Abstract
Background
High-concentration oxygen therapy causes increased arterial partial pressure of carbon dioxide (PaCO2) in patients with COPD, asthma, pneumonia, obesity and acute lung injury. The objective of these studies was to investigate whether this physiological response to oxygen therapy occurs in stable patients with neuromuscular disease or kyphoscoliosis, and bronchiectasis.
Methods
Three randomised cross-over trials recruited stable patients with neuromuscular disease or kyphoscoliosis (n = 20), bronchiectasis (n = 24), and COPD (n = 24). Participants were randomised to receive 50% oxygen and 21% oxygen (air), each for 30 min, in randomly assigned order. The primary outcome was transcutaneous partial pressure of carbon dioxide (PtCO2) at 30 min. The primary analysis was a mixed linear model.
Results
Sixty six of the 68 participants had baseline PtCO2 values < 45 mmHg. The intervention baseline adjusted PtCO2 difference (95% CI) between oxygen and room air after 30 min was 0.2 mmHg (− 0.4 to 0.9), P = 0.40; 0.5 mmHg (− 0.2 to 1.2), P = 0.18; and 1.3 mmHg (0.7 to 1.8), P < 0.001, in the neuromuscular/kyphoscoliosis, bronchiectasis and COPD participants respectively.
Conclusions
The small increase in PtCO2 in the stable COPD patients with high-concentration oxygen therapy contrasts with the marked increases in PaCO2 seen in the setting of acute exacerbations of COPD. This suggests that the model of studying the effects of high-concentration oxygen therapy in patients with stable respiratory disease is not generalisable to the use of oxygen therapy in the acute clinical setting. Appropriate studies of high-concentration compared to titrated oxygen in acute clinical settings are needed to determine if there is a risk of oxygen-induced hypercapnia in patients with neuromuscular disease, kyphoscoliosis or bronchiectasis.
Trial registration
Australian New Zealand Clinical Trials Registry ACTRN12615000970549 Registered 16/9/15, ACTRN12615000971538 Registered 16/9/15 and ACTRN12615001056583 Registered 7/10/15.
Similar content being viewed by others
Background
Oxygen has the potential to elevate arterial partial pressure of carbon dioxide (PaCO2) in patients with chronic obstructive pulmonary disease (COPD) [1,2,3,4,5]. The incidence and magnitude is variable and it can occur in both stable and acute exacerbations of COPD [1, 5]. Oxygen-induced hypercapnia is likely to be clinically important in the setting of acute exacerbations of COPD. A randomised-controlled trial (RCT) comparing high-concentration and titrated oxygen (to achieve arterial oxygen saturation (SpO2) of 88 to 92%) in patients with an acute COPD exacerbation identified an over two-fold increase in mortality with high-concentration oxygen [6]. Consequently oxygen therapy guidelines recommend titration of oxygen therapy within this SpO2 range in patients with acute exacerbations of COPD, to avoid the risks of both hypoxaemia and hyperoxaemia [2, 3].
A number of mechanisms have been proposed to explain how oxygen therapy increases PaCO2. These include a reduction in hypoxic drive to breathe leading to decreased ventilation, and the release of hypoxic pulmonary vasoconstriction leading to worsening of ventilation-perfusion mismatch and increased alveolar deadspace [5, 7]. RCTs report high-concentration oxygen therapy increases PaCO2 in patients with asthma [8], pneumonia [9], and obesity [10,11,12]. This suggests oxygen-induced hypercapnia might occur in a range of respiratory conditions with abnormal gas exchange and/or reduced ventilation with respiratory failure.
