Abstract
Background
To compare the microbiological culture within endotracheal aspirate specimens (ETAs) and endotracheal tube specimens (ETTs) in patients undergoing mechanical ventilation (MV) by statistical tools.
Method
ETAs and ETTs from a total number of 81 patients, who were undergoing MV at the intensive care unit (ICU) of Jiading Central Hospital Affiliated Shanghai University of Medicine & Health Sciences from September 1st, 2017 to August 31st, 2018, were collected for microbiological culture analysis. Correlation of ETAs and ETTs cultures were obtained by Spear-men correlation analysis, while the consistency of the two specimens was determined by Kappa analysis and principal component analysis (PCA).
Results
Microbiological culture from both ETAs and ETTs showed that Acinetobacter baumannii, Pseudomonas aeruginosa, Staphylococcus aureus, and Klebsiella pneumoniae were the main pathogens, with Spear-man correlation coefficients of 0.676, 0.951, 0.730 and 0.687 respectively (all P < 0.01), and the overall Spear-man correlation coefficient is 0.757 (P < 0.01). This result shows that two samples were positively correlated. Kappa analysis also revealed high consistency of the microbial culture results from the ETAs and the ETTs (overall κ = 0.751, P < 0.01). The κ values for the four bacteria detected were 0.670, 0.949, 0.723, and 0.687, respectively (all P < 0.001). PCA also revealed high similarity.
Conclusion
Combining microbiological culture and statistical analysis of samples collected from 81 patients who were undergoing MV in ICU, we showed that microbe found in the ETAs had high similarity with that found in the ETTs which collected at the end of the catheters. In clinical practice, ETAs analysis is easily accessible meanwhile provides a valuable information for MV patients.
Similar content being viewed by others
Background
Lower respiratory tract infections in mechanically ventilated patients are a primary cause of mortality remains stubbornly high in intensive-care units (ICU) [1,2,3,4]. A previous study has shown that well-timed and accurate identification of the microorganisms residing in the lungs could effectively diminish the mortality of such cases [5]. Because of this, microbiological culture analysis on patients’ endotracheal aspirate specimens (ETAs) is commonly used in clinical practices for determining the presence and the stain of the residing pathogen. However, it has been suggested that the microbiological culture analysis on ETAs alone could not distinguish the colonization from the infection [6, 7].
It was shown that there is a very high probability for biofilm to grow at the end of an endotracheal tubes intubated in patients receiving mechanical ventilation (MV) [8], in which corresponds to the change of the micro-environment inside the lower respiratory tract and enhance infection [9,10,11]. It was, therefore, suggested that the endotracheal tube specimens (ETTs) is more appropriate for etiological examination than ETAs [12]. Meanwhile, collection of ETTs from MV patients is neither clinically practical nor of sufficient simplicity [13]. This leads to the discussion whether ETTs or ETAs is more suitable for the etiology of infection of MV patients.
Recently, Pan et al. compared the pathogenic microbiology between ETAs and ETTs of patients on MV in pediatric ICU [14]. Their study showed that both results of microbiological culture are highly similar. It is interesting to ask if we could extend this conclusion to adult patients on MV. In this clinical study, we collected ETAs and ETTs from 81 adult patients on MV in ICU and performed microbiological culture analysis. By Spear-man correlation analysis, Kappa analysis as well as principle component analysis (PCA), we showed that microbial data in ETAs and ETTs are highly conserved. Therefore, we believe that it is not necessary to use ETTs instead of ETAs of clinical practices.
Methods
Subjects
Inclusion criteria: Patients treated with MV at the ICU of Jiading District Central Hospital Affiliated Shanghai University of Medicine & Health Sciences. All intubations were preformed according to “American Journal of respiratory and critical care medicine” [15]. Exclusion criteria: (1) Refusal by the patients’ guardian; (2) Patients showing inconsistency in the results of Gram staining and microbiological culture; (3) Other factors (Patients with no ETAs, ETTs contamination during extubation or transportation, etc.).
Diagnostic criteria for lower respiratory tract infections: (1) Patients with body temperature more than 38 °C, (2) leukocytosis, (3) microbiological culture showed a positive result, (4) lung density in physical examination in association with the presence or changes in radiographic infiltration [16, 17].
