Abstract
Background
Allergic rhinitis (AR) is a worldwide health problem. Allergen avoidance is strongly recommended for AR patients. Air purification can reduce concentrations of particles in indoor air, including those of allergens. Air purifiers have been recommended by clinicians for AR patients, but few studies have focused on the removal of airborne allergens from home environments. Such studies have been limited by a lack of blinding, small samples, or a failure to measure allergen levels, disease activity, or a combination of these factors. This study investigates the efficacy of a high-efficiency air purifier in reducing disease activity in the homes of AR patients sensitive to the allergens produced by Artemisia (mugwort) pollen.
Methods
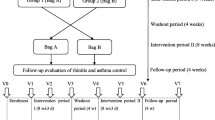
This is a randomized, double-blind, clinical controlled trial that will test active and inactive versions of an air purifier (Atmosphere®; Amway China). Sixty AR patients sensitive to the allergens produced by Artemisia pollen will be assigned randomly to two groups of equal numbers. All patients will undergo a 4-week treatment period and a 4-week observation period. Evaluation will be conducted at baseline (day 0) and on days 7, 14, 21, 28, and 56. The primary outcome measure will be the difference in visual analog scale scores from baseline. Secondary outcomes will be changes from baseline in nasal symptoms, allergy symptom scores, responses to the Rhinoconjunctivitis Quality of Life Questionnaire, Epworth Sleepiness Scale scores, and tolerability scores for the air purifier. Side effects of treatment will be recorded.
Discussion
Reducing exposure to allergens can reduce the risk of conditions such as AR. We hypothesise that AR patients sensitive to the allergens produced by Artemisia pollen will not suffer symptoms in a pollen-free environment. AR patients can remove pollen from their homes using air purifiers, decreasing the risk of symptoms. We expect that our study results will provide reliable evidence for determining the effects of air-purification therapy.
Trial registration
ChiCTR-INR-17012481. (Retrospectively registered 26 August 2017).
Similar content being viewed by others
Background
Allergic rhinitis (AR) is a global health problem. The reported incidence of AR is 11.8–46% worldwide [1, 2] and 11.1–19.1% [3, 4] in China. Allergen avoidance is strongly recommended for AR patients. In Japan, a country-wide map of pollen concentrations is published daily so that AR patients can avoid high-concentration areas [5]. Seasonal migration of AR patients is an effective but expensive option. We searched for more convenient and less expensive ways to avoid and mitigate allergen exposure.
Our research team focused on air purification because this strategy can reduce the concentrations of particles in indoor air. In addition, air purifiers can filter particles smaller than 1 μm in diameter [6], and the diameter of mugwort (Artemisia spp.) pollen is typically 19–25 μm [7] .Air purifiers are recommended by clinicians for AR patients [8], as they can reduce airborne indoor allergen levels significantly [9]. Few studies, however, have focused on the removal of airborne allergens in home environments. Moreover, those studies have been limited by a lack of blinding, small samples, and a failure to measure allergen levels, disease activity, or a combination of these factors.
Project aim
Studies have suggested that indoor particle purification may reduce clinical symptoms or improve clinical outcomes, particularly for individuals with allergies [10] .Little robust clinical evidence has been provided, however, for the efficacy of indoor-air purification as a treatment. We sought to investigate, by means of a double-blind, placebo-controlled protocol, the efficacy of a high-efficiency particulate air (HEPA) purifier in reducing disease activity in the homes of AR patients sensitive to the allergens produced by Artemisia pollen.
Methods/Design
Design
This is a randomised, double-blind, placebo-controlled clinical trial that tests active and inactive versions of an air purifier. The air purifiers are equipped with monitors to measure the number of hours they operate. AR patients sensitive to the allergens produced by Artemisia pollen will be assigned randomly to two groups of equal numbers. All patients will accept a 4-week treatment period and a 4-week observation period. Patient evaluation will be undertaken at baseline (day 0) and on days 7, 14, 21, 28, and 56 (Table 1). Figure 1 displays a flowchart of the study design. Specific tests that will be conducted at each follow-up occasion are outlined in Table 1.
Setting
Study activities will be conducted at different settings. Patient enrollment and specimen and data collection will be conducted in the Outpatient Department of the First Hospital of Yulin (Guangxi, China). Biochemical examinations will be undertaken by King Med Diagnostics (Guangzhou, China). Data analyses will be performed at the University of Toronto (Toronto, ON, Canada).