Neuromuscular disease and kyphoscoliosis can lead to hypoventilation and chronic respiratory failure; airflow obstruction and ventilation-perfusion mismatch are both features of bronchiectasis. As acute respiratory illnesses complicate both of these conditions and can result in hypoxia and the need for oxygen therapy, it is important to establish whether these patients are at risk of oxygen-induced hypercapnia. Two small sleep studies with a total of 17 participants [13, 14] and one exercise study in 22 participants [15] have been performed in patients with cystic fibrosis, which demonstrated average transcutaneous partial pressure of carbon dioxide (PtCO2) increases between 4 [15] and 7.5 mmHg [13] during oxygen therapy compared to room air. In patients with neuromuscular disease data are limited to retrospective case examples [16] and a retrospective case series in eight patients with a range of neuromuscular diseases [17]. In the case series, low-flow oxygen (0.5-2 L/min) was associated with an elevation in PaCO2 by an average 28 mmHg, however the measurements were made up to six days after oxygen therapy.
The purpose of our randomised cross-over trials was to investigate the effects of 50% oxygen compared to 21% oxygen in patients with stable neuromuscular disease or kyphoscoliosis and patients with stable bronchiectasis. To assess the applicability of the results to the clinical setting, we also studied stable COPD patients, matched by severity of airflow obstruction to the bronchiectasis patients. Our hypothesis was that oxygen therapy would increase PtCO2 in all three trials.
Methods
Overview
This series of three double-blind cross-over trials randomised patients to the order they received 50% oxygen (“oxygen” intervention) and medical grade air containing 21% oxygen (“air” intervention). The trials recruited 20 patients with neuromuscular disease or kyphoscoliosis (Neuromuscular/kyphoscoliosis Study), 24 patients with bronchiectasis (Bronchiectasis Study) and 24 patients with COPD (COPD Study). Each trial was prospectively registered on ANZCTR (ACTRN12615000970549, ACTRN12615000971538 and ACTRN12615001056583, respectively), and had Health and Disability Ethics Committee approval (see Online Supplement). Inclusion and exclusion criteria are presented in Table 1, including the criteria by which Bronchiectasis and COPD participants were matched by airflow obstruction severity.
Potentially eligible patients were recruited through Hutt Valley Hospital, Wellington Regional Hospital and the Medical Research Institute of New Zealand (MRINZ) patient lists, as well as newsletters and posters. Participants attended a single study visit at the MRINZ. After confirming eligibility, study baseline PtCO2, heart rate, and SpO2 were recorded via a SenTec transcutaneous monitor (SenTec AG, Switzerland), and respiratory rate by investigator observation.
Participants were then fitted with a full-face positive airway pressure mask (Respironics), attached to a Douglas Bag (Hans Rudolph) via CO2SMO adapter (Novametrix Medical Systems), one-way T valve, respiratory filter (Microgard II, Carefusion), tubing and three-way tap, all connected in series. Participants breathed room air for at least 5 mins to adjust to breathing through the equipment, and then breathed the intervention gas for 30 min. After each intervention, the mask was removed and there was a 30 min observation period breathing room air.
Endpoint measurement
The primary endpoint was PtCO2 after 30 min.
Heart rate and PtCO2 were measured via SenTec. Respiratory rate, end tidal carbon dioxide (ETCO2), minute ventilation and dead space to tidal volume (VD/VT) were measured via CO2SMO (Model 8100), from which the tidal volume, volume of dead space, alveolar volume and alveolar minute ventilation were calculated (see Online Supplement for further detail regarding equipment methodology). Measurements were taken at T = 0 (following mask stabilisation and immediately prior to intervention), and at 10 min intervals during the intervention and observation periods. SpO2 on the SenTec display was covered during the intervention and washout periods to maintain investigator blinding. PtCO2 was monitored continuously; the intervention was stopped if values rose by ≥10 mmHg from T = 0.
The study statistician, who was not involved in study recruitment or visits, created computerised 1:1 randomisation sequences for each study. Randomisation codes were placed in sealed opaque envelopes and opened by the unblinded investigator following T = 0 measurements. The blinded investigator recorded all measurements from this point onwards.
