Abstract
Background
Colonic stenosis is a rare cause of pediatric intestinal obstruction. The root cause underlying colonic stenosis is unclear and there is no fixed operation.
Case presentation
We reported on a male infant with progressive colonic stenosis caused by antibiotic-related colitis. The infant was admitted to our hospital with pneumonia but developed progressive abdominal distension and diarrhea following antibiotic treatment with meropenem. Initial testing of stool culture showed a Clostridium difficile infection. Additional testing with barium enema imaging showed stenosis at the junction of the sigmoid and descending colon at first and another stenosis occurred at the right half of the transverse colon 3 weeks later. Staged surgical treatment was performed with primary resections of the two parts suffering stenosis, ileostomy, and secondary intestinal anastomosis. A pathological exam then confirmed the diagnosis of colonic stenosis and the patient had an uneventful recovery and has been recovering well as evidenced by the 1-year follow-up.
Conclusions
Based on a review of the literature and our case report, we found that progressive colonic stenosis caused by colitis due to antibiotic-related Clostridium difficile infection is rare in infants. Infants with colitis and repeated abdominal distention, vomiting, and constipation should be treated with the utmost caution and screened. Despite this, clinical manifestations depended on the severity of the stenosis. Barium enema, colonoscopy, laprascopy or laparotomy and colonic biopsy are helpful for diagnosis and differential diagnosis. While both one-stage and multiple-stage operations are feasible, a staged operation should be used for multiple colonic stenoses.
Similar content being viewed by others
Background
Colonic stenosis is a rare cause of pediatric intestinal obstruction. The root cause underlying colonic stenosis is unclear. Both congenital and acquired colonic stenoses (e.g post-necrotizing enterocolitis) has been reported on [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Here we present an infant case of progressive colonic stenosis after antibiotic-related Clostridium difficile colitis and a review of the related literature, which to the best of our knowledge had not been reported previously.
Case presentation
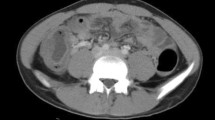
This male infant was the second child of a 39-year-old mother and was born via cesarean section during the 38th week of the pregnancy with a birth weight of 3300 g. The infant was admitted to our hospital 10 days after birth due to pneumonia and was treated with meropenem. He developed abdominal distension and diarrhea gradually from the 10th day of therapy on and stool culture revealed a Clostridium difficile infection. This was considered to be antibiotic-related and oral metronidazole and vancomycin were given. His symptoms were soon resolved but after discharge he gradually developed abdominal distension and constipation. A barium enema exam on the 42nd day after birth showed stenosis at the junction of the sigmoid and descending colon and a distended proximal bowel (Fig. 1a). Abdominal distension and constipation became more severe after 3 weeks of conservative treatment. A second barium enema exam then revealed another stenosis of the right transverse colon in addition to the previous stenosis (Fig. 1b).
Primary surgical exploration revealed two segments of stenoses. One was at the junction of the sigmoid and descending colon and was 3.5 cm in length, while the other one was at the right transverse colon and was 4 cm in length. The small intestine, however, was still intact. Both the two parts were resected and an ileostomy was conducted at the terminal ileum. A pathological exam showed fibrosis of lamina propria in the narrow segments. Ganglion cells were normal (Fig. 2a and b). Closure of ileostomy was performed 3 months later and he made uneventful recovery. At the 1-year follow-up, he exhibited a normal dietary intake and defecation. His state of growth and development was in the 70th percentile.