Study procedures
Staff training
Personnel who participate directly in this study will be trained to ensure the safety of patients, blinding of the study design, and data quality.
Sample size
The mean changes in findings before and after treatment were used as the indicator for efficacy evaluation in calculation of the sample size. We defined efficacy as changes in at least one score with the visual analog scale (VAS). Using a ratio of 1:1, α = 0.05, and 1 − β = 0.8 with a one-sided t-test, we calculated the appropriate trial sample size with 80% power. We found that a clinically significant difference could be detected using a sample of ≥21 patients in each group. We increased this number to 30 per group to allow for a predicted dropout rate of 30%.
Recruitment, screening, and enrollment
Clinical recruitment staff will be in charge of enrolling the 60 participants sensitive to the allergens produced by Artemisia pollen. We will follow specific criteria to recruit, screen, enroll, and exclude participants. Patients will be enrolled if they meet all inclusion criteria, and will not be enrolled if they meet any of the exclusion criteria, rejection criteria, or termination standards. Participants will be briefed on the purpose, procedures, treatments, and possible risks of the trial. They will be clearly informed of their rights to discontinue participation in this clinical trial. After patients sign consent forms, researchers will assign them to the intervention groups. All of the personal privacy will be protected.
Inclusion and exclusion criteria
Inclusion criteria are confirmed AR and sensitivity to allergens from Artemisia pollen. Patients must be aged 18–65 years, provide written informed consent, volunteer to participate in this clinical trial, and complete the case report form and other records. Inclusion and exclusion criteria are detailed in Table 2.
Rejection criteria and termination standards
Rejection refers to excluding patients from the analysis if they (i) do not meet the inclusion criteria; (ii) withdraw written informed consent; (iii) do not receive follow-up care after selection for the trial; (iv) violate the terms of the trial (such as by improper use of the air purifiers, leading to effects that cannot be evaluated).
Termination refers to censoring patients who have met the inclusion criteria but who halted their participation in the trial. Data from these patients will be included in the final statistical analyses. Termination standards include (i) symptoms (e.g., sneezing, runny nose, nasal obstruction, or nose itching) that become severe; (ii) the occurrence of a serious event, or (iii) other health reasons sufficient to halt participation in the study.
Consent
Patients who are eligible for the study will be referred by their attending doctor, then the research coordinator will carry out the informed consent process. The research doctor will explain the details of the study to the patients, including its objectives, process, duration, and possible risks and benefits. And patients will sign the consent forms that they are entirely voluntary and their decision to participate in the study will not affect their future medical care. Recruited patients can withdraw from the study at any time for any personal reason. Patients also reserve the right for questioning about the study and the hesitant period. The burden of the intervention assessed will be charged by the research group.
Follow-up
Upon completion of the 4-week treatment, patients will undergo a 4-week follow-up period. Research staff will continue to follow participants by telephone, short messaging via mobile telephone, or in the clinic. During the treatment period, follow-up will be conducted weekly. A record of symptom assessment and treatment compliance/changes will be maintained. If participants discontinue/deviate from the intervention protocols, research staff will record the reasons for the change. Such participants and their data will be excluded from the study.
Intervention in the treatment group
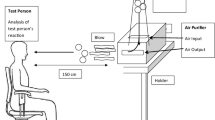
The patients will operate an Atmosphere® air purifier (Amway China, Guangzhou, China) in their bedrooms. This air purifier contains a HEPA two-way filter (model number 101076CH) with an airflow velocity of 100/200 cubic feet/minute at a filtration rate of 6000/12,000 cubic feet/hour. This represents 4/8 air changes per hour in a typical bedroom measuring 15 × 12 × 8 ft. The air purifier will be placed in the bedroom of the patient. Instructions will be given for the units to be left running continuously. The patients must stay in their bedrooms at night for 4 weeks (> 8 h each day).
Intervention in the control group
The patients will operate an Atmosphere air purifier in their bedrooms, but this air purifier will contain a placebo filter. This filter is also of a two-way design with an airflow velocity of 100/200 cubic feet/minute. Instructions will be given for the units to be left running continuously. The patients must stay in their bedroom at night for 4 weeks (> 8 h each day).