Analyses
The primary analysis was a mixed linear model with fixed effects for the T = 0 measurements, intervention, and randomisation order, and a random effect for participants to take into account the cross-over design. For all measures an interaction term was tested first to see if there was any difference between the interventions that depended on the time of measurement. As secondary analyses for all outcomes, the differences between interventions at each measurement time were analysed by similarly structured models, with addition of the fixed effect of the time of measurement and a random effect for each participant using a spatial exponential in time repeated measures variance-covariance matrix to account for the cross-over design. The results of this model for PtCO2 were compared between the COPD and Bronchiectasis study participants (as fixed effects), and were also adjusted for forced expiratory volume in one second (FEV1) percentage predicted. Finally the difference in proportions of participants with a change in PtCO2 of ≥ 4 mmHg and ≥ 10 mmHg from T = 0 were also estimated, as physiologically and clinically significant differences, respectively [8, 9, 23]. All estimates of differences are shown as oxygen minus room air.
Software used
SAS version 9.4 was used.
Sample size
The intended sample size for each cross-over study was 24 based on 80% power and a type I error rate of 5%, to detect a difference of 2.4 mmHg. This is half the difference found in a study of participants with obesity hypoventilation syndrome which reported a mean (SD) paired difference of 5 (4) mmHg [10].
Results
Participants
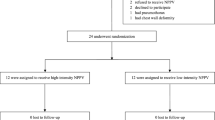
Participants were recruited between October 2015 and May 2017 and the CONSORT diagrams are shown in Fig. 1. The Neuromuscular/kyphoscoliosis study recruitment was stopped at 20 participants due to difficulty in recruitment. Participant characteristics are summarised in Table 2. The COPD group had higher smoking rates than the other two groups, and had similar severity of airflow obstruction as the Bronchiectasis group. One participant in the COPD study had a SpO2 of 87% at study baseline, all other participants had a SpO2 of ≥91%. All study baseline PtCO2 values were < 45 mmHg, with the exception of two participants in the Neuromuscular/kyphoscoliosis group.
Flow of participants through studies. BMI: Body mass index, COPD: Chronic obstructive pulmonary disease, CT: Computerised tomography, FEV1: Forced expiratory volume in 1 s, FVC: Forced vital capacity, SNIP: Sniff nasal inspiratory pressure. *Burkholderia or other result at the investigator’s discretion. ** T valve tubing malfunction for 1 participant in the Bronchiectasis study and Sentec maintenance error for 1 participant in the COPD study. See Online Supplement for further detail. See legend of Table 3 and Online Supplement for further N value details
PtCO2
PtCO2 rose after the mask was applied in both interventions, returning to study baseline within 10 min of removal. At T = 0, (i.e. after placement and stabilisation of the mask, but prior to receiving the intervention) the average PtCO2 increase was at least 1.3 mmHg higher than the last PtCO2 measurement prior to mask placement (Table 3).
Figure 2 demonstrates PtCO2 over the course of the study (see Online Supplement for individual time point data). The difference (95% CI) in PtCO2 at 30 min between oxygen and room air, adjusted for T = 0 PtCO2, was 0.2 mmHg (− 0.4 to 0.9), P = 0.40; 0.5 mmHg (− 0.2 to 1.2), P = 0.18; and 1.3 mmHg (0.7 to 1.8), P < 0.001, in the Neuromuscular/kyphoscoliosis, Bronchiectasis and COPD participants respectively (Table 3). PtCO2 did not increase or decrease by ≥4 mmHg from T = 0 during the interventions, with the exception of one Bronchiectasis and one COPD participant, during the oxygen intervention only (increases of 4.8 mmHg and 4.7 mmHg respectively). The interaction terms between the time points (10, 20 and 30 min) were not significantly different. The mixed linear model estimates for the differences in PtCO2 across all time points and adjusted for T = 0 were slightly higher during the oxygen intervention compared to room air in the Bronchiectasis and COPD studies (Table 3). When compared to the Bronchiectasis participants, the COPD participants had a greater mean difference in PtCO2 adjusted for T = 0 between the oxygen and air interventions: 0.90 mmHg (95% CI 0.5 to 1.3), P < 0.001. There was no change to this estimate after incorporation of FEV1 percentage predicted as a potential confounder.
