Abstract
Background
Bone loss with aging and menopause increases the risk of fragile vertebral fracture, osteoporotic vertebral compression fracture (OVCF). The fracture causes severe pain, impedes respiratory function, lower the quality of life, and increases the risk of new fractures and deaths. Various medications have been prescribed to prevent a secondary fracture, but few study summarized their effects. Therefore, we investigated their effects on preventing subsequent OVCF via meta-analyses of randomized controlled trials.
Methods
Electronic databases, including MEDLINE, EMBASE, CENTRAL, and Web of Science were searched for published randomized controlled trials from June 2015 to June 2019. The trials that recruited participants with at least one OVCF were included. We assessed the risk of bias of every study, estimated relative risk ratio of secondary OVCF, non-vertebral fracture, gastrointestinal complaints and discontinuation due to adverse events. Finally, we evaluated the quality of evidence.
Results
Forty-one articles were included. Moderate to high quality evidence proved the effectiveness of zoledronate (Relative Risk, RR: 0.34; 95% CI, 0.17–0.69, p = 0.003), alendronate (RR: 0.54; 95% CI: 0.43–0.68; p < 0.0001), risedronate (RR: 0.61; 95% CI: 0.51–0.73; p < 0.0001), etidronate (RR, 0.50; 95% CI, 0.29–0.87, p < 0.01), ibandronate (RR: 0.52; 95% CI: 0.38–0.71; p < 0.0001), parathyroid hormone (RR: 0.31; 95% CI: 0.23–0.41; p < 0.0001), denosumab (RR, 0.41; 95% CI, 0.29–0.57; p < 0.0001) and selective estrogen receptor modulators (Raloxifene, RR: 0.58; 95% CI: 0.44–0.76; p < 0.0001; Bazedoxifene, RR: 0.66; 95% CI: 0.53–0.82; p = 0.0002) in preventing secondary fractures. Moderate quality evidence proved romosozumab had better effect than alendronate (Romosozumab vs. alendronate, RR: 0.64; 95% CI: 0.49–0.84; p = 0.001) and high quality evidence proved that teriparatide had better effect than risedronate (risedronate vs. teriparatide, RR: 1.98; 95% CI: 1.44–2.70; p < 0.0001).
Conclusion
Zoledronate, alendronate, risedronate, etidronate, ibandronate, parathyroid hormone, denosumab and selective estrogen receptor modulators had significant secondary prevention effects on OVCF. Moderate quality evidence proved romosozumab had better effect than alendronate. High quality evidence proved PTH had better effect than risedronate, but with higher risk of adverse events.
Similar content being viewed by others
Background
Osteoporotic vertebral compression fracture (OVCF) is one of the most common fragile fractures, with a prevalence of 30 to 50% in people over 50 years of age [1]. It causes severe pain and disability, raises the risk of secondary fracture more than 4-fold [2, 3], and increases the risk of mortality [4]. Therefore, secondary prevention of OVCF was critical and should be emphasized to improve patients’ quality of life. However, though the primary prevention efficacy of medications have been well summarized [5,6,7,8,9,10], only one systematic review targeted on their secondary prevention effects [11]. Therefore, to investigate the efficacy of current medication therapies on preventing secondary OVCF, we conducted this study through systematically literature review and meta-analyses of randomized controlled trials (RCTs).
Methods
Search for studies
Four major electronic databases (MEDLINE, EMBASE, CENTRAL, and Web of Science) were searched with a developed search strategy that consisted of keywords “controlled trials”, “osteoporotic fracture”, “bisphosphonate”, “parathyroid hormone”, “denosumab” “calcitonin”, “Raloxifene”, “Bazedoxifene” “hormone replacement”, “romosozumab”, “abaloparatide”, etc., and others (Additional file 1). The search spanned the period from June 2015 to June 2019, with weekly alerts of updated published trials. Reference lists from other reviews and studies were also checked for relevant articles. The references were managed with Endnote X7 (Clarivate Analytics).
Selection of studies
Three authors (YZJ, BX, and MJC) independently screened the titles and abstracts of studies and evaluated their relevance to our study. A study was included if it involved patients with osteoporosis. A subsequent full-text assessment was done by three authors (YZJ, BX, and JHL) independently. Randomized controlled trials (RCTs) published in English that investigated the efficacy of currently approved medications for patients with OVCF were included. The studies that included osteoporosis patients without distinguishing their fracture history were included if the data of the participants with prevalent fractures was adequately presented. Studies that recruited patients with traumatic vertebral fracture, secondary osteoporosis, or did not report results in dichotomous data (i.e., patient-years, etc.), were excluded. The included medications were the approved ones, including zoledronate, alendronate, risedronate, etidronate, ibandronate, minodronate, pamidronate, calcitonin, hormone replacement therapy, parathyroid hormone, denosumab, romosozumab, raloxifene, and bazedoxifene [12,13,14]. Post hoc analyzed RCTs were also included, with taking care of duplicated data input. Disagreements between reviewers were resolved by discussion or, if unresolved, by consultation with consultation with librarians and a statistic professor from SMG-SNU Boramae Medical Center.
Data extraction and risk of bias
Basic characteristics of each study were independently extracted by YZJ, BX, and JHL with a designed table that contains the number of participants, interventions, comparisons, and outcomes. The primary outcome of this study was the vertebral fracture ratio in the final visit, and the secondary outcomes were gastrointestinal (GI) complaints of bisphosphonates, discontinuation due to adverse events (AEs), and non-vertebral fracture ratio.
The risk of bias was measured independently by YZJ, BX, and JHL with the tool recommended in updated guidelines of Cochrane Back and Neck Group [15]. The detection bias was rated for main result (vertebral fracture). The loss ratio was acceptable for a middle- or long-term trial (observational period > 1 year), if that was not exceeded 30%. The risk of other sources of bias was rated as low risk if the article stated both conflict of interest and sponsor of the trial and no other serious risk of bias was reported.