Concomitant care and interventions
During the treatment and observation periods, participants will be prohibited from taking medications such as antihistamines, corticosteroids, decongestants, or leukotriene receptor antagonists orally or intranasally. Only patients with severe symptoms would be permitted to use anti-allergy agents. The type of medication, dose, and use will be recorded by the patient for analysis. For other complex chronic diseases, patients must continue taking their routine medications and therapies. Research staff will record the details of the diseases, medications, and therapies in the case report.
Primary outcome measure
The visual analog scale (VAS) will be used to measure symptom severity and quality of life. The primary outcome measure is a difference in the VAS.
Secondary outcome measures
The secondary outcomes will be changes in nasal symptoms, allergy symptom scores, Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) scores, Epworth Sleepiness Scale scores, and tolerability scores for the Atmosphere air purifier.
Nasal symptoms
The most important symptom is a swelling of the turbinates, which will be graded as 1 (“mild”), 2 (“moderate”), or 3 (“severe”).
Allergy symptom scores
Allergy symptom scores will be used to grade symptoms as 0 (“no symptoms”), 1 (“mild symptoms”), 2 (“moderate symptoms”), and 3 (“severe symptoms”). The symptoms graded will be congestion, sneezing, nasal itch, rhinorrhea, eye itch, ear/palate itch, eye redness, and tearing.
RQLQ
The RQLQ contains 28 questions covering seven topics (daily life activities, sleep, non-eye/nasal symptoms, practical problems, nasal symptoms, eye symptoms, and emotional status). The questions will be graded on a scale from 0 (“none”) to 6 (“very often/always”).
Epworth sleepiness score
The Epworth Sleepiness Score comprises eight questions about the patient’s sleepiness, which are scored from 0 (“none”) to 3 (“probably”).
Tolerability of the atmosphere air purifier
Tolerability measure of the Atmosphere air purifier is based on answers to five questions, which will be graded on a scale from 1 (“complete disagreement”) to 5 (“complete agreement”).
Collection and management of data
Research staff is responsible for data collection. The baseline variables will be age, sex, highest education level achieved, dwelling environment, career, diagnosis, results of allergen examination, disease course, family history, VAS score, nasal symptoms, allergy symptom score, RQLQ score, Epworth Sleepiness Scale score, and tolerability of the Atmosphere air purifier. Participants will be required to record the intake of any medication during this study period.
Monitoring and management of data will be performed by a third party, which will build the study database and program settings. All data will be double-imported into an electronic database by two operators. Identified input errors will be corrected until there are no discrepancies in the database. Data organisation, data coding, range checking for data values, and data conversion to ensure quality will be the responsibility of the statistician.
Statistical analyses
Statistical analyses will be carried out using SPSS v. 17.0 (IBM, Armonk, NY, USA) in the Clinical Evaluation Center of Southern Medical University (Guangzhou, China). Data will be described as the mean and standard deviation for normally distributed data, median and interquartile range for non-normally distributed data, and frequency and proportion for categorical data. All statistical inferences will be determined using two-sided tests. We will set a significance level of 0.05 and use 95% confidence intervals to measure the uncertainty of the estimate. Efficacy analyses will use last observation carried forward (LOCF) methodology to impute for cases not fully followed up during treatment. Pearson’s χ2 test will be used to compare the differences between the dropout rate and the dropout rate attributable to adverse events. Baseline data analyses (two sets) will include demographic indicators and general, primary, and secondary indicators before intervention. Measurement data will be compared using a paired t-test. For analyses of efficacy, for quantitative variables, comparisons between groups will be made by repeated measurement variance analysis and covariance analysis. For qualitative variables, comparisons between groups will be made using Pearson’s χ2 test, whereas central effects will be tested by a mixed-effects model. For rating variables, comparisons between groups will be made using the Kruskal-Wallis test. In terms of analyses of centre effects, the quantitative indicators will be tested by the general linear method, and the qualitative indicators will be tested by the Cochran-Mantel-Haenszel test methods. A logistic regression model will be used to evaluate and correct the rating variables.
Trial status
This clinical trial was reviewed by the Ethics Committee of the First Hospital of Yulin in 2016. The first patient was enrolled in 2016. From the middle of 2016 to the end of 2017, 45 patients completed the treatment and observation periods. This clinical trial is expected to be completed at the end of 2018.
Dissemination plan
Researcher will assign the recruited patients anonymous recruitment numbers and de-identified data-sets will be used to perform all subsequent references and analyses. All of the collected patient information of this study will be kept confidential in compliance to the China Personal Data Protection Act. All the data will kept by the full-time manager. And the data will only be available to the researchers. All study data will be kept for 10 years.
