Abstract
Background
In non-small cell lung cancer (NSCLC) patients, concomitant idiopathic pulmonary fibrosis (IPF) and emphysema (CPFE) are independently related to poor survival. CPFE is a condition with features of both pulmonary fibrosis and emphysema. Here, we evaluated the effect of CPFE and IPF alone on the outcomes of NSCLC patients.
Patients and methods
We retrospectively evaluated 283 patients with CPFE or IPF who were diagnosed with NSCLC between November 2003 and February 2018 at two tertiary care hospitals in South Korea. Patients were classified into CPFE and IPF groups according to chest computed tomography findings.
Results
One-hundred-and-seven patients (37.8%; mean age: 70.1 years; men 97.2%) had CPFE. Compared with IPF patients, CPFE patients had a heavier smoking history; lower diffusing capacity of carbon monoxide (78.0% vs 64.8%, p < 0.001), and lower forced expiratory volume in 1 s. Of all patients with NSCLC, 71.7% overall died during the follow-up period; 71.6% died in the CPFE group and 72.0% in the IPF group. Multivariate logistic regression analysis showed that CPFE (odds ratio [OR]: 2.26, 95% confidence interval [CI]: 1.09–4.69; P = 0.029) was significantly correlated with acute exacerbations (AEs). In a Cox proportional hazards analysis, stage > III NSCLC, higher Eastern Cooperative Oncology Group performance status, and higher gender–age–physiology index score was related to higher mortality. However, CPFE was not related to a higher mortality rate in univariate (hazard ratio [HR]: 1.00; 95% CI: 0.75–1.32, P = 0.972) or multivariate analysis (HR: 0.89; 95% CI: 0.66–1.21, P = 0.466).
Conclusions
AE risk, but not all-cause mortality, was higher in patients with CPFE and NSCLC than in those with IPF and NSCLC. Physicians should be aware of the exaggerated risk of AE in patients with concomitant CPFE and NSCLC.
Similar content being viewed by others
Background
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive fibrosing interstitial pneumonia, characterized by progressively worsening dyspnea and lung function, and is associated with a poor prognosis [1]. IPF is reportedly associated with an increased risk of lung cancer [2]. Furthermore, in non-small cell lung cancer (NSCLC) patients, concomitant IPF is related to poor survival [2, 3].
Emphysema is defined as an enlargement of the air spaces distal to the terminal bronchioles due to destruction of the tissues comprising their walls, and can result in an obstructive pattern. Emphysema is also associated with an increased risk of lung cancer [4]. Furthermore, the presence of emphysema in NSCLC patients is also related to a poor prognosis [5].
Emphysema and IPF, which have different radiological, pathological, functional, and prognostic characteristics, have long been regarded as separate entities. However, the coexistence of emphysema and pulmonary fibrosis in individuals is being increasingly recognized [6]. In 2005, Cottin et al. first proposed defining a syndrome termed “combined pulmonary fibrosis and emphysema (CPFE),” which is characterized by a heavy smoking history, exercise hypoxemia, upper lobe emphysema and lower lobe fibrosis, unexpectedly subnormal lung volumes, and severe reduction of carbon monoxide transfer [7]. The pathogenesis of CPFE has not yet been fully elucidated. However, emphysema, IPF, and CPFE have common risk factors, such as smoking [6]. The survival rates of patients with CPFE are known to be poor [8, 9]. Several studies [6, 10,11,12,13,14,15,16,17] have evaluated the clinical course and complications of CPFE. Among them, some reports have stated that patients with CPFE have a higher risk of lung cancer development and death compared with emphysema patients [11, 16]. Nevertheless, the exact clinical course and complications of CPFE are unclear, especially when the condition is comorbid with lung cancer.
As the clinical course and complications of CPFE are not fully understood, physicians may be more reluctant to treat CPFE patients with concomitant NSCLC (CPFE-NSCLC) [17]. Therefore, we aimed to evaluate whether (1) CPFE-NSCLC patients are at higher odds of developing acute exacerbations (AEs) than are IPF patients with NSCLC (IPF-NSCLC) and whether (2) CPFE-NSCLC patients are at higher risk of mortality than are IPF-NSCLC patients.