Data analysis and quality of evidence
Relative risk (RR) and its 95% confidence intervals (CIs) were used to estimate the effect of interventions, with p-values < 0.05 considered significant. The overall effect size was calculated with a random effects model [15]. Heterogeneity between studies was identified and measured with p-value and I2 value from Chi-squared test, p-value < 0.1 was identified as significant, and I2 < 40% was considered as not important, I2 between 40 and 74% indicated moderate to substantial, I2 > 75% was identified as a considerable magnitude [15]. In studies with more than two arms, intervention groups were input into each subgroup and the data in the control groups were separated equally and then were compared to their counterparts. Sensitivity analyses were used to explore the interference from a study by excluding it from syntheses and the impact from loss to follow-up population by compositing the missing events according to event ratio in control groups [16]. The data was analyzed by two authors (YZJ and JHL) with RevMan 5.3.3 (Cochrane).
We evaluated five factors of the results to determine the quality of evidence, including study limitation, imprecision, indirectness, inconsistent and publication bias, followed the GRADE approach. The criteria for downregulating the level referred to the handbook of GRADE and guidelines from Cochrane Back and Neck Group [15, 16]. In the case that an outcome included one trial with no unclear or high risk of bias, the study limitation item was rated as not serious if its result remained same direction and signficancy with the pooled result.
Results
Characteristics of included studies and risk of bias
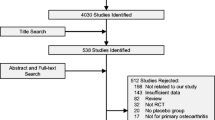
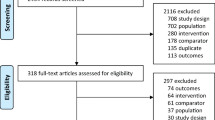
A total of 6850 articles were identified. Among them, 631 were subjected to full-text assessment. After full-text examination, 41 articles were finally included in this study (Fig. 1). Among them, 34 compared the effects of medications with control groups. Bisphosphonates (BPs) were compared in 19 RCTs [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33, 35, 36], calcitonin in 3 [37,38,39], hormone replacement therapy (HRT) in 3 [40,41,42], parathyroid hormone (PTH), teriparatide, or abaloparatide in 5 [43,44,45,46,47], denosumab in 2 [48, 49], and selective estrogen receptor modulators (SERMs) in 3 [50,51,52]. Five trials compared between the effects of medications, risedronate vs. etidronate [53], ibandronate vs. risedronate [54], romosozumab vs. alendronate [55], and teriparatide vs. risedronate [57, 58]. Follow-up duration in most trials was 2 to 3 years. Other basic characteristics of included studies were summarized in Table 1.
Approximately half of the biases were rated as unclear risk (Additional file 2). Risk of other sources of bias was rated as high in one study because the criteria used in its two clinical centers were different [21]. Performance bias was rated as high risk in 6 trials for significantly different compliance between groups [26, 46, 52] and the open-label study design used in 4 trials [20, 27, 37, 40].
Fujita et al. treated a teriparatide 1.4 μg/week group as a placebo group, and therefore, we followed their grouping and classified their data of teriparatide 1.2 μg/week group as the control group [45]. On the other side, since the control groups in other studies all received placebo, which was different from Fujita et al.’s study, it might affect the final result. Therefore, we performed a sensitivity analysis about this result, with excluding the Fujita el al.’s study from the original analysis and then compared the results from original analysis and sensitivity analysis (Table 1). Sorensen et al. reported a 2-year extension trial [23] of a 3-year original trial [22]. In the entension trial, the authors treated the initial time point of the extention trial as baseline. Therefore, while synthesizing the data, we deemed the data from the two studies were not duplicated and synthesized the data as from two studies. However, because the participants in experimental group and control group in the extension study had different medication history, the risk of selection bias of the extended trial was rated as high (Additional file 2).
Comparison with control group
Antiresorptive medications
The result of antiresorptive medications, including BPs, HRT, SERMs, calcitonin, and denosumab, were pooled together to investigate the effects of the medications. Thirty-three studies involving 21,012 participants were included. The result indicated that the administration of antiresorptive medications could significantly reduce the risk of the secondary OVCF (RR, 0.59; 95% CI, 0.53–0.65, p < 0.00001) (Table 2, Additional file 3a). Bisphosphonates did not significantly increase gastrointestinal (GI) complaints (RR, 1.02, p = 0.45; Additional file 3b). The result was treated as a secondary outcome because the heterogeneity in the comparison.
Zoledronate
Moderate quality evidence proved that zoledronate could significantly decrease the risk of secondary OVCF (RR, 0.34; 95% CI, 0.17–0.69, p = 0.003; Fig. 2a, Table 2), without significant increase in discontinuation due to medication (RR, 1.99; 95% CI, 0.76–5.25, p = 0.16; Table 3, Additional file 3c). Additionally, zoledronate could significantly decrease event ratio of non-vertebral fractures (RR, 0.54; 95% CI, 0.32–0.91; p = 0.02; Table 2, Additional file 3d).
Alendronate
High quality evidence proved that administrating alendronate significantly reduced the proportion of participants who had subsequent vertebral fractures (RR, 0.54; 95% CI, 0.43–0.68; p < 0.0001; heterogeneity, p = 0.63, I2 = 0%; Fig. 2b, Table 2). No significant increase in GI complaints or discontinuation was observed in the alendronate group (GI complaints, RR, 1.03; 95% CI, 0.93–1.15, p = 0.55; Discontinuation, RR, 0.88; 95% CI, 0.64–1.22, p = 0.46; Table 3, Additional file 3e and f). Alendronate had no significant effect on preventing non-vertebral fractures (RR, 0.81; 95% CI, 0.65–1.01, p = 0.07; Table 2, Additional file 3g).
Risedronate
Moderate quality evidence indicated that risedronate had a significant effect on preventing subsequent vertebral fractures (RR, 0.61; 95% CI, 0.51–0.73, p < 0.0001; Fig. 2c, Table 2). Risedronate administration did not significantly elevate GI complaints (RR, 1.09; 95% CI, 0.96–1.23, p = 0.18) or discontinuation rate (RR, 0.88; 95% CI, 0.69–1.12, p = 0.28) (Table 3, Additional file 3h and i). Risedronate had a significant effect on preventing non-vertebral fractures (RR, 0.71; 95% CI, 0.54–0.92, p = 0.01; Table 2, Additional file 3j).
