-CM:
-
International Classification of Diseases, Ninth Revision, Clinical Modification
References
Qin B, Wang J, Yang Z, Yang M, Ma N, Huang F, et al. Epidemiology of primary Sjogren's syndrome: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:1983–9.
Malladi AS, Sack KE, Shiboski SC, Shiboski CH, Baer AN, Banushree R, et al. Primary Sjogren's syndrome as a systemic disease: a study of participants enrolled in an international Sjogren's syndrome registry. Arthritis Care Res (Hoboken). 2012;64:911–8.
Roescher N, Tak PP, Illei GG. Cytokines in Sjogren's syndrome. Oral Dis. 2009;15:519–26.
Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86-A:963–74.
Goodman SM, Bass AR. Perioperative medical management for patients with RA, SPA, and SLE undergoing total hip and total knee replacement: a narrative review. BMC Rheumatol. 2018;2:2.
HCUP Databases. Healthcare Cost and Utilization Project (HCUP). Overview of the Nationwide Inpatient Sample (NIS). http://www.hcup-us.ahrq.gov/nisoverview.jsp. Last modified 8/13/18. Rockville: Agency for Healthcare Research and Quality; 2018. [cited 2019 04/26/2019].
Katz JN, Barrett J, Mahomed NN, Baron JA, Wright RJ, Losina E. Association between hospital and surgeon procedure volume and the outcomes of total knee replacement. J Bone Joint Surg Am. 2004;86-A:1909–16.
Bernatsky S, Linehan T, Hanly JG. The accuracy of administrative data diagnoses of systemic autoimmune rheumatic diseases. J Rheumatol. 2011;38:1612–6.
Giacovelli JK, Egorova N, Nowygrod R, Gelijns A, Kent KC, Morrissey NJ. Insurance status predicts access to care and outcomes of vascular disease. J Vasc Surg. 2008;48:905–11.
Young GJ, Cohen BB. The process and outcome of hospital care for Medicaid versus privately insured hospital patients. Inquiry. 1992;29:366–71.
Santaguida PL, Hawker GA, Hudak PL, Glazier R, Mahomed NN, Kreder HJ, et al. Patient characteristics affecting the prognosis of total hip and knee joint arthroplasty: a systematic review. Can J Surg. 2008;51:428–36.
Ravi B, Escott B, Shah PS, Jenkinson R, Chahal J, Bogoch E, et al. A systematic review and meta-analysis comparing complications following total joint arthroplasty for rheumatoid arthritis versus for osteoarthritis. Arthritis Rheum. 2012;64:3839–49.
Peltola M, Jarvelin J. Association between household income and the outcome of arthroplasty: a register-based study of total hip and knee replacements. Arch Orthop Trauma Surg. 2014;134:1767–74.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9.
Kurtz SM, Lau EC, Ong KL, Adler EM, Kolisek FR, Manley MT. Which hospital and clinical factors drive 30- and 90-day readmission after TKA? J Arthroplast. 2016;31:2099–107.
Kurtz SM, Lau EC, Ong KL, Adler EM, Kolisek FR, Manley MT. Hospital, patient, and clinical factors influence 30- and 90-day readmission after primary Total hip Arthroplasty. J Arthroplast. 2016;31:2130–8.
Hartkamp A, Geenen R, Godaert GL, Bootsma H, Kruize AA, Bijlsma JW, et al. Effect of dehydroepiandrosterone administration on fatigue, well-being, and functioning in women with primary Sjogren syndrome: a randomised controlled trial. Ann Rheum Dis. 2008;67:91–7.
Meijer JM, Meiners PM, Huddleston Slater JJ, Spijkervet FK, Kallenberg CG, Vissink A, et al. Health-related quality of life, employment and disability in patients with Sjogren's syndrome. Rheumatology (Oxford). 2009;48:1077–82.
Ramos-Casals M, Solans R, Rosas J, Camps MT, Gil A, Del Pino-Montes J, et al. Primary Sjogren syndrome in Spain: clinical and immunologic expression in 1010 patients. Medicine (Baltimore). 2008;87:210–9.
Fox RI. Sjogren's syndrome. Lancet. 2005;366:321–31.
Acknowledgements
None.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
JAS designed the study, developed study protocol, reviewed analyses and wrote the first draft of the paper. JDC performed the data abstraction and data analyses. All authors revised the manuscript, read, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The University of Alabama at Birmingham’s Institutional Review Board approved this study and all investigations were conducted in conformity with ethical principles of research (UAB X120207004). The IRB waived the need for an informed consent for this database study.
Consent for publication
Not required.
Competing interests
There are no financial conflicts related directly to this study. JAS has received consultant fees from Crealta/Horizon, Medisys, Fidia, UBM LLC, Trio health, Medscape, WebMD, Clinical Care options, Clearview healthcare partners, Putnam associates, Spherix, Practice Point communications, the National Institutes of Health and the American College of Rheumatology. JAS owns stock options in Amarin pharmaceuticals and Viking therapeutics. JAS is on the speaker’s bureau of Simply Speaking. JAS is a member of the executive of OMERACT, an organization that develops outcome measures in rheumatology and receives arms-length funding from 36 companies. JAS is a member of the Veterans Affairs Rheumatology Field Advisory Committee. JAS is the editor and the Director of the UAB Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis. JAS served as a member of the American College of Rheumatology’s (ACR) Annual Meeting Planning Committee (AMPC) and Quality of Care Committees, the Chair of the ACR Meet-the-Professor, Workshop and Study Group Subcommittee and the co-chair of the ACR Criteria and Response Criteria subcommittee. JDC has no conflicts. There are no non-financial competing interests for either author.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Multivariable-adjusted association of Primary versus Secondary Sjogren’s syndrome (SS) with complications and healthcare utilization outcomes after primary THA or primary TKA in the main model.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Singh, J.A., Cleveland, J.D. Sjogren’s syndrome is associated with higher rate of non-home discharge after primary hip arthroplasty and higher transfusion rates after primary hip or knee arthroplasty: a U.S. cohort study. BMC Musculoskelet Disord 21, 492 (2020). https://doi.org/10.1186/s12891-020-03514-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-020-03514-9