Information and sample collection
General information (gender, age) and past medical history (hypertension, diabetes, etc.) of patients were collected, and the Acute Physiology and Chronic Health Evaluation II (APACHE-II) score was calculated on the day of admission. The length of hospital stay of each patient were tracked until leaving the ICU or death.
ETTs were obtained immediately after extubation. Roughly 1 cm of the distal end of the ETTs was cut for microbiological culture analysis. ETAs was extracted by a steriled suction tube, which inserted into the lower respiratory tract through a catheter.
Materials and instruments
Blood agar plates, MacConkey plates, and chocolate agar plates were provided by Shanghai Yihua Biotechnology Co., Ltd. (Shanghai, China). The VITEK 2 compact automatic bacterial identification and drug susceptibility analysis systems (bioMérieux, Marcy-l’Étoile, France) was used. Staphylococcus aureus (ATCC29213) and Pseudomonas aeruginosa (ATCC27853) were purchased from the Shanghai Clinical Laboratory Center as the quality control strains.
Microbiological culture analysis
Microbiological culture analysis of ETAs and ETTs collected was preformed according to the CSLI method [18]. Briefly, part of the ETAs and ETTs collected was subjected to Gram staining, and the rest of the samples were immediately incubated onto a blood agar plate, a MacConkey plate, and a chocolate agar plate. All plates were incubated at 35 °C with 5% CO2 for 18–24 h before interpretation. Pure cultures and dominant pathogens were identified by the VITEK2 systems. The result of microbiological culture was included only when the dominant strains were consistent with the Gram staining result.
Statistical analysis
All statistical analyses were performed in the R environment [19]. Data were summarized as mean ± standard deviation (SD) or percentage. For the data of duration of ventilation days, the median was used, indicated as M (Q1~Q3). One-way analysis of variance and chi-square test were used for statistical analysis. Spear-man correlation analysis, coincidence rate, kappa coefficient and PCA were performed to analyze the results of microbiological culture from the two kinds of samples.
Results
Study population
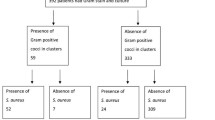
There were 332 patients admitted to the ICU of Jiading District Central Hospital Affiliated Shanghai University of Medicine & Health Sciences from September 1st, 2017 to August 31st, 2018, of which 144 were treated with MV. 81 of them matched the criteria and were included in this study. The reasons of hospitalization were summarized as followed: severe pneumonia (n = 24), severe cerebrovascular disease (n = 19), multiple injuries (n = 15), acute exacerbation of chronic obstructive pulmonary disease (n = 9), cerebral trauma (n = 9), and others (n = 5; Fig. 1). The mean age of patients was 62.1 ± 19.7 years, in which 63 patients were males (77.8%) and 18 patients were females (22.2%; Table 1). ETTs was extubated when: (i) Patients recovered (n = 49, 60.5%); (ii) change of ETTs (n = 3, 3.7%); and (iii) patients succumbed to mortality (n = 29, 35.8%).
Microbiological culture results
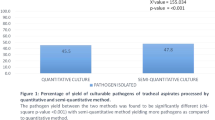
Within 81 ETTs, 61 of them (75.3%) grew one (50) or two (11) major populations of pathogenic bacteria, while the other 20 samples grew single major population of normal respiratory track flora. i.e. A total number of 92 major populations of cells were observed, in which 72 of them were pathogenic bacteria and 20 were normal respiratory track flora. Bacterial identification results showed that the main pathogens observed were A. baumannii (39.1%, 36/92), P. aeruginosa (12.0%, 11/92), S. aureus (9.8%, 9/92), and K. pneumoniae (7.6%, 7/92) respectively (Fig. 2a).
Microbial distribution pie chart. ETTs distribution pie chart (2a); ETAs distribution pie chart (2b). A.baumannii:Acinetobacter baumannii; K.peneumoniae:Klebsiella pneumoniae; P.Aeruginosa: Pseudomonas aeruginosa; S. aureus: Staphylococcus aureus; E. cloacae: Enterobacter cloacae; S. mitis:Streptococcus mitis; A.lwoffii:Acinetobacter lwoffii; E. faecalis: Enterococcus faecalis; E.faecium: Enterococcus faecium; P. mirabilis:Proteus mirabilis; S. haemolyticus:Staphylococcus haemolyticus; S. maltophilia:Stenotrophomonas maltophilia ; M. morganii: Morganella morganii; K. oxytoca: Klebsiella oxytoca; P. oryzihabitans: Pseudomonas oryzihabitans; X. maltophilia: Xanthomonas maltophilia; F. fungi: Filamentous fungi
For ETAs analysis, 57 specimens (70.4%) grew pathogenic bacteria as major cell population, while 24 of them grew normal respiratory track flora as major population of cell. Totally 89 populations of cells were observed, of which 63 were pathogenic bacteria and 26 were normal respiratory track flora. The main pathogenic bacteria stains were A. baumannii (34.8%, 31/89), P. aeruginosa (13.5%, 12/89), S. aureus (7.9%, 7/89), and K. pneumoniae (7.9%, 7/89; Fig. 2b).