Etidronate
Moderate quality evidence showed that the administration of etidronate could significantly reduce the risk of subsequent vertebral fractures (RR, 0.50; 95% CI, 0.29–0.87, p < 0.01; Fig. 2d, Table 2). The result was consistent with that of sensitivity test, in which a study [29] with a small sample size and big variance was excluded (Additional file 3k). No significant difference was observed in GI complaints (RR, 0.57; 95% CI, 0.28–1.15, p = 0.12) or discontinuation (RR, 0.40; 95% CI, 0.03–5.48, p = 0.50) between intervention and control groups (Table 3, Additional file 3l and m). Etidronate did not have a significant effect on preventing non-vertebral fractures (RR, 0.95; 95% CI, 0.59–1.53, p = 0.83; Table 2, Additional file 3n).
Ibandronate
Moderate quality evidence proved that ibandronate administrated 2.5 mg daily or 20 mg intermittently could significantly reduce the subsequent fracture risk (RR, 0.52; 95% CI, 0.38–0.71, p < 0.0001; Fig. 2e, Table 2), while insufficient dosages (0.5 mg or 1 mg per 3 months) did not (RR, 0.87; 95% CI, 0.69–1.11, p = 0.27; Fig. 2f, Table 2). Ibandronate did not significantly raise the risk of discontinuation due to adverse events (sufficient dose: RR, 0.90; 95% CI, 0.69–1.18, p = 0.45; insufficient dose: RR, 1.27; 95% CI, 0.98–1.66, p = 0.07) (Additional file 3o and p, Table 3). Neither sufficient nor insufficient dosage of ibandronate had significant effect on preventing non-vertebral fractures (sufficient: RR, 1.10; 95% CI, 0.85–1.41, p = 0.47; insufficient, only hip fracture: RR, 0.59; 95% CI, 0.26–1.31; p = 0.19; Table 2, Additional file 3q and r).
Minodronate
Low quality evidence proved minodronate had significant effect in reducing secondary fracture (RR, 0.44; 95% CI, 0.31–0.63; p < 0.001; Fig. 2g, Table 2). Minodronate did not have a significant effect on preventing non-vertebral fractures (RR, 0.80; 95% CI, 0.35–1.84, p = 0.60; Table 2, Additional file 3s).
Pamidronate
Very low quality evidence indicated significantly lower risk of secondary fracture due to pamidronate (RR, 0.33; 95% CI, 0.13–0.84, p = 0.02; Fig. 2h, Table 2). Pamidronate did not have significant effect on preventing non-vertebral fractures (RR, 0.33; 95% CI, 0.04–3.10, p = 0.33; Table 2, Additional file 3t).
Calcitonin
Very low quality evidence proved calcitonin had no significant effect on preventing secondary fracture (RR, 1.02; 95% CI, 0.14–7.36, p = 0.98) (Table 2).
HRT
Low quality evidence proved HRT had no significant effect on prevention of secondary vertebral or non-vertebral fracture (vertebral: RR, 0.88; p = 0.78; non-vertebral: RR, 0.37; 95% CI, 0.04–3.05, p = 0.36; Table 2, Additional file 3u). HRT did not significantly elevate the risk of discontinuation (RR, 0.53, 95% CI, 0.17–1.61, p = 0.26; Table 3, Additional file 3v).
Parathyroid (PTH)
Moderate quality evidence proved that the administration of teriparatide 28.2 μg/week or 56.5 μg/week, abaloparatide 80 μg/day, and recombinant human (rh)PTH 20 μg/day or rhPTH 40 μg/day could significantly reduce the risk of secondary fracture (Table 2). The synthesized RR was 0.31 (95% CI, 0.23–0.41; p < 0.0001) and the heterogeneity between different doses was insignificant (p = 0.45, Fig. 3a). The result of the sensitive analysis that excluded the trial had teriparatide 1.4 μg/week group as its control group [45] showed no significant change (RR, 0.31, 95% CI, 0.22–0.44, p < 0.00001). The risk of discontinuation due to medication was significantly raised by PTH administration (RR, 1.54; 95% CI, 1.11–2.13, p < 0.009; Additional file 3w). Forty μg/day rhPTH significantly elevated the risk of discontinuation, while 20 μg/day or 56.5 μg/week did not, but no significant heterogeneity was observed between groups. PTH had significant effect on preventing non-vertebral fractures (RR, 0.52; 95% CI, 0.36–0.75; p = 0.0005; Table 2, Additional file 3x).
Denosumab
Moderate quality evidence proved that the administration of denosumab significantly reduced the risk of secondary fracture (RR, 0.41; 95% CI, 0.29–0.57; p < 0.0001; Fig. 3b, Table 2). No significant increase in discontinuation due to medication was observed (RR, 0.75; 95% CI, 0.44–1.27, p = 0.29; Table 3, Additional file 3y). Denosumab did not have a significant effect on preventing non-vertebral fractures (RR, 0.45; 95% CI, 0.20–1.03, p = 0.06; Table 2, Additional file 3z).
SERMs
Both raloxifene (RLX) and bazedoxifene (BZA) could significantly reduce risk of secondary fracture (RLX: RR, 0.58; 95% CI, 0.44–0.76, p < 0.0001. BZA: RR, 0.66; 95%CI, 0.53–0.82, p = 0.0002; Fig. 3c and d). Heterogeneity between 60 μg/day and 120 μg/day of RLX was significant and substantial (test for subgroup differences, p = 0.06, I2 = 72.1%; Fig. 3c). The effect of BZA was proved by moderate quality evidence and the effect of RLX was supported by high quality evidence (Table 2).