Discussion
The Atmosphere air purfier has been approved for daily use. The Atmosphere air purifier filter (101076CH) was certified to filter pollen allergens by Allergy UK. Before treatment commences, we will explain to patients in detail the research purpose, methods, and possible risks of the clinical trial, and also inform them explicitly of their right to discontinue their participation. Researchers will accept the research plan, treatment procedure, patients, research schedule, case report forms, and written informed consent forms. After the trial, if the participants are willing to continue treatment, we will provide appropriate therapies. Patients who suffer harm from participation in this clinical trial will be provided a certain amount of economic compensation or free treatment, especially if they suffer adverse events. Any moderate/serious adverse events will be reported to the Ethics Committee of the First Hospital of Yulin.
Reducing exposure to allergens can reduce the risk of conditions such as AR. We hypothesise that AR patients sensitive to the allergens produced by Artemisia pollen will not suffer symptoms in a pollen-free environment. AR patients can remove pollen from their homes using air purifiers, decreasing the risk of symptoms.
The city of Yulin is part of Guangxi Province in western China, south of the Mu Us Desert. In recent years, Artemisia desertorum Sprengel has been sown extensively in and around Yulin, increasing the prevalence of allergic reaction [11,12,13]. We therefore selected Yulin as the study site. Morris et al. (2006) recommended that individuals with seasonal AR use indoor air purifiers during the ragweed-pollen season [14]. Stillerman and colleagues conducted a 12-week air-purification treatment in patients with perennial AR [15], and found that nasal congestion, sneezing, runny nose, itchy eyes, tearing, and other symptoms improved, as did quality of life. They therefore suggested that reducing allergen exposure effectively has clinical value and benefits AR patients [15]. Therapy based on reducing allergic sensitisation by avoiding allergen exposure at night may have clinical applications. Considering that plant pollen can be removed by air purification, we selected AR patients sensitive to the allergens of Artemisia pollen as our research subjects. The treatment periods in the studies cited above were 1–12 weeks, and the follow-up period was short, which may have resulted in inadequate evaluation of the efficacy of air-purification treatments. The duration of follow-up in our study will be 4 weeks, to ensure an adequate evaluation of treatment efficacy. In addition, those studies were not randomised controlled trials. Hence, we will conduct a double-blind, parallel-design, air purifier and placebo controlled randomised clinical trial. We expect that our study results will provide reliable evidence for determining the effects of air-purification treatment.
Abbreviations
- AR:
-
Allergic rhinitis
- HEPA:
-
High-efficiency particulate air
- LOCF:
-
Last observation carried forward
- RQLQ:
-
Rhinoconjunctivitis Quality of Life Questionnaire
- VAS:
-
Visual analog scale
References
Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, van Weel C, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(Suppl 86):8–160.
Bousquet PJ, Leynaert B, Neukirch F, Sunyer J, Janson CM, Anto J, Jarvis D, Burney P. Geographical distribution of atopic rhinitis in the European Community Respiratory Health Survey I. Allergy. 2008;63(10):1301–9.
Han DM, Zhang L, Huang D, Wu YF, Dong Z, Xu G, Kong WJ, Bao JM, Zhou B, Wang SQ, et al. Self-reported prevalence of allergic rhinitis in eleven cities in China. Chinese Journal of otorhinolaryngology head and neck. Surgery. 2007;42(5):378–84. http://en.cnki.com.cn/Journal_en/EE073-ZHEB-2007-05.htm.
Zheng M, Wang X, Bo M, Wang K, Zhao Y, He F, Cao F, Zhang L, Bachert C. Prevalence of allergic rhinitis among adults in urban and rural areas of China: a population-based cross-sectional survey. Allergy, Asthma Immunol Res. 2015;7(2):148–57.
Suzuki M, Murayama K, Tonouchi M, Kumagai H, Komatsu S: Automatic mesurements of Japanese cedar / cypress pollen concentration and the numerical forecasting at Tokyo metropolitan area. 2018. https://www.researchgate.net/publication/267260519.
Hacker DW, Sparrow EM. Use of air-cleaning devices to create airborne particle-free spaces intended to alleviate allergic rhinitis and asthma during sleep. Indoor Air. 2005;15(6):420–31.