Transcutaneous partial pressure of carbon dioxide over time. COPD: Chronic obstructive pulmonary disease, PtCO2: Transcutaneous partial pressure of carbon dioxide. Blue lines represent oxygen intervention data, red represent air intervention data. Values are mean plus/minus 1SD. The solid grey line represents study baseline PtCO2 (prior to the start of the first intervention while breathing room air without a mask). T = 0 to T = 30 are measurements taken while wearing the study mask. T = 40 to T = 60 are washout measurements breathing room air, not wearing the study mask. See legend of Table 3 and Online Supplement for N value details
Changes in other respiratory measures
Secondary outcomes are presented in Table 4. In the Bronchiectasis participants, the mean ETCO2 decreased by 1.0 mmHg during the oxygen intervention, compared with air. This was associated with a small increase in dead space (0.01 L) and VD/VT (0.03). In the COPD group the mean ETCO2 decreased by 1.1 mmHg, and this was associated with a small reduction in alveolar minute ventilation (0.21 L/min) and increase in VD/VT (0.023).
Discussion
These randomised cross-over studies have shown that 50% oxygen for 30 min did not result in clinically significant increases in PtCO2 at 30 min in patients with stable COPD, Bronchiectasis or Neuromuscular disease/Kyphoscoliosis. In the patients with stable COPD the mean PtCO2 increase with high-concentration oxygen therapy was 1.3 mmHg; while this was statistically significant, the magnitude of the change is not of clinical significance. This is in contrast with the marked increases in PaCO2 seen in the setting of acute exacerbations of COPD [1, 5, 6]. This suggests that the results of all three studies in stable patients are unlikely to be generalisable to the use of oxygen therapy in the acute clinical setting.
To our knowledge there have been no RCTs investigating the effects of oxygen on PaCO2 in acutely unwell patients with neuromuscular disease, kyphoscoliosis or bronchiectasis. However the risk of oxygen-induced hypercapnia has been well established through RCTs comparing high-concentration and titrated oxygen regimens in patients with acute COPD exacerbations [6]. We undertook the current studies in patients with neuromuscular disease, kyphoscoliosis or bronchiectasis while stable, recognising that the results from stable patients in the laboratory setting may not translate to the clinical setting. We therefore conducted the study in COPD patients, as a comparator group in which clinically relevant oxygen-induced PaCO2 elevations have been demonstrated [1, 5, 6]. The study baseline SpO2 and PtCO2 values were comparable across all three studies, and FEV1 percentage predicted matching between the COPD and Bronchiectasis patients ensured recruitment of patients with similar physiological impairment in terms of airflow obstruction. Contrary to findings in the acute setting [1, 5, 6], oxygen administration did not result in a clinically significant change in PtCO2 in the stable COPD patients, indicating that the study model was not an appropriate method to detect the potential for oxygen-induced hypercapnia in patients with neuromuscular disease, kyphoscoliosis or bronchiectasis in clinical practice.