Comparison between interventions
Comparison between BPs
Moderate quality evidence proved no significant difference in the effects on preventing vertebral fracture between risedronate and etidronate (RR, 1.12; 95% CI, 0.69–1.81, p = 0.66; Additional file 4a). High quality evidence proved no significant difference between ibandronate and risedronate in preventing vertebral fracture (RR, 1.01; 95% CI, 0.78–1.32, p = 0.92; Additional file 4b, Table 2) and no significant difference was observed between ibandronate and risedronate in preventing non-vertebral fracture (RR, 1.12; 95% CI, 0.75–1.66, p = 0.59; Additional file 3aa, Table 2).
Hormone therapy vs. BPs
Very low quality evidence indicated no significant difference between HRT and etidronate (RR, 0.63; 95% CI, 0.12–3.32, p = 0.59; Additional file 4c). Moderate quality evidence indicated teriparatide (20 μg/week) showed a significantly superior effect on preventing vertebral fracture and non-vertebral fracture than risedronate (vertebral fracture: RR, 1.98; 95% CI, 1.44–2.7, p < 0.0001; Additional file 4d), without significantly increasing ratio of discontinuation (RR, 0.75; 95% CI, 0.57–1.00, p = 0.05; Table 3, Additional file 3bb). No significant difference in effects of non-vertebral fracture was observed (RR, 1.28; 95% CI, 0.94–1.73, p = 0.12; Additional file 3cc).
Monoclonal antibody medication vs. BPs
Low quality evidence proved the difference between the effects of alendronate and denosumab on preventing vertebral fracture was not significant (RR, 0.69; 95% CI, 0.41–1.17, p = 0.17; Additional file 4e). Moderate quality evidence proved romosozumab had significantly better effect on preventing secondary vertebral fracture than alendronate (RR, 0.64, 95% CI, 0.49–0.84, p = 0.001; Additional file 4f).
Difference between the effects of alendronate and denosumab on preventing non-vertebral fracture was not statistically different (RR, 1.49; 95% CI, 0.52–4.24; p = 0.46; Additional file 3dd), neither was between romosozumab and alendronate (RR, 0.74; 95% CI, 0.54–1.00, p = 0.05; Additional file 3ee).
Discussion
In this study, we focused on osteoporosis patients with a history of OVCF. We collected related RCTs, synthesized their results, and finally estimated the secondary prevention effects of the medications on OVCF. We found zoledronate, alendronate, risedronate, etidronate, ibandronate, minodronate, pamidronate, PTH, denosumab, romosozumab and SERMs had significant secondary prevention effect on OVCF. In the comparisons between the medications, teriparatide had a significantly superior effect to risedronate, and the quality of evidence was high. The effects of risedronate, ibandronate, PTH, and SERMs were supported by moderate quality evidence and the effects of alendronate, denosumab were supported by high quality evidence.
In the result of discontinuation due to adverse events, PTH was the only intervention that significantly elevated the ratio. None of the bisphosphonates increased the risk of GI complaints. Zoledronate, risedronate, and PTH had significant effect on preventing non-vertebral fracture in patients with prevalent OVCF.
Most of widely used BPs, include zoledronate, alendronate, risedronate, etidronate and ibandronate, had significant effect, which were supported by moderate quality evidences. Among the medications, risedronate and ALN are first line osteoporosis medications, whose effects have been proved by substantial evidence [5, 7]. Ibandronate is a nitrogen-containing BPs and IV injection of it allows for a dosing interval even longer than 2 months [59]. Zoledronate is another nitrogen-containing BPs that has the highest potency among clinical use BPs [60]. According to our result, 5 mg/year iv injection of zoledronate could significantly reduce the risk of secondary OVCF. The extremely low medication frequency could be its another advantage that might improve patients’ compliance rate. Significantly elevated adverse events ratio or rare adverse events caused by BPs (e.g. osteonecrosis of jaw or atypical fracture, etc.) was not 7reported in any trial. Insignificant difference in GI complaints between BPs and control group indicated properly administrated BPs might help avoiding the risk of GI complaints, which was consistent with previous studies [61].
PTH is a bone anabolic medication that has significant efficacy against OVCF [62]. In this study, moderate quality evidence proved that the injection of PTH or teriparatide significantly reduce the risk of secondary OVCF and even the lowest dosage (28.2 μg/week) showed a significant effect. Compare with risedronate, teriparatide showed significantly better effect, which indicated PTH might have better effect on preventing secondary OVCF. It was consistent with previous studies that proved PTH had better effect on spine BMD compare with bisphosphonate [63, 64]. But on the other side, the superiority of PTH over bisphosphonate on hip BMD remains controversial, and it has been showed the PTH had inferior effect on BMD of distal radius [63, 64]. .Also, PTH treatment was the only medication that was associated with a series of adverse events that increased the risk of discontinuation. The most frequent adverse event was nausea, and other complaints included vomiting, headache, dizziness, and leg cramps [43, 46].
SERMs included in the study were raloxifene and bazedoxifene. Both showed a significant effect in preventing secondary fracture. Raloxifene seemed to have a better effect when prescribed at a higher dosage, which was indicated by the significant and substantial heterogeneity between the two groups. Besides beneficial skeletal effects, SERMs reduce the risk of breast cancer [65]. However, an elevated risk of venous thromboembolic events due to raloxifene and bazedoxifene has been described [52]. Additionally, raloxifene significantly raises the risk of discontinuation [50]. Therefore, SERMs should be prescribed with an awareness of their risk of side effects.
Denosumab is a RANKL inhibitor that was proved to possess significant effect on preventing secondary OVCF. Side effects of it include skin rashes, infections, and osteonecrosis of the jaw [62], but presently, there was no significant difference in adverse events compared with control group. Additionally, Boonen et al. reported a significant reduction of fatal adverse events ratio with denosumab in patients with prevalent vertebral fracture [49]. One advantage of denosumab is its low dosing frequency, which might elevate compliance. Romosozumab is a sclerostin inhibitor that has been proved to have better effect on preventing secondary OVCF than alendronate. However, it should be noticed that the cardiac ischemic events and cerebrovascular events ratio were higher in romosozumab group. The role of sclerostin in vessels remains unclear, and the results from basic studies were controversial [66,67,68]. Therefore, further evaluation of safety profile of romosozumab is needed.