Methods
Study design and population
The study was conducted retrospectively in two tertiary hospitals in South Korea. We evaluated all chest computed tomography (CT) scans of patients with a diagnosis of IPF and lung cancer that were obtained in the Severance Hospital and Seoul National University Bundang Hospital between November 2003 and February 2018. The inclusion criteria were as follows: (1) fulfilment of diagnostic criteria of IPF and CPFE, (2) availability of CT scan images at the time of diagnosis in the institutional radiology database; and (3) availability of clinical data from medical records. In total, 435 patients were considered after applying the inclusion criteria. Among these, patients were excluded if they (1) were diagnosed with small cell lung cancer (n = 59); (2) had non-confirmed pathology (n = 17); (3) had incomplete data available (n = 76); or (4) were lost to follow-up (n = 20). Finally, 283 patients were included in the analysis.
Clinical and laboratory data were collected retrospectively from medical records. Data on age, smoking history, pulmonary function test results, underlying diseases, height and weight at the time of the diagnosis, gender–age–physiology (GAP) index score [18], Eastern Cooperative Oncology Group performance status (ECOG) [19], histological type of NSCLC, and clinical and/or pathologic staging of NSCLC were collected for all patients. Information on the treatment modality used for NSCLC; date of treatment; AE after surgery, chemotherapy, or radiotherapy (including the date AE was confirmed); and mortality data (including mortality due to AE) was also collected for all patients. According to smoking status, patients were categorized into two groups (never-smoker vs. ever-smoker [a person who had smoked at least 100 cigarettes and cigars during the course of their life]). The GAP score was calculated based on gender (0–1 points), age (0–2 points), forced vital capacity (FVC) (0–2 points), and diffusing capacity of carbon monoxide (DLco) (0–3 points), and was classified into stages I (0–3 points), II (4–5 points), or III (6–8 points), as previously described [18]. Early NSCLC was defined as stage I or II, while advanced NSCLC was defined as stage III or IV according to the eighth edition lung cancer stage classification [20]. The primary outcome was the development of AE and overall survival. Overall survival was estimated from the date of diagnosis of NSCLC and IPF/CPFE until death. Death registration data were provided by the Ministry of Security and Public Administration of Korea.
Diagnostic criteria for IPF, CPFE, and AE
IPF was diagnosed using the criteria for the usual interstitial pneumonia (UIP) pattern as described in an official ATS/ERS/JRS/ALAT statement [21], as follows: subpleural, basal, predominantly reticular abnormality or honeycombing, with or without traction bronchiectasis, and the absence of an inconsistent UIP pattern. Diagnosis was confirmed at each hospital by a multi-disciplinary team consisting of specialists in pulmonary medicine, radiology, and pathology.
CPFE was defined according to Cottin et al.’s and Ryerson et al.’s definitions, namely the presence of classic features of centrilobular and/or paraseptal emphysemas (≥ 10%) in the upper lobes and pulmonary fibrosis (mainly IPF/UIP) in the lower lobes radiographically [7, 13]. Classification of CPFE and IPF was based on radiologic findings on chest CT scans. All CT scans of the included patients were reviewed by two radiologist and four pulmonologists, independently.
AE was defined according to the International Working Group Report by Collard et al. [22]: previous or concurrent diagnosis of IPF, acute worsening or development of dyspnea, typically < 1 month in duration, and CT scan with new bilateral ground-glass opacity and/or consolidation, with the deterioration not fully explained by cardiac failure or fluid overload. In the present study, acute exacerbation of IPF or CPFE within 1 month after treatment (chemotherapy, surgery, or radiotherapy) was defined as AE, in order to evaluate the effect of CPFE on the treatment of NSCLC.
Statistical analysis
Baseline characteristics of CPFE-NSCLC and IPF-NSCLC patients were compared using an unpaired t-test for continuous variables or χ2 test for categorical variables and are presented as mean ± standard deviation and numbers (percentage). Multiple logistic regression models were used to estimate odds ratios (ORs) for AE. Survival times were estimated using the Kaplan–Meier method and compared with the log-rank test. Multivariate Cox proportional hazards models were performed to investigate the relationships between clinical parameters and mortality. Variables that overlapped (e.g., age, gender, FVC, and DLCO in the GAP index) were excluded in the multivariate Cox proportional hazards models. An adjusted P-value < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS version 23.0 (SPSS Inc., Chicago, IL, USA).