A number of factors may explain the minimal PtCO2 change in the COPD participants. Firstly, oxygen delivery was via a closed-circuit system, rather than standard masks used in clinical practice. This ensured precise fraction of inspired oxygen (FiO2) administration and allowed deadspace and ventilation measurement. However, this method of delivery may have affected participant’s responses to the interventions, particularly as breathing through the study mask consistently resulted in a small increase in PtCO2. Secondly, the studies were conducted in stable, rather than acutely unwell, patients. This allowed randomised cross-over trial design and meant that participants were more likely to tolerate study procedures. However, the physiological response to oxygen in stable patients may not translate to the acute setting. Previous studies investigating oxygen delivery to stable COPD patients have had variable results, ranging from no or small changes in mean PaCO2 or PtCO2 [24,25,26,27,28,29,30,31] to marked increases [23, 32,33,34,35,36,37,38]. Two studies have compared the effects of identical oxygen regimens in patients when having an acute exacerbation of their respiratory disease and when stable. Rudolf et al. found that an FiO2 of up to 0.28 for 1 h increased PaCO2 by 9, 15 and 31 mmHg compared with air in three patients with exacerbation of chronic respiratory failure [39]. However, the same oxygen regimen did not alter PaCO2 more than 3 mmHg when the same three patients were stable. Similarly, Aubier et al. found 30 min of oxygen via a mouthpiece increased average PaCO2 by 10.1 mmHg in 12 patients during a COPD exacerbation [25]. It increased by only 2.8 mmHg when the same patients were stable. The differences in response between acute and stable disease may relate to lower tidal volumes and/or a greater degree of hypoxic pulmonary vasoconstriction and ventilation/perfusion mismatch that occur in acute COPD exacerbation, which are further modified by oxygen therapy [40, 41]. Additionally, acutely unwell patients are more likely to have lower SpO2 levels and elevated PaCO2 levels. While hypercapnia and hypoxaemia are not necessarily prerequisites for oxygen-induced hypercapnia [38, 42], both have been associated with increased likelihood and magnitudes of oxygen-induced elevations in PaCO2 [10, 12, 23, 25, 33, 38]. In support of this, previous studies in stable COPD demonstrating significant oxygen-induced increases in PaCO2 have had participants with lower baseline blood oxygen levels [32, 34, 35, 38], and/or higher baseline PaCO2 values [23, 32,33,34,35,36,37,38] than the participants in the current three studies. Additionally, only one of the participants, from the COPD study, had a study baseline SpO2 of 87%. All other participants had saturations ≥91%, meaning they were well above the SpO2 level at which initiation of oxygen therapy is recommended in the acute clinical setting [2, 3].
Oxygen therapy has previously been demonstrated to increase VD/VT [10, 11, 23, 27, 35] and reduce ETCO2 [43]. However, caution is needed in interpreting the small increases in VD/VT and reductions in ETCO2 during the oxygen intervention in the Bronchiectasis and COPD studies. These values were recorded by CO2SMO and increased oxygen concentrations in the respiratory circuit could systematically decrease the displayed ETCO2 while still keeping it within the manufacturer’s error range of up to 5% (User manual Oct 10, 1997). This decrease could explain the small changes in VD/VT and ETCO2 observed.
There are a number of methodological issues relevant to interpretation of the study findings. Transcutaneous monitoring was used as a surrogate for PaCO2 by arterial blood gas (ABG). ABGs and capillary blood gas sampling were not used to measure PaCO2 during the interventions as they do not provide continuous measurement and cause discomfort. Additionally, ABG sampling carries risk of ischaemia. Our study outcome measures were change in PtCO2 over time, which the SenTec has been demonstrated to accurately determine [44, 45], with an estimate of bias for change in PtCO2 of − 0.03 mmHg (95% CI − 0.44 to 0.38) p = 0.89 when compared to arterialised blood gas values in COPD patients [45]. Only 20 participants were recruited to the Neuromuscular/kyphoscoliosis study, however this did not affect the power to detect a difference in PtCO2 between the interventions, given the SD was lower than that used for sample size calculation.