Unlike the superior effects on OVCF of most medications, only zoledronate, risedronate, and PTH had a significant effect on preventing non-vertebral fractures in patients with prevalent OVCF. Combined with the effects of medications on OVCF, the findings might indicate zoledronate, risedronate, and PTH might be better options for patients with prevalent OVCF. Additionally, denosumab and alendronate showed marginally significant effects. The results might have less credibility than the main outcome because of missed information concerning the non-vertebral fracture status of the participants. But, the patients included in this study could still be considered as having a high risk of non-vertebral fracture because prevalent vertebral fracture and low bone mineral density are potential risk factors of non-vertebral fractures [69, 70]. Therefore, the data might be instructive for clinical usage of the medications.
It must be noted that many phase 3 studies were excluded from this meta-analysis because the data of patients with prevalent fractures were not reported. The exclusion might cause an underestimation of the effects of some newly developed medications like denosumab and zoledronate. One limitation of this study include the absence of searching the gray literature, which might increase the risk of publication bias that might lead to an overestimation of the effect of newly developed medications like romosozumab and bazedoxifene. Also, we only included English written manuscript in this study. Though no solid evidence showed a bias caused by the language restriction [71,72,73], the manuscript written in other languages should be included in further studies for a more comprehensive understanding of the effects of the medications. The generalizability of results of GI complaints was limited, because most of the trials excluded patients with upper GI disease at baseline. Additionally, our criteria for assessing the risk of bias might be too stringent, which might underestimate the quality of evidence. Also, it should be noticed that the most common domains that downregulated the GRADE was the study limitation and imprecision. The same scenario has been reported in a review of systematic reviews, in which the authors indicated the need of high quality RCTs with large sample size for better clinical decisions [74]. In the aspect of study limitation, the two main categories of risk of bias that were rated as unclear to high risk of bias were performance bias and selection bias. For a higher quality of evidence, the report of study might better follow the guidance, like the CONSORT, and report the procedure of randomization and blinding could be great help. To decrease the impact from imprecision, RCTs with higher sample sizes were needed. Also, a report of a subgroup of population with prevalent fracture would help in expanding the sample size.
Most systematic reviews and meta-analyses included osteoporosis patients, regardless their fracture history that introduces indirectness in the results [5,6,7,8,9,10]. The results might be overestimated on patients had fracture history, and for optimized treatment, accurate analyses of OVCF patients is urged. However, only one systematic review satisfied the demand [11]. Compared with that, we included 14 more RCTs and new medicines such as romosozumab and abaloparatide that allowed for a more comprehensive review and comparisons between different medications. Also, our results included vertebral fracture, non-vertebral fracture, GI complaints of BPs and discontinuation due to AEs. In the end, we evaluated the quality of evidence. The updated information could offer more practical evidence for clinical use.
Our results are consistent with those from other systematic reviews about primary prevention of OVCF [5,6,7, 9, 75,76,77]. This could indicate that the medications have a consistent effect on osteoporosis patients, regardless their OVCF history. Also, medications used to prevent osteoporotic fracture had a low risk of severe adverse events in most of the 2–3 years follow-ups. Therefore, the benefits from reducing the risk of fracture, disability, and mortality very likely outweigh the disadvantages. But, careful evaluation of risk factors and arrangement of drug holidays are also necessary to minimize the risk of adverse events [78].
Lack of RCTs that compared interventions of secondary prevention effect limited our assessment of differences between interventions. Although indirect comparisons could be conducted through statistical analyses, high quality RCTs that provide direct evidence are necessary for a solid conclusion.
Conclusion
High to moderate quality evidence proved zoledronate, alendronate, risedronate, etidronate, ibandronate, PTH, denosumab and SERMs have significant effect on preventing secondary OVCF. Among them, zoledronate, risedronate and PTH also had significant effects on preventing non-vertebra fracture. Moderate quality evidence proved romosozumab had better effect than alendronate. High quality evidence proved that PTH had superior effect to risedronate, but that medication should be prescribed with caution because of its significantly higher risk of adverse events.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AEs:
-
Adverse events
- BPs:
-
Bisphosphonates
- BZA:
-
Bazedoxifene
- CIs:
-
Confidence intervals
- GI:
-
Gastrointestinal
- HRT:
-
Hormone replacement therapy
- OVCF:
-
Osteoporotic vertebral compression fracture
- PTH:
-
Parathyroid hormone
- RCTs:
-
Randomized controlled trials
- RLX:
-
Raloxifene
- SERMs:
-
Selective estrogen receptor modulators
References
Ballane G, Cauley JA, Luckey MM, El-Hajj Fuleihan G. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos Int. 2017;28(5):1531–42.
Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–33.
Naves M, Diaz-Lopez JB, Gomez C, Rodriguez-Rebollar A, Rodriguez-Garcia M, Cannata-Andia JB. The effect of vertebral fracture as a risk factor for osteoporotic fracture and mortality in a Spanish population. Osteoporos Int. 2003;14(6):520–4.
Teng GG, Curtis JR, Saag KG. Mortality and osteoporotic fractures: is the link causal, and is it modifiable? Clin Exp Rheumatol. 2008;26(5 Suppl 51):S125–37.
Wells G, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;(1):Cd004523.
Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P. Etidronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;(1):Cd003376.
Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;(1):Cd001155.
Murad MH, Drake MT, Mullan RJ, Mauck KF, Stuart LM, Lane MA, Abu Elnour NO, Erwin PJ, Hazem A, Puhan MA, et al. Comparative effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2012;97(6):1871–80.
Chen JF, Yang KH, Zhang ZL, Chang HC, Chen Y, Sowa H, Gurbuz S. A systematic review on the use of daily subcutaneous administration of teriparatide for treatment of patients with osteoporosis at high risk for fracture in Asia. Osteoporos Int. 2015;26(1):11–28.