Ethics statement
This research protocol was approved by the Institutional Review Board of Severance Hospital, South Korea (IRB No. 4–2018-0770). The study design was approved by the appropriate ethics review boards. The requirement to obtain informed patient consent was waived.
Results
Baseline characteristics
Baseline characteristics of the study subjects are provided in Table 1. Men constituted 95.1% of the study cohort. The mean age for the patients overall was 70.3 years (range, 46–89 years); the mean ages of CPFE and IPF patients were 70.4 years (range, 46–89 years) and 70.1 years (range, 51–87 years), respectively. Compared with IPF patients, CPFE patients had a heavier smoking history, lower DLco (78.0% vs. 64.8%, P < 0.001), and lower forced expiratory volume in 1 s (FEV1)/FVC ratio (75.1% vs. 71.2%, P = 0.001). Body mass index (BMI), FVC, NSCLC histology, FVC predicted %, FEV1 predicted %, NSCLC stage, ECOG status, treatment modality, and GAP score did not differ significantly between IPF and CPFE patients.
Of the NSCLC patients overall, 71.7% died during the follow-up period; 71.6% died in the CPFE group and 72.0% in the IPF group. The respective follow-up periods were 18.6 months and 24.1 months (P = 0.975). CPFE patients had an increased tendency to develop AE, although this was not statistically significant in the univariate analysis (12.5% vs. 21.3%, P = 0.098). The time elapsed between diagnosis of CPFE or IPF and NSCLC was analyzed but did not differ significantly between the two groups (16.1 months vs. 16.4 months, P = 0.937).
AE and CPFE
Additional file 1: Table S1 shows the incidences of AE after treatment according to the treatment modality. Nine patients had undergone surgery and adjuvant chemotherapy, 22 patients had undergone concurrent chemoradiation therapy, and four patients had undergone surgery, followed by concurrent chemoradiation therapy. All AE were calculated separately after the respective treatments. NSCLC patients with CPFE tended to have more AE after surgery (IPF 8.6% vs. CPFE 21.6%, P = 0.073). Although the incidence of AE was higher in the CPFE group (13.1% vs. 21.3%, P = 0.098), no statistically significant difference was found.
Table 2 shows the relationship between CPFE and AE in the logistic regression model. GAP stage, smoking status, NSCLC histology, NSCLC stage, and CPFE were included in the regression model. In the analysis, due to the small number of patients in the GAP stage III group (n = 14), this group was combined with the GAP stage II group. CPFE (OR: 2.26, 95% CI: 1.09–4.69, P = 0.029) and GAP stage > II (OR: 2.20; 95% CI: 1.05–4.64; P = 0.037) showed a significant correlation with AE. NSCLC histology, smoking history (ever-smoker), and NSCLC stage > III did not show significant correlations with AE.
Survival and CPFE
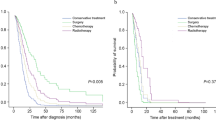
There was no significant difference in survival rates between the IPF and CPFE groups (P = 0.972), according to analysis of the Kaplan–Meier survival curves (Fig. 1).
The relationships between all-cause mortality and clinical parameters, including CPFE, were evaluated using Cox proportional hazards analysis (Table 3). Univariate analysis showed that advanced stage NSCLC, higher GAP index score (P < 0.001), AE (P < 0.001), lower FVC predicted, lower FEV1 predicted, lower DLco predicted, higher FEV1/FVC, %, and histological type other than adenocarcinoma or squamous cell carcinoma were significantly correlated with all-cause mortality. BMI, smoking history, time elapsed between diagnosis of IPF or CPFE and diagnosis of NSCLC, and CPFE did not correlate with all-cause mortality.