Conclusion
Delivery of 50% oxygen for 30 min did not result in a clinically significant increase in PtCO2 in stable outpatients with neuromuscular disease, kyphoscoliosis, bronchiectasis or COPD. This indicates the model used is an inappropriate method for evaluating the risks of oxygen-induced hypercapnia in the acute clinical setting and highlights the limitations of interpreting results from studies in stable patients in the laboratory setting. It is recommended that future studies into the risks of oxygen-induced hypercapnia are undertaken through comparison of high-concentration oxygen to titrated oxygen in the acute respiratory illnesses that complicate neuromuscular disease, kyphoscoliosis and bronchiectasis. In the interim, current evidence of the potential for oxygen-induced hypercapnia to occur across a range of respiratory conditions [6, 8, 9, 12] supports guideline recommendations to titrate oxygen therapy in all patients to avoid the risks of hyperoxaemia as well as hypoxaemia.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body Mass Index
- COPD:
-
Chronic Obstructive Pulmonary Disease
- CT:
-
Computerised Tomography
- ETCO2 :
-
End Tidal Carbon Dioxide
- FEV1 :
-
Forced Expiratory Volume in 1 s
- FiO2 :
-
Fraction of Inspired Oxygen
- FVC:
-
Forced Vital Capacity
- MRINZ:
-
Medical Research Institute of New Zealand
- PaCO2 :
-
Arterial Partial Pressure of Carbon Dioxide
- PtCO2 :
-
Transcutaneous Partial Pressure of Carbon Dioxide
- RCT:
-
Randomised Controlled Trial
- SNIP:
-
Sniff Nasal Inspiratory Pressure
- SpO2 :
-
Oxygen Saturation
- VC:
-
Vital Capacity (slow)
- VD/VT:
-
Ratio of Dead Space to Tidal Volume
References
Murphy R, Driscoll P, O’Driscoll R. Emergency oxygen therapy for the COPD patient. Emerg Med J. 2001;18:333–9.
O’Driscoll BR, Howard LS, Earis J, Mak V, British Thoracic Society Emergency Oxygen Guideline Group, BTS Emergency Oxygen Guideline Development Group. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72:ii1–ii90.
Beasley R, Chien J, Douglas J, Eastlake L, Farah C, King G, Moore R, Pilcher J, Richards M, Smith S, Walters H. Thoracic Society of Australia and New Zealand oxygen guidelines for acute oxygen use in adults: ‘swimming between the flags’. Respirology. 2015;20:1182–91.
Pilcher J, Perrin K, Beasley R. The effect of high concentration oxygen therapy on PaCO2 in acute and chronic respiratory disorders. Transl Respir Med. 2013;1:1–3.
Pilcher J, Weatherall M, Perrin K, Beasley R. Oxygen therapy in acute exacerbations of chronic obstructive pulmonary disease. Expert Rev Respir Med. 2015;9:287–93.
Austin MA, Wills KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:c5462.
New A. Oxygen: kill or cure? Prehospital hyperoxia in the COPD patient. Emerg Med J. 2006;23:144–6.
Perrin K, Wijesinghe M, Healy B, Wadsworth K, Bowditch R, Bibby S, Baker T, Weatherall M, Beasley R. Randomised controlled trial of high concentration versus titrated oxygen therapy in severe exacerbations of asthma. Thorax. 2011;66:937–41.
Wijesinghe M, Perrin K, Healy B, Weatherall M, Beasley R. Randomized controlled trial of high concentration oxygen in suspected community-acquired pneumonia. J R Soc Med. 2011;105:208–16.
Wijesinghe M, Williams M, Perrin K, Weatherall M, Beasley R. The effect of supplemental oxygen on hypercapnia in subjects with obesity-associated hypoventilation: a randomized, crossover, clinical study. Chest. 2011;139:1018–24.
Hollier CA, Harmer AR, Maxwell LJ, et al. Moderate concentrations of supplemental oxygen worsen hypercapnia in obesity hypoventilation syndrome: a randomised crossover study. Thorax. 2014;69:346–53.
Pilcher J, Richards M, Eastlake L, McKinstry SJ, Bardsley G, Jefferies S, Braithwaite I, Weatherall M, Beasley R. High flow or titrated oxygen for obese medical inpatients: a randomised crossover trial. Med J Aust. 2017;207:430–4.
Gozal D. Nocturnal ventilatory support in patients with cystic fibrosis: comparison with supplemental oxygen. Eur Respir J. 1997;10:1999–2003.
Spier S, Rivlin J, Hughes D, Levison H. The effect of oxygen on sleep, blood gases, and ventilation in cystic fibrosis. Am Rev Respir Dis. 1984;129:712–8.
Marcus CL, Bader D, Stabile MW, Wang CI, Osher AB, Keens TG. Supplemental oxygen and exercise performance in patients with cystic fibrosis with severe pulmonary disease. Chest. 1992;101:52–7.