Discussion and conclusions
Intestinal obstruction caused by colonic stenosis is rare in children. We reviewed literature relating to pediatric colonic stenoses since 1961 (Tables 1 and 2) [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Pathogenesis of pediatric colonic stenoses varied among patients but congenital stenosis featured prominently among the literature. George Ekema and Reyes C introduced cases with congenital cytomegavirus (CMV) infection which involved the gastrointestinal tract and finally developed into colonic stenosis [30, 31]. Many researchers accepted the theory that fetal intestinal injury in the uterus due to disturbance in the blood supply was key. The causes of ischemia included emboli originating in the placenta, fetal herniation, kinks, intussusceptions, drugs (particularly cocaine) and placental causes [7, 10, 14, 16, 32,33,34]. Some colonic stenoses were secondary to necrotizing enterocolitis (NEC) [17,18,19,20,21,22,23,24,25,26,27,28,29], which were the most common type of non-congenital colon stenoses. The present case did not have complications during the pregnancy and perinatal period though. TORCH exam of the child and the mother was also negative. Colonic stenoses developed after antibiotic-related cololitis caused by Clostridium difficile progressively, which was confirmed by two barium exams. Sahara K and Kawaratani H reported that it is adult inflammatory bowel disease that causes colonic stenosis, and stool culture suggests Clostridium difficile [35, 36], but in our case the underlying disease wasn’t present. This patient did have a history of intestinal infection of Clostridium difficile prior to onset of symptoms though. The second barium exam showed a new site of stenosis compared to the first barium exam. This evidence shows that stenoses occurred secondary to infection rather than being congenital.
It has been suggested that infants who have had abdominal distension, vomiting, and constipation should be suspected to suffer from colon stenosis. The barium enema was important in the diagnosis colonic stenosis in this case as it could determine the site of obstruction and severity of stenosis. However, a colonoscopy would be an alternative method to help with diagnosis [6]. The major differential diagnosis was Hirschsprung’s disease confirmed by pathological exam. In humans and other mammals, both domestic and wild, Clostridium difficile takes hold of the large intestine. While toxigenic and nontoxigenic strains do exist, toxigenic forms are responsible for causing disease in humans. Toxin A (TcdA) and toxin B (TcdB), are two closely related diarrhea-causing toxins and their presence is seen is a cause of pathogenicity. TcdB is found in all toxigenic strains, regardless of whether TcdA occurs concurrently. In addition to this, inactivation of Rho GTPases through enzymatic glucosylation of a conserved threonine residue is a similar molecular mechanism of action found in both of these toxins. Most often actin depolymerization and cell death follow, and the mechanism leads to the stimulation of an inflammatory cascade, with the end result being tissue damage, diarrhea, and pseudomembranous colitis [37, 38]. Moreover, significantly correlated with this tissue damage, diarrhea, and pseudomembranous colitis was the occurrence of a progression to fibrosis at the lamina propria.
Surgery is the major treatment of colon stenosis (Tables 1 and 2). For stenoses in both the right and left half of the colon, resection and primary anastomosis or proximal diversion could be successfully performed [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Pelizzo G reported three cases of colonic stenoses with norovirus infection in preterm babies. All patients received primary ileostomy followed by an immediate or staged coloectomy. Proximal diversion of intestinal contents is recommended to help to preserve colon integrity [19]. In our patient, primary resections of strictures of the colon with proximal diversion had successfully preserved the rest of the colon. This was important as the colonic stenoses was proved to be progressive in this case. The main reason why we chose to perform ileostomy rather than colon anastomosis was due to the fact of the colon stenosis being progressive. We didn’t know whether new stenosis would occur. Barium enema imaging before enterostomy didn’t reveal another colonic stenosis and the patient had an uneventful recovery followed by a clean check of health at the 1-year follow-up.
Based on a review of the literature and our case report, we found that progressive colonic stenosis caused by colitis due to antibiotic-related Clostridium difficile infection is rare in infants. Infants with colitis and repeated abdominal distention, vomiting, and constipation should be suspected and screened. Clinical manifestations depended on the severity of the stenosis. Barium enema, colonoscopy, laprascopy or laparotomy and colonic biopsy are helpful for diagnosis and differential diagnosis. Both one-stage surgery and multiple-stage operations are feasible, however staged operation should be used for multiple colonic stenoses.
Abbreviations
- CMV:
-
Congenital cytomegavirus
- NEC:
-
Necrotizing enterocolitis
References
Elisa Z, Cinzia C, Sergio S, Giuseppe LV, Fortunato S. Multiple congenital colonic stenosis:a rare gastrointestinal malformation. Case Rep Pediatr. 2016:6329793. Published online 2016 Mar 15. https://doi.org/10.1155/2016/6329793.
Saha N, Shezote T, Alam S. Congenital stenosis in the descending colon causing intestinal obstruction in a one and half years male child. Mymensingh Med J. 2013;22(3):574–7.
