Abstract
Background
Ménétrier’s disease (MD) is a protein-losing gastropathy characterized by gastric hypertrophy, foveolar hyperplasia and hypoalbuminemia. MD is uncommon in childhood with nonspecific clinical symptoms, and the exact cause of pediatric MD is still unclear.
Case presentation
Here, we reported a 4 year and 10-month boy presenting with MD from China. The patient was suffered with vomiting, abdominal pain, hypoproteinemia and edema. Laboratory tests showed that the boy was infected with Clostridium difficile (CD). Gastrointestinal endoscopy revealed giant gastric folds, and histological gastric biopsies showed foveolar hyperplasia with glandular atrophy, infiltration of eosinophils in the lamina propria of the patient. Finally, the boy was recovered after supportive therapy with intravenous albumin and CD eradication.
Conclusion
For the nonspecific clinical symptoms of MD, gastrointestinal endoscopic evaluations with gastric tissue biopsies are required to establish the diagnosis of MD in children with unexplained hypoalbuminemia.
Similar content being viewed by others
Background
Ménétrier’s disease (MD) is a rare form of acquired gastropathy that characterized by gastric hypertrophy and hypoalbuminemia, which was first described in 1888 by French pathologist Pierre Ménétrier [1, 2]. The common clinical symptoms of MD include epigastric pain, anorexia, weight loss, nausea, gastrointestinal bleeding, diarrhea, vomiting, fatigue, and peripheral edema [3]. Blood tests of patients with MD show hypoproteinemia and hypoalbuminemia, endoscopy usually reveals giant gastric mucosal folds, and gastric biopsy shows foveolar hyperplasia and decreased oxyntic glands [3]. The etiology of MD is still unknown, but has been associated with some gastric diseases, including gastric bacterial and viral infections [4]. MD can occur both in adults and children. In adults, MD usually presents with an insidious onset and a progressive clinical course, which are related to surgical resection and potential risk for malignant transformation [5,6,7]. Pediatric MD is generally characterized by abrupt onset and spontaneous regression without any special treatment [8,9,10]. However, uncommon case of non self-limited pediatric MD needing specific treatment has been reported in previous study [11]. Up to date, limited number of pediatric MD cases was reported in the literature. Here, we report a pediatric case of MD from China. The clinical features of the MD patient were described in the study.
Case presentation
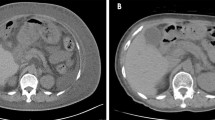
The patient was a 4 year and 10-month old boy presented to gastroenterology department of our hospital because of 4 days abdominal pain and vomiting, and 1 day eyelid edema. The boy was born at term with unremarkable family history. Two weeks before the onset of abdominal pain and vomiting, the boy was suffered a course of severe pneumonia caused by Mycoplasma pneumoniae. A regimen of ceftriaxone, azithromycin, amoxicillin and potassium clavulanate were given to eradicate the Mycoplasma pneumonia infection in respiratory department of our hospital. On admission, physical examination revealed obvious bilateral periorbital edema, abdominal pain around umbilical cord, mild edema of both lower extremities. Laboratory tests showed low levels of total protein (32.99 g/L, reference range: 60-80 g/L), albumin (24.82 g/L, reference range: 38-54 g/L), and globulin (14 g/L, reference range: 22-34 g/L). Hemoglobin level was normal, reticulocyte was slightly up-regulated, and eosinophil percentage was increased (11%). Coagulation function, erythrocyte sedimentation rate were normal, tumor markers (alpha-fetoprotein, carcinoembryonic antigen) and autoantibodies were negative. Parasites antigens, CMV-DNA, and EBV-DNA were all negative. The 13C urea breath test was negative. Clostridium difficile (CD) toxin test and culture were positive. Serological tests revealed decreased levels of IgG (1.42 g/L, reference range: 3.82–14.04 g/L), complement component 1q (67.27 mg/L, reference range: 159-233 mg/L), C3 (0.43 g/L, reference range: 0.79–1.52 g/L), and C4 (0.08 g/L, reference range: 0.1–0.4 g/L). Abdominal ultrasonography suggested diffuse thickening of the gastric wall. CT showed giant cerebriform enlargement of rugal folds in the gastric fundus and body (Fig. 1), and minimal effusion in the pelvic cavity. Gastrointestinal endoscopy revealed enlarged gastric folds, erythema, and hemorrhagic erosions covered with whitish mucus throughout the gastric body (Fig. 2). Histological findings of the gastric mucosa showed foveolar hyperplasia with glandular atrophy, infiltration of eosinophils, plasmocytes and neutrophils in the lamina propria (Fig. 3). HP was not detected in the gastric biopsies. Combined with the laboratory examination and gastrointestinal endoscopy findings, the patient was diagnosed as giant hypertrophic gastritis (MD), CD infection and hypoproteinemia. The patient was administered with intravenous albumin for six consecutive days (a total of 110 g). A 2-week regimen of oral vancomycin was given to eradicate CD infection. The patient was discharged at the 16th day of admission without notable symptoms, the albumin level was normal (43 g/L) and CD toxin and culture tests were negative. After 2 months of discharge, follow-up gastrointestinal endoscopy showed a mild superficial gastritis, no manifestation of gastric fold hypertrophy and no ulcer. The boy did not complain of any notable symptoms during 6-month of follow-up.