Chiou M, Bach JR, Saporito R, Albert O. Quantitation of oxygen-induced hypercapnia in respiratory pump failure. Revista Portuguesa de Pneumologia (English Edition). 2016;22:262–5.
Gay PC, Edmonds LC. Severe hypercapnia after low-flow oxygen therapy in patients with neuromuscular disease and diaphragmatic dysfunction. Mayo Clin Proc. 1995;70:327–30.
Johnston CE, Richards BS, Sucato DJ, Bridwell KH, Lenke LG, Erickson M. Correlation of preoperative deformity magnitude and pulmonary function tests in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2011;36:1096–102.
Benditt J, Boitano L. Pulmonary issues in patients with chronic neuromuscular disease. Am J Respir Crit Care Med. 2013;187:1046–55.
Uldry C, Fitting JW. Maximal values of sniff nasal inspiratory pressure in healthy subjects. Thorax. 1995;50:371–5.
Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. General considerations for lung function testing. Eur Respir J. 2005;26:153–61.
NICE guidelines. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. Clinical guideline published: 23 June 2010. https://www.nice.org.uk/guidance/cg101/resources/chronic-obstructive-pulmonary-disease-in-over-16s-diagnosis-and-mana. Accessed Apr 2020.
O’Donnell DE, D’Arsigny C, Fitzpatrick M, Webb KA. Exercise hypercapnia in advanced chronic obstructive pulmonary disease: the role of lung hyperinflation. Am J Respir Crit Care Med. 2002;166:663–8.
Mithoefer JC, Karetzky MS, Mead GD. Oxygen therapy in respiratory failure. N Engl J Med. 1967;277:947–9.
Aubier M, Murciano D, Fournier M, Milic-Emili J, Pariente R, Derenne JP. Central respiratory drive in acute respiratory failure of patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1980;122:191–9.
Mithoefer JC, Keighley JF, Cook WR. The AaDO2 and venous admixture at varying inspired oxygen concentrations in chronic obstructive pulmonary disease. Crit Care Med. 1978;6:131–5.
Castaing Y, Manier G, Guenard H. Effect of 26% oxygen breathing on ventilation and perfusion distribution in patients with cold. Clin Respir Physiol. 1985;21:17–23.
O’Donnell DE, Bain DJ, Webb KA. Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitation. Am J Respir Crit Care Med. 1997;155:530–5.
Saadjian AY, Philip-Joet FF, Levy S, Arnaud A. Vascular and cardiac reactivity in pulmonary hypertension due to chronic obstructive lung disease: assessment with various oxygen concentrations. Eur Respir J. 1992;5:525–30.
Saadjian AY, Philip-Joet FF, Barret A, Levy S, Amaud AG. Effect of almitrine bismesylate on pulmonary vasoreactivity to hypoxia in chronic obstructive pulmonary disease. Eur Respir J. 1994;7:862–8.
King A, Cooke N, Leitch A, Flenley D. The effects of 30% oxygen on the respiratory response to treadmill exercise in chronic respiratory failure. Clin Sci. 1973;44:151–62.
Schiff MM, Massaro D. Effect of oxygen administration by a Venturi apparatus on arterial blood gas values in patients with respiratory failure. N Engl J Med. 1967;277:950–3.
Lopez-Majano V, Dutton RE. Regulation of respiration during oxygen breathing in chronic obstructive lung disease. Am Rev Respir Dis. 1973;108:232–40.
Prime FJ, Westlake EK. The respiratory response to CO2 in emphysema. Clin Sci. 1954;13:321–32.
Sassoon CS, Hassell KT, Mahutte CK. Hyperoxic-induced hypercapnia in stable chronic obstructive pulmonary disease. Am Rev Respir Dis. 1987;135:907–11.
Vos PJ, Folgering HT, de Boo TM, Lemmens WJ, van Herwaarden CL. Effects of chlormadinone acetate, acetazolamide and oxygen on awake and asleep gas exchange in patients with chronic obstructive pulmonary disease (COPD). Eur Respir J. 1994;7:850–5.