Galván-Montaño A, Suárez-Roa Mde L, Carmona-Moreno E. Congenital stenosis of the colon with foreign bodies. Case report. Cir Cir. 2010;78(3):259–61.
Ruggeri G, Libri M, Gargano T, Pavia S, Pasini L, Tani G, Lima M. Congenital colonic stenosis: a case of late-onset. Pediatr Med Chir. 2009;31(3):130–3.
Mizuno M, Kato T, Hebiguchi T, Yoshino H. Congenital membranous colonic stenosis-case report of an extremely rare anomaly. J Pediatr Surg. 2003;38(8):E13–5.
García Vázquez A, Cano Novillo I, Portela Casalod E, Benavent Gordo MI, Berchi García FJ. Congenital colonic stenosis. An Esp Pediatr. 2002;56(3):258–60.
Abu-Judeh HH, Methratta S, Ybasco A, Garrow E, Ali S. Congenital colonic stenosis. South Med J. 2001;94(3):344–6.
Dalla Vecchia LK, Grosfeld JL, West KW, Rescorla FJ, Scherer LR, Engum SA. Intestinal atresia and stenosis: a 25-year experience with 277 cases. Arch Surg. 1998;133(5):490–6.
Murphree SM, Dunkley AS. Colon atresia and stenosis in Zimbabwe: case reports and a review of the literature. Cent Afr J Med. 1992;38(12):463–5.
Sax EJ. Pediatric case of the day. Congenital colonic stenosis. Am J Roentgenol. 1991;156(6):1315–7.
Pai GK, Pai PK. A case of congenital colonic stenosis presenting as rectal prolapse. J Pediatr Surg. 1990;25(6):699–700.
Rescorla FJ, Grosfeld JL. Intestinal atresia and stenosis:analysis of survival in 120 cases. Surgery. 1985;98(4):668–76.
Schiller M, Aviad l, Freund H. Congenital colonic atresia and stenosis. Am J Surg. 1979;138(5):721–4.
Erskine JM. Colonic stenosis in the newborn: the possible thromboembolic etiology of intestinal stenosis and atresia. J Pediatr Surg. 1970;5(3):321–33.
Benson CD, Lotfi MW, Brouch AJ. Congenital atresia and stenosis of the colon. J Pediatr Surg. 1968;3(2):253–7.
Santulli TV, Blanc WA. Congenital atresia of the intestine: pathogenesis and treatment. Ann Surg. 1961;154(6):939–48.
Marseglia L, Manti S, D’Angelo G, Lima M, Impellizzeri P, Romeo C, Gitto E. Colonic stenosis post-necrotizing enterocolitis in term newborn with acquired cytomegalovirus infection. Chirurgia (Bucur). 2015;110(2):175–8.
Gaudin A, Farnoux C, Bonnard A, Alison M, Maury L, Biran V, Baud O. Necrotizing enterocolitis (NEC) and the risk of intestinal stricture: the value of C-reactive protein. PLoS One. 2013;8(10):e76858.
Pelizzo G, Nakib G, Goruppi I, Fusillo M, Scorletti F, Mencherini S, Parigi GB, Stronati M, Calcaterra V. Isolated colon ischemia with norovirus infection in preterm babies: a case series. J Med Case Rep. 2013;7:108.
Martinez-Ferro M, Rothenberg S, St. Peter S, Bignon H, Holcomb G. Laparoscopic treatment of postnecrotizing enterocolitis colonic strictures. J Laparoendosc Adv Surg Tech A. 2010;20(5):477–80.
Baudon JJ, Josset P, Audry G, Benlagha N, Fresco O. Intestinal stenosis during ulceronecrotizing enterocolitis. Arch Pediatr. 1997;4(4):305–10.
Vilariño Mosquera A, CanoNovillo I, Benavent Gordo I, Jiménez MA, Delga Manzanares M, Barrios C, Berchi García FJ. Retrospective analysis of 80 cases of neonatal necrotizing enterocolitis. Cir Pediatr. 1995;8(4):148–50.
Schimpl G, Höllwarth ME, Fotter R, Becker H. Late intestinal strictures following successful treatment of necrotizing enterocolitis. Acta Paediatr Suppl. 1994;396:80–3.