Discussion and conclusions
MD is a rare disease characterized by hypertrophic folds in the body of the stomach, foveolar hyperplasia and hypoproteinemia due to selective loss of serum proteins across the gastric mucosa [7]. Although MD can occur both in adults and children, pediatric MD presents a distinct clinical course that differs with MD in adults. It usually presents with an insidious onset and tends to progress over time in adults [5]. Although pediatric MD typically has an abrupt onset, self-limited, and an overall benign course that can spontaneously resolve within 2 to 10 weeks with supportive therapy only [8, 12], uncommon case of non self-limited pediatric MD needing specific treatment was also reported in previous study [11]. Di Nardo et al. reported a non self-limited pediatric MD needing endoscopic mucosal resection for diagnosis which was then successfully treated with octreotide long-acting release (LAR) [11]. MD usually occurs in children younger than 10 years, and boys are affected more often than girls [2]. The mean duration of pediatric MD is less than 6 weeks [10]. The child with MD described in this study was a boy with aged less than 10 years. Studies [10, 13] suggested that pediatric MD patients may present with a variety of nonspecific symptoms. The common symptoms of pediatric MD include abdominal pain, nausea and frequent vomiting, diarrhea, loss of appetite, weight loss, and malnutrition [13]. Peripheral edema due to hypoalbuminemia is also present frequently in pediatric MD patients [8, 10, 13]. In this report, the patient presented with symptoms of abdominal pain and vomiting, and eyelid edema. Radiologic, endoscopic and pathologic findings further supported the diagnosis of MD. Finally, after 2 weeks of supportive therapy, the disease was resolved and the boy was asymptomatic during the follow-up of 6 months.
The exact cause of pediatric MD is still unclear. Previous studies [8, 14,15,16,17] have suggested that MD is associated with several gastric infections. The most common infection associated pediatric MD is CMV. It has been reported that around 70% of pediatric MD patients were infected with CMV [8, 18]. Pediatric MD cases with HP infection were also reported in the literature [16]. It was shown that the clinical and biochemical resolution of MD achieved after the eradication therapy for HP infection [14, 15]. Furthermore, Mycoplasma pneumoniae infection is reported associated with MD in children [17]. Two weeks before the diagnosis of MD, the patient was infected with Mycoplasma pneumoniae, indicating that Mycoplasma pneumoniae infection may play a role in the onset of MD.
CD infection is a leading cause of antibiotic-associated and healthcare-associated infective diarrhea, which is determined by the presence of diarrhea and either a detection of toxin producing CD in stool, or findings of pseudomembranous colitis [19]. The prevalence and severity of CD infection in children has been increased in past decades [20]. Antibiotic (metronidazole, vancomycin or fidaxomicin) is the current first line treatment for CDI [19]. Interestingly, the MD case presented here was infected with CD which confirmed by CD toxin test and culture. The CDI may be caused by the large amount of antibiotics using in previous pneumonia episode of the patients. To the best of our knowledge, the association of CD infection with MD has not been reported in the literature. However, the possibility of gastric lesions caused by CD infection-mediated immune disorder can’t be excluded in this reported case. Further studies are needed to investigate the role of CDI on the pathogenesis of MD.
In summary, we described the clinical features of a pediatric case with MD from China in this study. Given the nonspecific symptoms, gastrointestinal endoscopic evaluations with gastric tissue biopsies are required to establish the diagnosis of MD in children with unexplained hypoalbuminemia.
Availability of data and materials
The data of the current study are available from the corresponding author on reasonable request.
Abbreviations
- CD:
-
Clostridium difficile
- CMV:
-
Cytomegalovirus
- CT:
-
Computed tomography
- EBV:
-
Epstein-Barr virus
- HP:
-
Helicobacter pylori
- MD:
-
Ménétrier’s disease
References
Ménétrier P. Des polyadenomes gastriques et deleurs rapports avec le cancer del’estomac. Arch Physiol Normal Pathol. 1888;1:232–62.
Friedman J, Platnick J, Farruggia S, Khilko N, Mody K, Tyshkov M. Menetrier disease. Radiographics. 2009;29(1):297–301.
Rich A, Toro TZ, Tanksley J, Fiske WH, Lind CD, Ayers GD, Piessevaux H, Washington MK, Coffey RJ. Distinguishing Menetrier's disease from its mimics. Gut. 2010;59(12):1617–24.