Bone RC, Pierce AK, Johnson RL Jr. Controlled oxygen administration in acute respiratory failure in chronic obstructive pulmonary disease: a reappraisal. Am J Med. 1978;65:896–902.
Chiang LL, Hung TC, Ho SC, Lin HC, Yu CT, Wang CH, Kuo HP. Respiratory response to carbon dioxide stimulation during low flow supplemental oxygen therapy in chronic obstructive pulmonary disease. J Formosan Med Assoc. 2002;101:607–15.
Rudolf M, Banks RA, Semple SJ. Hypercapnia during oxygen therapy in acute exacerbations of chronic respiratory failure. Hypothesis revisited. Lancet (London, England). 1977;2:483–6.
Calverley PM. Respiratory failure in chronic obstructive pulmonary disease. Eur Respir J. 2003;47(Suppl):26s–30s.
Stradling JR. Hypercapnia during oxygen therapy in airways obstruction: a reappraisal. Thorax. 1986;41:897–902.
Bardsley G, Pilcher J, McKinstry S, Shirtcliffe P, Berry J, Fingleton J, Weatherall M, Beasley R. Oxygen versus air-driven nebulisers for exacerbations of chronic obstructive pulmonary disease: a randomised controlled trial. BMC Pulmonary Medicine. 2018. https://doi.org/10.1186/s12890-018-0720-7.
Yamauchi H, Ito S, Sasano H, Azami T, Fisher J, Sobue K. Dependence of the gradient between arterial and end-tidal P CO2 on the fraction of inspired oxygen. Br J Anaesth. 2011;107:631–5.
Rodriguez P, Lellouche F, Aboab J, Buisson CB, Brochard L. Transcutaneous arterial carbon dioxide pressure monitoring in critically ill adult patients. Intens Care Med. 2006;32:309–12.
Fingleton J, McKinstry S, Pilcher J, Weatherall M, Beasley R, Bardsley G. Accuracy of transcutaneous carbon dioxide measurement for change over time. Respirology. 2017;22:18–100. https://doi.org/10.1111/resp.13009.
Acknowledgements
We would like to give special thanks to all of the participants for their involvement in our study. Leonie Eastlake, James Gilchrist, Emily Tweedale, Joe Singer and Alice McDouall (all of the Medical Research Institute of New Zealand) were all Investigators who made significant contributions to the conduct of one or more of the studies.
Funding
The studies received funding from the Health Research Council of New Zealand (Independent Research Organisations Capability Fund and Clinical Research Fellowship for JP) and Capital and Coast District Health Board. The Neuromuscular/ kyphoscioliosis disease study specifically received funding from the Neuromuscular Research Foundation Trust, funded by the Richdale Charitable Trust. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
RB was the principal investigator for the study, is guarantor for the study, and affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. DT, SE, MW1, GB, HC, MR, SB, RS and JP were investigators on the study and collected the data. MW2 performed the statistical analysis. JP wrote the first draft of the manuscript. JP and RB conceived the study and wrote the first draft of the protocol. All authors contributed to study design, interpretation of results, manuscript writing, and reviewed the final manuscript prior to submission. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. No writing assistance was received.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained from the Health and Disability Ethics Committee, New Zealand (References: Neuromuscular disease/ Kyphoscoliosis CEN/11/11/065; Bronchiectasis CEN/11/12/075; COPD 13/STH/200). Written informed consent was obtained before any study-specific procedures. No participant was under the age of 16.
Consent for publication
Not applicable.
Competing interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf. All authors have no competing interests to declare, other than the MRINZ receiving research funding from Health Research Council of New Zealand.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pilcher, J., Thayabaran, D., Ebmeier, S. et al. The effect of 50% oxygen on PtCO2 in patients with stable COPD, bronchiectasis, and neuromuscular disease or kyphoscoliosis: randomised cross-over trials. BMC Pulm Med 20, 125 (2020). https://doi.org/10.1186/s12890-020-1132-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-020-1132-z