Gobet R, Sacher P, Schwöbel MG. Surgical procedures in colonic strictures after necrotizing enterocolitis. Acta Paediatr Suppl. 1994;396:77–9.
Radhakrishnan J, Blechman G, Shrader C, Patel MK, Mangurten HH, McFadden JC. Colonic strictures following successful medical management of necrotizing enterocolitis: a prospective study evaluating early gastrointestinal contrast studies. J Pediatr Surg. 1991;26(9):1043–6.
D’Agostino S, Stracca-Pansa V, Drei F, Valli F, Colombo B, Guarise P. Post-necrotizing enterocolitis stenosis of the colon associated with cytomegalovirus infection. Description of a clinical case. Pediatr Med Chir. 1988;10(6):637–9.
Schwartz MZ, Keith Hayden C, Joan Richardson C, Tyson KRT, Lobe TE. A prospective evaluation of intestinal stenosis following necrotizing enterocolitis. J Pediatr Surg. 1982;17(6):764–70.
Kosloske AM, Burstein J, Bartow SA. Intestinal obstruction due to colonic stricture following neonatal necrotizing enterocolitis. Ann Surg. 1980;192(2):202–7.
Bonte C, Barberis D, Maillard E, Rousselle B, Dervaux D. Colonic stenosis following necrotizing enterocolitis in newborn infants. Apropos of 3 cases. Arch Fr Pediatr. 1977;34(7):622–31.
Ekema G, Pedersini P, Milianti S, Ubertazzi M, Minoli D, Manciana A. Colonic stricture mimicking Hirschsprung’s disease: a localized cytomegalovirus infection. J Pediatr Surg. 2006;41(4):850–2.
Reyes C, Pereira S, Warden MJ, Sills J. Cytomegalovirus enteritis in a premature infant. J Pediatr Surg. 1997;32(11):1545–7.
Powell RW, Raffensperger JG. Congenital colonic atresia. J Pediatr Surg. 1982;17(2):166–70.
Louw JH, Barnard CN. Congenital intestinal atresia:observation on its origin. Lancet. 1955;269(6899):1065–7.
Hall TR, Zaninovi A, Lewin D, Barrett C, Boechat MI. Neonatal intestinal ischemia with bowl perforation:an in utero complication of maternal cocaine abuse. AJR Am J Roentgenol. 1992;158(6):1303–4.
Sahara K, Yamada R, Fujiwara T, Koizumi K, Horiguchi S-i, Hishima T, Yamaguchi T. Idiopathic myointimal hyperplasia of mesenteric veins: rare case of ischemic colitis mimicking inflammatory bowel disease. Dig Endosc. 2015;27(7):767–70.
Kawaratani H, Tsujimoto T, Toyohara M, Kin K, Taniguchi T, Shirai Y, Ikenaka Y, Nakayama M, Fujii H, Fukui H. Pseudomembranous colitis complicating ulcerative colitis. Dig Endosc. 2010;22(4):373–5.
Vedantam G, Clark A, Chu M, McQuade R, Mallozzi M, Viswanathan VK. Clostridium difficile infection: toxins and non-toxin virulence factors, and their contributions to disease establishment and host response. Gut Microbes. 2012;3(2):121–34.
Pothoulakis C, Lamont JT. Microbes and microbial toxins: paradigms for microbial-mucosal interactions II. The integrated response of the intestine to Clostridium difficile toxins. Am J Physiol Gastrointest Liver Physiol. 2001;280(2):G178–83.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Contributions
Study conception and design: XJ. Data acquisition: XX, YW, YZ, QW. Analysis and data interpretation: BX, XJ. Drafting of the manuscript: XX. Critical revision: XJ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board and Ethical Committee at the West China Hospital of Sichuan University in China with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Written informed consent was obtained from the patient’s parents to publish this case report and any accompanying images. This material is original research. It has not been previously published and has not been submitted for publication elsewhere while under consideration.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Xie, X., Xiang, B., Wu, Y. et al. Infant progressive colonic stenosis caused by antibiotic-related Clostridium difficile colitis – a case report and literature review. BMC Pediatr 18, 320 (2018). https://doi.org/10.1186/s12887-018-1302-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-018-1302-9