Fiori R, Velari L, Di Vito L, Della Gatta F, Bianchi M, Capurso L, Simonetti G. Menetrier's disease diagnosed by enteroclysis CT: a case report and review of the literature. Abdom Imaging. 2011;36(6):689–93.
Scharschmidt BF. The natural history of hypertrophic gastrophy (Menetrier's disease). Report of a case with 16 year follow-up and review of 120 cases from the literature. Am J Med. 1977;63(4):644–52.
Sundt TM 3rd, Compton CC, Malt RA. Menetrier's disease. A trivalent gastropathy. Ann Surg. 1988;208(6):694–701.
Wolfsen HC, Carpenter HA, Talley NJ. Menetrier's disease: a form of hypertrophic gastropathy or gastritis? Gastroenterology. 1993;104(5):1310–9.
Occena RO, Taylor SF, Robinson CC, Sokol RJ. Association of cytomegalovirus with Menetrier's disease in childhood: report of two new cases with a review of literature. J Pediatr Gastroenterol Nutr. 1993;17(2):217–24.
Megged O, Schlesinger Y. Cytomegalovirus-associated protein-losing gastropathy in childhood. Eur J Pediatr. 2008;167(11):1217–20.
Chouraqui JP, Roy CC, Brochu P, Gregoire H, Morin CL, Weber AM. Menetrier's disease in children: report of a patient and review of sixteen other cases. Gastroenterology. 1981;80(5 pt 1):1042–7.
Di Nardo G, Oliva S, Aloi M, Ferrari F, Frediani S, Marcheggiano A, Cucchiara S. A pediatric non-protein losing Menetrier’s disease successfully treated with octreotide long acting release. World J Gastroenterol. 2012;18(21):2727–9.
Shah KJ. Transient protein-losing gastropathy (Menetrier’s disease) in childhood. Pediatr Radiol. 1988;18(3):248.
Baker A, Volberg F, Sumner T, Moran R. Childhood Menetrier's disease: four new cases and discussion of the literature. Gastrointest Radiol. 1986;11(2):131–4.
Kawasaki M, Hizawa K, Aoyagi K, Nakamura S, Fujishima M. Menetrier’s disease associated with helicobacter pylori infection: resolution of enlarged gastric folds and hypoproteinemia after antibacterial treatment. Am J Gastroenterol. 1997;92(10):1909–12.
Yamada M, Sumazaki R, Adachi H, Ahmed T, Matsubara T, Hori T, Nakahara A, Takita H. Resolution of protein-losing hypertrophic gastropathy by eradication of helicobacter pylori. Eur J Pediatr. 1997;156(3):182–5.
Jun DW, Kim DH, Kim SH, Song MH, Lee HH, Jo YJ, Park YS. Menetrier's disease associated with herpes infection: response to treatment with acyclovir. Gastrointest Endosc. 2007;65(7):1092–5.
Ben Amitai D, Zahavi I, Dinari G, Garty BZ. Transient protein-losing hypertrophic gastropathy associated with mycoplasma pneumoniae infection in childhood. J Pediatr Gastroenterol Nutr. 1992;14(2):237–9.
Tard C, Madhi F, Verlhac S, Hagege H, Epaud R, Jung C. Protein-losing gastropathy associated with cytomegalovirus in two sisters - case reports and review of the literature. Arch Pediatr. 2019;26(4):232–5.
McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, Dubberke ER, Garey KW, Gould CV, Kelly C, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987–94.
Khanna S, Baddour LM, Huskins WC, Kammer PP, Faubion WA, Zinsmeister AR, Harmsen WS, Pardi DS. The epidemiology of Clostridium difficile infection in children: a population-based study. Clin Infect Dis. 2013;56(10):1401–6.
Acknowledgements
The authors thank the family for participating and supporting this study.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (grant number 81870373, 81500449), Shanghai Hospital Development Center New Frontier Technology Joint Research Project (grant number SHDC12017115), and Shanghai Municipal Commission of Health and Family Planning, China (2017ZZ02019). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
JZ and YW drafted the manuscript. JZ, HL, TZ, and YX acquired, analyzed, and interpreted the data. YX and TZ edited the manuscript. All authors agreed to be accountable for all aspects of the work. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consents were obtained from the parents of the patients for the publication of this study. This study was in compliance with the Helsinki Declaration and was approved by the Ethical Review Board of Shanghai Children’s Hospital.
Consent for publication
The parents of the patients consented to the publication of the case and any accompanying images with written consent.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, J., Wang, Y., Liu, H. et al. Ménétrier’s disease in childhood: a case report from China. BMC Pediatr 20, 110 (2020). https://doi.org/10.1186/s12887-020-2005-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-020-2005-6