Correlation analysis
Spear-man correlation analysis showed that in general the results of microbiological culture from ETAs were positively correlated with those from ETTs (Spear-man γ = 0.757, P < 0.01). In addition, P. aeruginosa, normal respiratory flora, S. aureus, K. pneumoniae, and A. baumannii showed positively correlation between two kinds of specimens (Spear-man γ = 0.951, 0.757, 0.730, 0.687, 0.676, all P < 0.01). Lower respiratory tract infection rate was positively correlated with age (Spear-man γ = 0.561, P < 0.01). The presence of normal respiratory flora is negatively correlated with the growth of pathogens in either ETTs (Spear-man γ = − 1, P < 0.01) or ETAs (Spear-man γ = − 0.970, P < 0.01) samples (Table 2) as expected. Meanwhile, we analyzed factors including diabetes mellitus, hypertension, duration of ventilation days, APACHE-II and the cause of hospital admission with the results of microbiological culture from the two samples types (Additional file 1: Table S1, S2), the results showed low correlation between those factors and the type and diversity of bacterial species found within either type of the two samples.
Comparison of microbial data between both specimens
The comparison between culture data of ETAs and ETTs showed that the total microbial coincidence rate was 96.7%. The coincidence rate of K. pneumoniae, P. aeruginosa, and A. baumannii were 100, 90.9, and 86.1%, respectively (Table 3).
Kappa analysis showed that microbial data of ETAs and ETTs were highly conserved in general (κ = 0.751); The κ values of P. aeruginosa, S. aureus, K. pneumoniae, and A. baumannii were 0.949, 0.723, 0.687, and 0.670, respectively (all P < 0.001).
PCA was performed and the result was as shown in Fig. 3. Each pathogen and the overall culture results showed a high degree of similarity.
Discussion
In this study, we compared the microbiological data between ETTs and ETAs. Statistical tools (Spear-man correlation analysis, Kappa analysis and PCA) were applied and the results showed that the microbial components of ETAs and ETTs were highly conserved.
Microbial data showed that Gram negative pathogenic bacteria A. baumannii, K. pneumonia and P. aeruginosa were observed most frequently, which conserved with several recent studies on ETAs microbial components of ETAs [5, 7]. A detailed study suggested that the multi-resistant property of these Gram negative pathogens is a key for the high prevalence of biofilm of ETTs [13, 20]. These pathogens are often related to the infection of MV patients in ICU [8].
By directly counting the match rate, Gil-Perotin et al. showed that there was only 56% of similarity between ETAs and ETTs [10]. And by excluding Candida spp., which was considered to prefer attaching to the mucosa rather than the prosthesis, the match rate was 69%. The Candida spp. excluded match rate indicated that the microbial components of ETTs and ETAs should be similar, which is conserved to our conclusion.
Both Spear-man correlation coefficient (Spear-man γ = 0.757) and Kappa analysis showed that the ETAs is highly correlated to the ETTs in general, with κ = 0.751. The partially inconsistency is possibly due to: (1) The uneven distribution of microbial colonies due to the limited movement and cough reflex of patients undergoing MV [12, 21], (2) Microbials have higher resistance to antibiotics when forming biofilm on ETTs than suspending in ETAs, causing the differences in the microbial component between ETAs and ETTs [10, 22].