Multivariate Cox proportional hazards analyses were performed to compare the contributions of these indices (Table 4). Stepwise Cox proportional hazards analysis demonstrated that higher ECOG status (HR: 1.30; 95% CI: 1.16–1.55; P = 0.003), advanced stage NSCLC (HR: 3.15; 95% CI: 2.21–4.49; P < 0.001), and higher GAP score (HR: 1.31; 95% CI: 1.16–1.48; P < 0.001) were risk factors for all-cause mortality. CPFE (HR: 0.89; 95% CI: 0.66–1.21; P = 0.466) was not a significant risk factor for all-cause mortality in multivariate analysis.
Discussion
In this study, we have described differences in clinical features and outcomes between NSCLC-IPF and NSCLC-CPFE. Although previous studies have compared CPFE to IPF, few studies have compared CPFE-NSCLC to IPF-NSCLC. We demonstrated that CPFE is related to AE, but is not a significantly greater risk factor of all-cause mortality compared with IPF in NSCLC patients.
In this study, the prevalence of CPFE was 37.8% in patients with NSCLC and pulmonary fibrosis. In previously reported studies, the proportion of patients with CPFE detectable on a high-resolution CT scan ranged from 8 to 51% in IPF patients [13, 23]. As the prevalence of lung cancer in CPFE is reported to be higher than that in IPF patients (50% vs. 14.5%) [24], the prevalence reported in this study is in accordance with the findings of previous studies. Cigarette smoking is the main risk factor for NSCLC, IPF, and emphysema, and IPF and emphysema are additional independent risk factors for NSCLC; thus, it is plausible that CPFE patients have a heavier smoking history and higher prevalence of NSCLC. The average smoking pack-years were higher in the CPFE group than in the IPF group in our study. These conditions also share many pathogenic pathways, including genetic and epigenetic alterations, tissue invasion, uncontrolled proliferation, and activation of specific signal transduction pathways [25].
We assessed the median duration from the diagnosis of CPFE/IPF and diagnosis of NSCLC in patients who developed CPFE/IPF prior to developing NSCLC. The duration did not differ between the CPFE and IPF groups (20.01 months vs. 21.06 months, P = 0.618). This may suggest that CPFE patients do not necessarily need a shorter follow-up period compared with IPF patients to monitor for the presence of lung cancer. The result did not differ when the patients who were diagnosed concurrently (i.e., diagnosed with NSCLC within 1 month before or after the diagnosis of IPF or CPFE) were excluded (43.0 months vs. 38.5 months, P = 0.670).
The annual incidence of AE in patients with IPF has been reported as 5–15% [26]. Additionally, the incidence rate of AE triggered by chemotherapy [27], surgery [28], and radiotherapy [29] is increased in patients with IPF. In our study, the total incidence of AE was 15.7%. We found that CPFE was an independent risk factor of AE after treatment of NSCLC, similar to advanced IPF (higher GAP stage). In a retrospective study of 487 patients who underwent lobectomy for lung cancer, Saito et al. found that seven of 10 post-lobectomy acute respiratory distress syndrome cases (70%) had CPFE [30]. In Japan, Minegishi et al. have reported that the incidence of AE associated with anticancer treatment in lung cancer patients with interstitial lung disease is 10–30% for surgical resection and 9–21% for chemotherapy [12]. In our study, surgical resection patients with CPFE were at higher odds of developing AEs than were patients with IPF. Our study adds evidence that, regardless of whether invasive or non-invasive treatment is used, CPFE may increase the risk of AE.
In IPF and lung cancer patients, efforts are being made to prevent the development of AE. Iwata et al. showed that perioperative pirfenidone for lung cancer surgery in patients with IPF significantly decreased the incidence of AE after surgery [31]. This may also apply to CPFE patients, but further studies are required.