Hou XJWC, Guangfa Q, et al. Temporal and spatial changes of airborne pollen concentration of Artemisia in the suburbs of Beijing. Journal of Northeast Forestry University. 2010;38(4):77–9. http://en.cnki.com.cn/Journal_en/DD049-DBLY-2010-04.htm.
Wood RA, Johnson EF, Van Natta ML, Chen PH, Eggleston PA. A placebo-controlled trial of a HEPA air cleaner in the treatment of cat allergy. Am J Respir Crit Care Med. 1998;158(1):115–20.
Myatt TA, Minegishi T, Allen JG, Macintosh DL. Control of asthma triggers in indoor air with air cleaners: a modeling analysis. Environ Health. 2008;7:43. https://doi.org/10.1186/1476-069x-7-43
Fisk WJ. Health benefits of particle filtration. Indoor Air. 2013;23(5):357–68.
Yin J, Yue FM, Wang LL, He HJ, Xu T, Li H, Wen LP, Sun JL, Gu JQ, Han SM, et al. Natural course from rhinitis to asthma in the patients with autumnal pollinosis: a clinical study of 1096 patients. Zhonghua Yi Xue Za Zhi. 2006;86(23):1628–32. https://www.researchgate.net/publication/6932117.
Yin J, Yue FM, Wang LL, He HJ, Xu T, Zhang HY, Li H, Wen LP, Sun JL, Gu JQ, et al. The clinical study of the relationship between allergic rhinitis and allergic asthma in the patients with autumnal pollinosis. Zhonghua Yi Xue Za Zhi. 2005;85(24):1683–7. https://www.researchgate.net/publication/7515757.
Wen ZHYJ. Correlation of the Artemisia and humulus scandens pollen count in the air andthe severity of asthma symptoms in patients with autumnal pollinosis. In: Chinese journal of allergy & clinical immunology; 2012. http://en.cnki.com.cn/Journal_en/EE059-OZHL-2012-01.htm.
Morris RJ, Helm TJ, Schmid W, Hacker D. A novel air filtration delivery system improves seasonal allergic rhinitis. Allergy Asthma Proc. 2006;27(1):63–7. https://www.ncbi.nlm.nih.gov/pubmed/16598995.
Stillerman A, Nachtsheim C, Li W, Albrecht M, Waldman J. Efficacy of a novel air filtration pillow for avoidance of perennial allergens in symptomatic adults. Annals of allergy, asthma & immunology : official publication of the American College of Allergy. Asthma Immunol. 2010;104(5):440–9. https://doi.org/10.1016/j.anai.2010.03.006
Acknowledgements
Special thanks should go to the patient advisers Li Li, Li Zhang, Qiao-yan Chen, Yun-ying Li and Ji-yan Xia, who have put considerable time and effort into our study.
Funding
This work was supported by the Technical Research and Development Program Project in 2016. This funder will provided grant to cover programme development but have no role in study design, collection, management, analysis, and interpretation of data; writing of the report, and the decision to submit the report for publication. The air purifiers (active and placebo versions) were provided by Amway (China). This funder only provided the air purifiers for free and have no role in study design, collection, management, analysis, and interpretation of data; writing of the report, and the decision to submit the report for publication.
Availability of data and materials
The datasets which will be used and/or analyzed during the current research are available from the corresponding author on reasonable request especially.
Author information
Authors and Affiliations
Contributions
ZFY, XLW, and LL conceived and designed the study and are responsible for study coordination. QYC and LL developed the study design and revised the protocol. QYC, LL, and LZ participated in patient enrollment and data collection. LL obtained funding and ethical approval. LL, LZ, QYC, YYL and JYX also participated in as patient advisers. JHM, YYL, JYX, XBB, and PFX provided suggestions and contributed to the design of the study. All authors contributed to the writing of the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This clinical trial was reviewed by the Ethics Committee of the First Hospital of Yulin in 2016. The study has been retrospectively registered in the Chinese Clinical Trials Registry (ChiCTR-INR-17012481, 26 August 2017). All of the patients will be required to sign consent forms to participate in the study. All of the personal privacy will be protected.
Competing interests
LL obtained funding of Technical Research and Development Program Project in 2016. The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chen, Qy., Li, L., Zhang, L. et al. Efficacy of indoor air purification in treating Artemisia (mugwort) pollen allergic rhinitis: study protocol for a randomised controlled trial. BMC Public Health 18, 841 (2018). https://doi.org/10.1186/s12889-018-5678-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-018-5678-0