In this study, we demonstrated that the microbial components of ETAs and ETTs are highly correlated through microbiological culture analysis and statistical tools. The sample collection processes from MV patients could be formidable in clinical practices [13, 23]. And yet, a timely determination of the pathogen is crucial for proper treatment and therefore eventually reduce mortality [8]; and this become even more important when the function of immune system of patients decreases with ages [1], resulting an increase of infection rate. Due to the reasons mentioned above ETAs is more preferable for its accessibility in clinical practice, while its accuracy is highly conserved with the ETTs. Moreover, Hashemi et al. suggested that Gram stains of the ETAs help to establish the presence of inflammation based on numbers of polymorphnuclear cells [24], and quantitative culture (bacterial growth ≥105 CFU/mL) may provide a better standard for comparison and greater diagnostic specificity to discriminate between colonization and infection in lower airway [17].
To further confirm the consistency among microbial components of ETTs and ETAs, a larger sample size could be used, e.g. combining data from ICUs of different hospitals. Up to date microbiological culture analysis is still a major technique for inspection in the hospitals of China, introducing biomarkers and molecular diagnostic techniques could enhance the inspection processes.
Conclusions
In summary, the microbial data of ETAs and ETTs of MV patients in the ICU are highly correlated. The ETAs is more preferable for etiological examination of lower respiratory tract infections in MV patients.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- A. baumannii/ Ab:
-
Acinetobacter baumannii
- A. lwoffii :
-
Acinetobacter lwoffii
- AECOPD:
-
Acute exacerbation of chronic obstructive pulmonary disease
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation II
- CVD:
-
Cerebrovascular disease
- E. cloacae :
-
Enterobacter cloacae
- E. faecalis :
-
Enterococcus faecalis
- E. faecium :
-
Enterococcus faecium
- ETAs/ A:
-
Endotracheal aspirate specimens
- ETTs/ T:
-
Endotracheal tube specimens
- F. fungi :
-
Filamentous fungi
- ICU:
-
Intensive care unit
- K. oxytoca :
-
Klebsiella oxytoca
- K. pneumoniae/ Kpn:
-
Klebsiella pneumoniae
- LRTI:
-
Lower respiratory tract infection
- M. morganii :
-
Morganella morganii
- MV:
-
Mechanical ventilation
- NM:
-
Normal microbiota
- P. aeruginosa/ PA:
-
Pseudomonas aeruginosa
- P. mirabilis :
-
Proteus mirabilis
- P. oryzihabitans :
-
Pseudomonas oryzihabitans
- PCA:
-
principal component analysis
- S. aureus/ SA:
-
Staphylococcus aureus
- S. haemolyticus :
-
Staphylococcus haemolyticus
- S. maltophilia :
-
Stenotrophomonas maltophilia
- S. mitis :
-
Streptococcus mitis
- X. maltophilia :
-
Xanthomonas maltophilia
References
Hough CL, Caldwell ES, Cox CE, et al. Development and validation of a mortality prediction model for patients receiving 14 days of mechanical ventilation. Crit Care Med. 2015;43(11):2339–45.
Vincent JL, Rello J, Marshall J, et al, for the EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329.
Alireza A, Saeed S, Nasrin S. Microorganisms’ colonization and their antibiotic resistance pattern in oro - tracheal tube. Iran J Microbiol. 2013;5(2):102–7.
Rello J, Lisboa T, Koulenti D. Respiratory infections in patients undergoing mechanical ventilation. Lancet Respir Med. 2014;2(9):764–74.
Willson DF, Conaway M, Kelly R, et al. The lack of specificity of tracheal aspirates in the diagnosis of pulmonary infection in intubated children. Pediatr Crit Care Med. 2014;15(4):299–05.
May RM, Hoffman MG, Sogo MJ, et al. Micro-patterned surfaces reduce bacterial colonization and biofilm formation in vitro: potential for enhancing endotracheal tube designs. Clin Tranal Med. 2014;3:8.
Brusselaers N, Labeau S, Vogelaers D, et al. Value of lower respiratory tract surveillance cultures to predict bacterial pathogens in ventilator-associated pneumonia: systematic review and diagnostic test accuracy meta-analysis. Intensive Care Med. 2013;39(3):365–75.
Hotterbeekx A, Xavier BB, Bielen K, et al. The endotracheal tube microbiome associated with Pseudomonas aeruginosa or Staphylococcus epidermidis. Sci Rep. 2016;6:36507.
Loss SH, de Oliveira RP, Maccari JG, et al. The reality of patients requiring prolonged mechanical ventilation: a multicenter study. Rev Bras Ter Intensiva. 2015;27(1):26–35.
Gil-Perotin S, Ramirez P, Marti V, et al. Implications of endotracheal tube biofilm in ventilator-associated pneumonia response: a state of concept. Crit Care. 2012;16(3):R93.