CPFE did not affect the mortality rate of NSCLC patients, although AE has been associated with increased mortality. AEs of IPF are a well-described complication after lung resection, with an incidence of approximately 15% and mortality of 33.3–100% in some studies [32]. It is possible that, in IPF-NSCLC and CPFE-NSCLC patients, the main prognostic factor is the NSCLC stage. Similarly, Goto et al. [33] showed that IPF is a prognostic factor in stage I/II NSCLC, but not in stage III/IV NSCLC. Another possibility is that the outcomes of AEs of CPFE could be more favorable than those of IPF. Toyoshima et al. [34] have reported that, in a comparison of AEs of CPFE and IPF, the AEs of CPFE had a more favorable outcome. Lastly, it is possible that the general prognosis of CPFE-NSCLC patients is better than that of IPF-NSCLC patients, without AEs. Some studies have indicated a much worse prognosis for CPFE than for IPF [35, 36]. However, there are data indicating that CPFE patients have the same [10] or even longer survival [37] compared with IPF subjects. Further studies are needed on this matter.
Our study had some limitations. First, it included only Korean patients and only two centers participated in the study. Some studies of drug-induced lung injury have suggested ethnic differences in the susceptibility to acute progressive respiratory failure during the course of IPF [38]. Large multiregional prospective studies are needed to eliminate an ethnic bias. Second, there might have been bias in patient selection. Of the patients who met the inclusion criteria, the proportion of patients who had incomplete data or who were lost to follow-up was high (22%). Furthermore, as our study population comprised patients who underwent chest CT scans at a tertiary hospital, the population may not be fully representative of the disease population.
Conclusions
In conclusion, the risk of AE was higher in patients with CPFE and NSCLC, but all-cause mortality was not higher in NSCLC patients with concomitant CPFE than in those with concomitant IPF. Physicians should be aware of the increased risk of AE when treating NSCLC patients with CPFE. A multidisciplinary approach is required for treating these patients.
Availability of data and materials
The datasets used and/or analyzed are available from corresponding author upon reasonable request.
Abbreviations
- AE:
-
Acute exacerbation
- CPFE:
-
Combined pulmonary fibrosis and emphysema
- CT:
-
Computed tomography
- DLCO :
-
Diffusing capacity of carbon monoxide
- ECOG:
-
Eastern Cooperative Oncology Group performance status
- FEV1:
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- GAP:
-
Gender–Age–Physiology
- IPF:
-
Idiopathic pulmonary fibrosis
- NSCLC:
-
Non-small cell lung cancer
- UIP:
-
Usual interstitial pneumonia
References
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824.
Lee T, Park JY, Lee HY, Cho YJ, Yoon HI, Lee JH, Jheon S, Lee CT, Park JS. Lung cancer in patients with idiopathic pulmonary fibrosis: clinical characteristics and impact on survival. Respir Med. 2014;108(10):1549–55.
Kanaji N, Tadokoro A, Kita N, Murota M, Ishii T, Takagi T, Watanabe N, Tojo Y, Harada S, Hasui Y, et al. Impact of idiopathic pulmonary fibrosis on advanced non-small cell lung cancer survival. J Cancer Res Clin Oncol. 2016;142(8):1855–65.
Li Y, Swensen SJ, Karabekmez LG, Marks RS, Stoddard SM, Jiang R, Worra JB, Zhang F, Midthun DE, de Andrade M, et al. Effect of emphysema on lung cancer risk in smokers: a computed tomography-based assessment. Cancer Prev Res (Phila). 2011;4(1):43–50.
Gullon JA, Suarez I, Medina A, Rubinos G, Fernandez R, Gonzalez I. Role of emphysema and airway obstruction in prognosis of lung cancer. Lung Cancer. 2011;71(2):182–5.
Lin H, Jiang S. Combined pulmonary fibrosis and emphysema (CPFE): an entity different from emphysema or pulmonary fibrosis alone. J Thorac Dis. 2015;7(4):767–79.
Cottin V, Nunes H, Brillet PY, Delaval P, Devouassoux G, Tillie-Leblond I, Israel-Biet D, Court-Fortune I, Valeyre D, Cordier JF. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J. 2005;26(4):586–93.
Kurashima K, Takayanagi N, Tsuchiya N, Kanauchi T, Ueda M, Hoshi T, Miyahara Y, Sugita Y. The effect of emphysema on lung function and survival in patients with idiopathic pulmonary fibrosis. Respirology. 2010;15(5):843–8.