Ellis AG, Reginster JY, Luo X, Bushmakin AG, Williams R, Sutradhar S, Mirkin S, Jansen JP. Indirect comparison of bazedoxifene vs oral bisphosphonates for the prevention of vertebral fractures in postmenopausal osteoporotic women. Curr Med Res Opin. 2014;30(8):1617–26.
Saito T, Sterbenz JM, Malay S, Zhong L, MacEachern MP, Chung KC. Effectiveness of anti-osteoporotic drugs to prevent secondary fragility fractures: systematic review and meta-analysis. Osteoporos Int. 2017;28(12):3289–300.
Rizzoli R. Postmenopausal osteoporosis: assessment and management. Best Pract Res Clin Endocrinol Metab. 2018;32(5):739–57.
Zanatta LB, Marcatto C, Ramos CS, Mañas N, Moreira C, Borba V. Use of pamidronate for osteoporosis treatment in public health care in Brazil. Rev Bras Reumatol (Engl Ed). 2017;57(6):514–20.
Ohishi T, Matsuyama Y. Minodronate for the treatment of osteoporosis. Ther Clin Risk Manag. 2018;14:729–39.
Furlan AD, Malmivaara A, Chou R, Maher CG, Deyo RA, Schoene M, Bronfort G, van Tulder MW. 2015 updated method guideline for systematic reviews in the Cochrane Back and neck group. Spine (Phila Pa 1976). 2015;40(21):1660–73.
Higgins JPT, Green S. CCochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Nakamura T, Fukunaga M, Nakano T, Kishimoto H, Ito M, Hagino H, Sone T, Taguchi A, Tanaka S, Ohashi M, et al. Efficacy and safety of once-yearly zoledronic acid in Japanese patients with primary osteoporosis: two-year results from a randomized placebo-controlled double-blind study (ZOledroNate treatment in efficacy to osteoporosis; ZONE study). Osteoporos Int. 2017;28(1):389–98.
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–41.
Kushida K, Shiraki M, Nakamura T, Kishimoto H, Morii H, Yamamoto K, Kaneda K, Fukunaga M, Inoue T, Nakashima M, et al. Alendronate reduced vertebral fracture risk in postmenopausal Japanese women with osteoporosis: a 3-year follow-up study. J Bone Miner Metab. 2004;22(5):462–8.
Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J, Favus M. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The alendronate phase III osteoporosis treatment study group. N Engl J Med. 1995;333(22):1437–43.
Clemmesen B, Ravn P, Zegels B, Taquet AN, Christiansen C, Reginster JY. A 2-year phase II study with 1-year of follow-up of risedronate (NE-58095) in postmenopausal osteoporosis. Osteoporos Int. 1997;7:488–95.
Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral efficacy with Risedronate therapy (VERT) study group. Osteoporos Int. 2000;11(1):83–91.
Sorensen OH, Crawford GM, Mulder H, Hosking DJ, Gennari C, Mellstrom D, Pack S, Wenderoth D, Cooper C, Reginster JY. Long-term efficacy of risedronate: a 5-year placebo-controlled clinical experience. Bone. 2003;32(2):120–6.
Fogelman I, Ribot C, Smith R, Ethgen D, Sod E, Reginster JY. Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial. BMD-MN study group. J Clin Endocrinol Metab. 2000;85(5):1895–900.
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH 3rd, Brown J, Eriksen EF, Hoseyni MS, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral efficacy with Risedronate therapy (VERT) study group. Jama. 1999;282(14):1344–52.
Guanabens N, Farrerons J, Perez-Edo L, Monegal A, Renau A, Carbonell J, Roca M, Torra M, Pavesi M. Cyclical etidronate versus sodium fluoride in established postmenopausal osteoporosis: a randomized 3 year trial. Bone. 2000;27(1):123–8.
Lyritis GP, Tsakalakos N, Paspati I, Skarantavos G, Galanos A, Androulakis C. The effect of a modified etidronate cyclical regimen on postmenopausal osteoporosis: a four-year study. Clin Rheumatol. 1997;16(4):354–60.
Montessori ML, Scheele WH, Netelenbos JC, Kerkhoff JF, Bakker K. The use of etidronate and calcium versus calcium alone in the treatment of postmenopausal osteopenia: results of three years of treatment. Osteoporos Int. 1997;7(1):52–8.
Shiota E, Tsuchiya K, Yamaoka K, Kawano O. Effect of intermittent cyclical treatment with etidronate disodium (HEBP) and calcium plus alphacalcidol in postmenopausal osteoporosis. J Orthop Sci. 2001;6(2):133–6.
Watts NB, Harris ST, Genant HK, Wasnich RD, Miller PD, Jackson RD, Licata AA, Ross P, Woodson GC 3rd, Yanover MJ, et al. Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. N Engl J Med. 1990;323(2):73–9.
Chesnut IC, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, Felsenberg D, Huss H, Gilbride J, Schimmer RC, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19(8):1241–9.
Recker R, Stakkestad JA, Chesnut CH 3rd, Christiansen C, Skag A, Hoiseth A, Ettinger M, Mahoney P, Schimmer RC, Delmas PD. Insufficiently dosed intravenous ibandronate injections are associated with suboptimal antifracture efficacy in postmenopausal osteoporosis. Bone. 2004;34(5):890–9.
Matsumoto T, Hagino H, Shiraki M, Fukunaga M, Nakano T, Takaoka K, Morii H, Ohashi Y, Nakamura T. Effect of daily oral minodronate on vertebral fractures in Japanese postmenopausal women with established osteoporosis: a randomized placebo-controlled double-blind study. Osteoporos Int. 2009;20(8):1429–37.
Reid IR, Wattie DJ, Evans MC, Gamble GD, Stapleton JP, Cornish J: Continuous Therapy With Pamindronate, A Potent Bisphosphonate, In Postmenopausal Osteoporosis. Journal of Clinical Endocrinology & Metabolism 1994;(6):1595–99.