Wunderink RG. Surrogate markers and microbiologic end points. Clin Infect Dis. 2010;51 Suppl:S126–30.
Danin PE, Girou E, Legrand P, et al. Description and microbiology of endotracheal tube biofilm in mechanically ventilated subjects. Respir Care. 2015;60(1):21–9.
Vandecandelaere I, Matthijs N, Nelis HJ, et al. The presence of antibiotic-resistant nosocomial pathogens in endotracheal tube biofilms and corresponding surveillance cultures. Pathog Dis. 2013;69(2):142–8.
Pan Y, Du L, Ai Q, et al. Microbial investigations in throat swab and tracheal aspirate specimens are beneficial to predict the corresponding endotracheal tube biofilm flora among intubated neonates with ventilator-associated pneumonia. Exp Ther Med. 2017;14(2):1450–8.
Richard JC, Lefebvre JC, Tassaux D, et al. Update in mechanical ventilation 2011. Am J Respir Crit Care Med. 2011;184(1):32–6.
Klompas M. What can we learn from international ventilator-associated pneumonia rates? Crit Care Med. 2012;40:3303–4.
Craven DE, Hjalmarson KI. Ventilator-associated tracheobronchitis and pneumonia: thinking outside the box. Clin Infect Dis. 2010;51(Suppl 1):S59–66.
Humphries RM, Ambler J, Mitchell SL, et al. CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J Clin Microbiol. 2018;56(4):e01934–17.
Jalal H, Pechlivanoglou P, Krijkamp E, et al. An overview of R in health decision sciences. Med Decis Mak. 2017;37(7):735–46.
Rello J, Sonora R, Jubert P, et al. Pneumonia in intubated patients: role of respiratory airway care. Am J Respir Crit Care Med. 1996;154(1):111–5.
Abu Samra M, El Bendary H, Hayes SM, et al. Role of topical antibiotic prophylaxis in prevention of bacterial translocation into upper trachea in nasally intubated patients undergoing tonsillectomies. Int J Pediatr Otorhinolaryngol. 2013;77(2):270–4.
Roberts CG. The role of biofilms in reprocessing medical devices. Am J Infect Control. 2013;41(5 Suppl):S77–80.
Sottile FD, Marrie TJ, Prough DS, et al. Nosocomial pulmonary infection: possible etiologic significance of bacterial adhesion to endotracheal tubes. Crit Care Med. 1986;14:265–70.
Hashemi SH, Hashemi N, Esna-Ashari F, et al. Clinical features and antimicrobial resistance of bacterial agents of ventilator-associated Tracheobronchitis in Hamedan, Iran. Oman Med J. 2017;32(5):403–8.
Acknowledgements
The authors would like to thank Dr. Shuang Sha, Dongyu Liang and Qingqing Yi for their great help in this paper.
Funding
The work was supported by the Natural Science Foundation of Shanghai, China (16ZR1430200), the special program for collaborative innovation in Shanghai University of Medicine and Health Sciences (SFP-18-21-003), the Training Program for young Scholar of Jiading District Central Hospital (ZQN704) and Foundation of the Public Health Bureau of Jiading (2017-KY-06); which had role in study design, analysis, or interpretation of the data, or in writing of the manuscript.
Author information
Authors and Affiliations
Contributions
SL, WF and SJ assisted with data analysis, interpretation, and drafting of the manuscript. CQ and JS assisted with study design, statistical analysis and drafting of the manuscript. XW assisted with statistical analysis of the manuscript. JT, YH and CS contributed to measuring samples in this study. YX, TH, HS and WX contributed to data interpretation and critically reviewed the manuscript. All authors planned the study design, contributed to the interpretation of the data, drafted and approved the submitted manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This protocol was approved by the Ethics Review Board of Jiading District Central Hospital. All the participation in this study were written consent by the patients or patients’ guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Table S1. Correlation analysis of two specimens and variables. Table S2. Correlation analysis of between the cause of hospitalization and the type of bacterial species. (DOCX 40 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shen, L., Wang, F., Shi, J. et al. Microbiological analysis of endotracheal aspirate and endotracheal tube cultures in mechanically ventilated patients. BMC Pulm Med 19, 162 (2019). https://doi.org/10.1186/s12890-019-0926-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-019-0926-3