Mejia M, Carrillo G, Rojas-Serrano J, Estrada A, Suarez T, Alonso D, Barrientos E, Gaxiola M, Navarro C, Selman M. Idiopathic pulmonary fibrosis and emphysema: decreased survival associated with severe pulmonary arterial hypertension. Chest. 2009;136(1):10–5.
Jankowich MD, Rounds S. Combined pulmonary fibrosis and emphysema alters physiology but has similar mortality to pulmonary fibrosis without emphysema. Lung. 2010;188(5):365–73.
Kwak N, Park CM, Lee J, Park YS, Lee SM, Yim JJ, Yoo CG, Kim YW, Han SK, Lee CH. Lung cancer risk among patients with combined pulmonary fibrosis and emphysema. Respir Med. 2014;108(3):524–30.
Minegishi Y, Kokuho N, Miura Y, Matsumoto M, Miyanaga A, Noro R, Saito Y, Seike M, Kubota K, Azuma A, et al. Clinical features, anti-cancer treatments and outcomes of lung cancer patients with combined pulmonary fibrosis and emphysema. Lung Cancer. 2014;85(2):258–63.
Ryerson CJ, Hartman T, Elicker BM, Ley B, Lee JS, Abbritti M, Jones KD, King TE Jr, Ryu J, Collard HR. Clinical features and outcomes in combined pulmonary fibrosis and emphysema in idiopathic pulmonary fibrosis. Chest. 2013;144(1):234–40.
Sugino K, Nakamura Y, Ito T, Isshiki T, Sakamoto S, Homma S. Comparison of clinical characteristics and outcomes between combined pulmonary fibrosis and emphysema associated with usual interstitial pneumonia pattern and non-usual interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32(2):129–37.
Oh JY, Lee YS, Min KH, Hur GY, Lee SY, Kang KH, Shim JJ. Presence of lung cancer and high gender, age, and physiology score as predictors of acute exacerbation in combined pulmonary fibrosis and emphysema: a retrospective study. Medicine. 2018;97(31):e11683.
Li C, Wu W, Chen N, Song H, Lu T, Yang Z, Wang Z, Zhou J, Liu L. Clinical characteristics and outcomes of lung cancer patients with combined pulmonary fibrosis and emphysema: a systematic review and meta-analysis of 13 studies. J Thorac Dis. 2017;9(12):5322–34.
Girard N, Marchand-Adam S, Naccache JM, Borie R, Urban T, Jouneau S, Marchand E, Ravel AC, Kiakouama L, Etienne-Mastroianni B, et al. Lung cancer in combined pulmonary fibrosis and emphysema: a series of 47 Western patients. J Thorac Oncol. 2014;9(8):1162–70.
Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684–91.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649–55.
Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung Cancer stage classification. Chest. 2017;151(1):193–203.
Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, Behr J, Cottin V, Danoff SK, Morell F, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44–68.
Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, Lee JS, Maher TM, Wells AU, Antoniou KM, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med. 2016;194(3):265–75.
Ye Q, Huang K, Ding Y, Lou B, Hou Z, Dai H, Wang C. Cigarette smoking contributes to idiopathic pulmonary fibrosis associated with emphysema. Chin Med J. 2014;127(3):469–74.
Usui K, Tanai C, Tanaka Y, Noda H, Ishihara T. The prevalence of pulmonary fibrosis combined with emphysema in patients with lung cancer. Respirology. 2011;16(2):326–31.
Vancheri C, Cottin V, Kreuter M, Hilberg O. IPF, comorbidities and management implications. Sarcoidosis Vasculitis Diffuse Lung Dis. 2015;32 Suppl 1:17–23.
Ryerson CJ, Cottin V, Brown KK, Collard HR. Acute exacerbation of idiopathic pulmonary fibrosis: shifting the paradigm. Eur Respir J. 2015;46(2):512–20.
Kobayashi H, Naito T, Omae K, Omori S, Nakashima K, Wakuda K, Ono A, Kenmotsu H, Murakami H, Endo M, et al. ILD-NSCLC-GAP index scoring and staging system for patients with non-small cell lung cancer and interstitial lung disease. Lung Cancer. 2018;121:48–53.