Brumsen C, Papapoulos SE, Lips P, Geelhoed-Duijvestijn PH, Hamdy NA, Landman JO, McCloskey EV, Netelenbos JC, Pauwels EK, Roos JC, et al. Daily oral pamidronate in women and men with osteoporosis: a 3-year randomized placebo-controlled clinical trial with a 2-year open extension. J Bone Miner Res. 2002;17(6):1057–64.
Harris ST, Watts NB, Jackson RD, Genant HK, Wasnich RD, Ross P, Miller PD, Licata AA, Chesnut CH. Four-year study of intermittent cyclic etidronate treatment of postmenopausal osteoporosis: three years of blinded therapy followed by one year of open therapy. Am J Med. 1993;95(6):557–67.
Peichl P, Rintelen B, Kumpan W, Broll H. Increase of axial and appendicular trabecular and cortical bone density in established osteoporosis with intermittent nasal salmon calcitonin therapy. Gynecol Endocrinol. 1999;13(1):7–14.
Hodsman AB, Fraher LJ, Watson PH, Ostbye T, Stitt LW, Adachi JD, Taves DH, Drost D. A randomized controlled trial to compare the efficacy of cyclical parathyroid hormone versus cyclical parathyroid hormone and sequential calcitonin to improve bone mass in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 1997;82(2):620–8.
Chesnut CH 3rd, Majumdar S, Newitt DC, Shields A, Van Pelt J, Laschansky E, Azria M, Kriegman A, Olson M, Eriksen EF, et al. Effects of salmon calcitonin on trabecular microarchitecture as determined by magnetic resonance imaging: results from the QUEST study. J Bone Miner Res. 2005;20(9):1548–61.
Gutteridge DH, Stewart GO, Prince RL, Price RI, Retallack RW, Dhaliwal SS, Stuckey BG, Drury P, Jones CE, Faulkner DL, et al. A randomized trial of sodium fluoride (60 mg) +/− estrogen in postmenopausal osteoporotic vertebral fractures: increased vertebral fractures and peripheral bone loss with sodium fluoride; concurrent estrogen prevents peripheral loss, but not vertebral fractures. Osteoporos Int. 2002;13(2):158–70.
Wimalawansa SJ. A four-year randomized controlled trial of hormone replacement and bisphosphonate, alone or in combination, in women with postmenopausal osteoporosis. Am J Med. 1998;104(3):219–26.
Lufkin EG, Wahner HW, O'Fallon WM, Hodgson SF, Kotowicz MA, Lane AW, Judd HL, Caplan RH, Riggs BL. Treatment of postmenopausal osteoporosis with transdermal estrogen. Ann Intern Med. 1992;117(1):1–9.
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–41.
Nakamura T, Sugimoto T, Nakano T, Kishimoto H, Ito M, Fukunaga M, Hagino H, Sone T, Yoshikawa H, Nishizawa Y, et al. Randomized Teriparatide [human parathyroid hormone (PTH) 1-34] once-weekly efficacy research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J Clin Endocrinol Metab. 2012;97(9):3097–106.
Fujita T, Fukunaga M, Itabashi A, Tsutani K, Nakamura T. Once-weekly injection of low-dose Teriparatide (28.2 mug) reduced the risk of vertebral fracture in patients with primary osteoporosis. Calcif Tissue Int. 2014;94(2):170–5.
Greenspan SL, Bone HG, Ettinger MP, Hanley DA, Lindsay R, Zanchetta JR, Blosch CM, Mathisen AL, Morris SA, Marriott TB. Effect of recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146(5):326–39.
Cosman F, Hattersley G, Hu MY, Williams GC, Fitzpatrick LA, Black D. Effects of abaloparatide-sc on fractures and bone mineral density in subgroups of postmenopausal women with osteoporosis and varying baseline risk factors. J Clin Densitom. 2018;21(1):28.
Nakamura T, Matsumoto T, Sugimoto T, Hosoi T, Miki T, Gorai I, Yoshikawa H, Tanaka Y, Tanaka S, Sone T, et al. Clinical trials express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: denosumab fracture intervention randomized placebo controlled trial (DIRECT). J Clin Endocrinol Metab. 2014;99(7):2599–607.
Boonen S, Adachi JD, Man Z, Cummings SR, Lippuner K, Torring O, Gallagher JC, Farrerons J, Wang A, Franchimont N, et al. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab. 2011;96(6):1727–36.
Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple outcomes of Raloxifene evaluation (MORE) investigators. Jama. 1999;282(7):637–45.
Lufkin EG, Whitaker MD, Nickelsen T, Argueta R, Caplan RH, Knickerbocker RK, Riggs BL. Treatment of established postmenopausal osteoporosis with raloxifene: a randomized trial. J Bone Miner Res. 1998;13(11):1747–54.
Palacios S, Silverman SL, de Villiers TJ, Levine AB, Goemaere S, Brown JP, De Cicco NF, Williams R, Hines TL, Mirkin S, et al. A 7-year randomized, placebo-controlled trial assessing the long-term efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: effects on bone density and fracture. Menopause. 2015;22(8):806–13.
Kushida K, Fukunaga M, Kishimoto H, Shiraki M, Itabashi A, Inoue T, Kaneda K, Morii H, Nawata H, Yamamoto K, et al. A comparison of incidences of vertebral fracture in Japanese patients with involutional osteoporosis treated with risedronate and etidronate: a randomized, double-masked trial. J Bone Miner Metab. 2004;22:469–78.
Ito M, Tobinai M, Yoshida S, Hashimoto J, Nakamura T. Effect of monthly intravenous ibandronate injections on vertebral or non-vertebral fracture risk in Japanese patients with high-risk osteoporosis in the MOVER study. J Bone Miner Metab. 2017;35(1):58–64.
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377(15):1417–27.
Nakamura T, Nakano T, Ito M, Hagino H, Hashimoto J, Tobinai M, Mizunuma H, Grp MS: Clinical Efficacy on Fracture Risk and Safety of 0.5 mg or 1 mg/month Intravenous Ibandronate Versus 2.5 mg/day Oral Risedronate in Patients with Primary Osteoporosis. Calcified tissue international 2013;(2):137–146.