Sato S, Shimizu Y, Goto T, Kitahara A, Koike T, Ishikawa H, Watanabe T, Tsuchida M. Survival after repeated surgery for lung cancer with idiopathic pulmonary fibrosis: a retrospective study. BMC Pulm Med. 2018;18(1):134.
Kolek V, Vasakova M, Sterclova M, Cwiertka K, Vrana D, Kudlacek A, Skrickova J, Pesek M, Petera J. Radiotherapy of lung Tumours in idiopathic pulmonary fibrosis. Klin Onkol. 2017;30(4):303–6.
Saito H, Minamiya Y, Nanjo H, Ito M, Ono T, Motoyama S, Hashimoto M, Ogawa J. Pathological finding of subclinical interstitial pneumonia as a predictor of postoperative acute respiratory distress syndrome after pulmonary resection. Eur J Cardiothorac Surg. 2011;39(2):190–4.
Iwata T, Yoshida S, Nagato K, Nakajima T, Suzuki H, Tagawa T, Mizobuchi T, Ota S, Nakatani Y, Yoshino I. Experience with perioperative pirfenidone for lung cancer surgery in patients with idiopathic pulmonary fibrosis. Surg Today. 2015;45(10):1263–70.
Watanabe A, Kawaharada N, Higami T. Postoperative acute exacerbation of IPF after lung resection for primary lung Cancer. Pulm Med. 2011;2011:960316.
Goto T, Maeshima A, Oyamada Y, Kato R. Idiopathic pulmonary fibrosis as a prognostic factor in non-small cell lung cancer. Int J Clin Oncol. 2014;19(2):266–73.
Tsuchiya K, Suda T: Characteristic Features of Acute Exacerbation in Combined Pulmonary Fibrosis and Emphysema. In: B43 ILD: CLINICAL STUDIES, REGISTRIES AND MORE. edn.: A3450-A3450.
Zhang L, Zhang C, Dong F, Song Q, Chi F, Liu L, Wang Y, Che C. Combined pulmonary fibrosis and emphysema: a retrospective analysis of clinical characteristics, treatment and prognosis. BMC Pulm Med. 2016;16(1):137.
Jankowich MD, Rounds SIS. Combined pulmonary fibrosis and emphysema syndrome. Chest. 2012;141(1):222–31.
Todd NW, Jeudy J, Lavania S, Franks TJ, Galvin JR, Deepak J, Britt EJ, Atamas SP. Centrilobular emphysema combined with pulmonary fibrosis results in improved survival. Fibrogenesis Tissue Repair. 2011;4(1):6.
Azuma A, Hagiwara K, Kudoh S. Basis of acute exacerbation of idiopathic pulmonary fibrosis in Japanese patients. Am J Respir Crit Care Med. 2008;177(12):1397–8 author reply 1398.
Acknowledgements
We thank Dr. M. Song (Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.) for her contribution to the statistical analysis and assessment of the patients’ clinical data.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2018R1C1B5043991). The funding grant was used as a partial income of a researcher who only participated in collecting retrospective data but was not included in the authors list.
Author information
Authors and Affiliations
Contributions
HIY., SHL., and SWM. conceived and designed the study; SWM., SHL., HIY., MSP., YSK., JJ., YSJ., JHL., and CTL. acquired the clinical data; SWM. and SYK. conducted the statistical analysis; HSS. was involved in pathologic support; and KWL., JHC., and SSK. were involved in radiologic support. HIY., SHL. and SWM. had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the analysis. All authors designed the study, interpreted the data, critically revised the manuscript for important intellectual content, and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This research protocol was approved by the Institutional Review Board of Severance Hospital, South Korea (IRB No. 4–2018-0770). The study design was approved by the appropriate ethics review boards. The requirement to obtain informed patient consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1:
Table S1. Incidence of acute exacerbation according to treatment modality.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Moon, S., Park, M., Kim, Y. et al. Combined pulmonary fibrosis and emphysema and idiopathic pulmonary fibrosis in non-small cell lung cancer: impact on survival and acute exacerbation. BMC Pulm Med 19, 177 (2019). https://doi.org/10.1186/s12890-019-0951-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-019-0951-2