Hadji P, Zanchetta JR, Russo L, Recknor CP, Saag KG, McKiernan FE, Silverman SL, Alam J, Burge RT, Krege JH, et al. The effect of teriparatide compared with risedronate on reduction of back pain in postmenopausal women with osteoporotic vertebral fractures. Osteoporos Int. 2012;23:2141–50.
Kendler DL, Marin F, Zerbini CAF, Russo LA, Greenspan SL, Zikan V, Bagur A, Malouf-Sierra J, Lakatos P, Fahrleitner-Pammer A et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): A multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391(10117):230–40.
Di Munno O, Delle Sedie A. Efficacy of ibandronate: a long term confirmation. Clin Cases Miner Bone Metab. 2010;7(1):23–6.
Ruza I, Mirfakhraee S, Orwoll E, Gruntmanis U. Clinical experience with intravenous zoledronic acid in the treatment of male osteoporosis: evidence and opinions. Ther Adv Musculoskelet Dis. 2013;5(4):182–98.
Cryer B, Bauer DC. Oral bisphosphonates and upper gastrointestinal tract problems: what is the evidence? Mayo Clin Proc. 2002;77(10):1031–43.
Langdahl BL, Harslof T. Medical treatment of osteoporotic vertebral fractures. Ther Adv Musculoskelet Dis. 2011;3(1):17–29.
Shen L, Xie X, Su Y, Luo C, Zhang C, Zeng B. Parathyroid hormone versus bisphosphonate treatment on Bone mineral density in osteoporosis therapy: a meta-analysis of randomized controlled trials. PLoS One. 2011;6(10):e26267.
Liu CL, Lee HC, Chen CC, Cho DY. Head-to-head comparisons of bisphosphonates and teriparatide in osteoporosis: a meta-analysis. Clin Invest Med. 2017;40(3):E146–e157.
McClung MR. New management options for osteoporosis with emphasis on SERMs. Climacteric. 2015;18(Suppl 2):56–61.
Zhu D, Mackenzie NC, Millan JL, Farquharson C, MacRae VE. The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS One. 2011;6(5):e19595.
Claes KJ, Viaene L, Heye S, Meijers B, d’Haese P, Evenepoel P. Sclerostin: another vascular calcification inhibitor? J Clin Endocrinol Metab. 2013;98(8):3221–8.
Ominsky MS, Boyd SK, Varela A, Jolette J, Felx M, Doyle N, Mellal N, Smith SY, Locher K, Buntich S, et al. Romosozumab improves Bone mass and strength while maintaining Bone quality in Ovariectomized Cynomolgus monkeys. J Bone Miner Res. 2017;32(4):788–801.
Siris ES, Genant HK, Laster AJ, Chen P, Misurski DA, Krege JH. Enhanced prediction of fracture risk combining vertebral fracture status and BMD. Osteoporos Int. 2007;18(6):761–70.
Finigan J, Greenfield DM, Blumsohn A, Hannon RA, Peel NF, Jiang G, Eastell R. Risk factors for vertebral and nonvertebral fracture over 10 years: a population-based study in women. J Bone Miner Res. 2008;23(1):75–85.
Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, Mierzwinski-Urban M, Clifford T, Hutton B, Rabb D. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28(2):138–44.
Walpole SC. Including papers in languages other than English in systematic reviews: important, feasible, yet often omitted. J Clin Epidemiol. 2019;111:127–34.
Moher D, Pham B, Klassen TP, Schulz KF, Berlin JA, Jadad AR, Liberati A. What contributions do languages other than English make on the results of meta-analyses? J Clin Epidemiol. 2000;53(9):964–72.
Pandis N, Fleming PS, Worthington H, Salanti G. The quality of the evidence according to GRADE is predominantly low or very low in Oral health systematic reviews. PLoS One. 2015;10(7):e0131644.
Bianchi G, Sambrook P. Oral nitrogen-containing bisphosphonates: a systematic review of randomized clinical trials and vertebral fractures. Curr Med Res Opin. 2008;24(9):2669–77.
MacLean C, Newberry S, Maglione M, McMahon M, Ranganath V, Suttorp M, Timmer M. Systematic Review: Comparative Effectiveness of Treatments to Prevent Fractures in Men and Women with Low Bone Density or Osteoporosis. Ann Intern Med. 2008;143(3):17.
Crandall CJ, Newberry SJ, Diamant A, Lim YW, Gellad WF, Booth MJ, Motala A, Shekelle PG. Comparative effectiveness of pharmacologic treatments to prevent fractures: an updated systematic review. Ann Intern Med. 2014;161(10):711–23.
Varenna M, Bertoldo F, Di Monaco M, Giusti A, Martini G, Rossini M. Safety profile of drugs used in the treatment of osteoporosis: a systematical review of the literature. Reumatismo. 2013;65(4):143–66.
Acknowledgements
Not applicable.
Funding
This work was supported by Mid-career Researcher Program through NRF grant (2016R1A2B3015048) funded by the Korea government (MSIP).
Author information
Authors and Affiliations
Contributions
JHL conceived the study, both JHL and YZJ participated in the designing the study. BX, MJC and YZJ participated in searching and selection of the studies. BX, JHL and YZJ analyzed data. JHL and YZJ participated in interpreting the data and preparing the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Searching strategy. (DOC 19 kb)
Additional file 2:
Risk of bias summary. (DOCX 43 kb)
Additional file 3:
Forest plot of secondary outcomes. (DOCX 407 kb)
Additional file 4:
Comparison between bisphosphonate. (JPG 1284 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jin, YZ., Lee, J.H., Xu, B. et al. Effect of medications on prevention of secondary osteoporotic vertebral compression fracture, non-vertebral fracture, and discontinuation due to adverse events: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord 20, 399 (2019). https://doi.org/10.1186/s12891-019-2769-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-019-2769-8