Abstract
Background
Increasing evidences indicated that diabetes might increase the incidence of gallbladder cancer. However, no sufficient data has ever clarified the impact of diabetes on the survival of patients with gallbladder cancer.
Methods
We comprehensively searched PubMed, Embase, and the Cochrane Library databases through July 2019 in order to find sufficient eligible researches. The pooled hazard risks (HRs) and relative risks (RRs) with 95% confidence intervals (CIs) were calculated with either fix-effects or random-effects model. Due to the low gallbladder cancer mortality in general population, the RRs and standard mortality ratios (SMRs) were considered the similar estimates of the HRs.
Results
Ten eligible studies were included in this meta-analysis. Analysis of eight cohorts found that diabetes was closely associated with the mortality of gallbladder cancer (HR = 1.10; 95% CI: 1.06–1.14; P < 0.00001). However, the mortality in male diabetes patients was not higher than female patients (RR = 1.08, 95%CI = 0.57–2.04, P = 0.80).
Conclusions
These findings indicated that diabetes patients had a higher mortality of gallbladder cancer compared with non-diabetes.
Similar content being viewed by others
Background
Gallbladder cancer (GBC) is one of the most common biliary tract malignancies worldwide [1]. By and large, poor prognosis seriously affects the mortality of patients with gallbladder cancer [2]. Gallbladder cancer patients survive the mean survival rate of 6 months and a 5-year survival rate of 5% [3]. Generally, women are two to six times more likely to be attacked by gallbladder cancer [4]. The prognosis of patients with GBC is affected by a growing number of factors, including age, gender, smoking, ethnic, and menopause [5,6,7,8,9]. Advancing age partly demonstrates the prevalence of gallbladder cancer [10]. Finding an optimal prognostic indicator would be helpful to improve the survival rate of GBC.
Diabetes mellitus (DM) is a costly chronic disease worldwide. The incremental increase in costs of this disease have laid economic burdens on both financial expenditure in most countries and patients themselves. In the United State, the newly diagnosed patients spent approximately $8941 more than subjects who were not diagnosed with DM over a period of 5 years [11]. Approximately 415 million people suffered from diabetes in 2015 while 5 million patients died from diabetes [12]. By 2040, the number of diabetes patients are predicted to ascend to 642 million. DM is always regarded as a pivotal risk factor linked to cancer at different sites, including lung [13], liver [14], esophagus [15], stomach [16], colorectum [17], kidney [18], breast [19], leukemia, non-Hodgkin lymphoma, myeloma [20], ovary [21], and prostate [22]. As several studies and meta-analyses have pointed out, DM was closely associated with the onset risk of gallbladder cancer [23, 24]. However, rare study has focused on the relationship between DM and the mortality of gallbladder cancer. This meta-analysis aimed to figure out if DM patients had a higher risk of dying from GBC and if male and female patients had a different risk of die from GBC.
Methods
Search strategy
A comprehensive search has been made on the PubMed, Embase, web of science, and the Cochrane Library databases to find all the eligible studies up to July 13th 2019. The following text words were used in the PubMed: (“diabetes” OR “glucose intolerance” OR “insulin resistance” OR “hyperglycemia” OR “hyperinsulinemia” OR “metabolic syndrome”) AND (“gallbladder cancer” OR “gallbladder carcinoma” OR “gall bladder cancer” OR “gall bladder carcinoma”). Correlative key words were used in the Embase, web of secience, and the Cochrane Library. To comprehensively search eligible studies, we simultaneously searched the reference lists of relevant reviews or included publications for further studies.
Inclusion and exclusion criteria
The included literatures met the following criteria: (1) cohort design; (2) investigated gallbladder cancer outcomes; (3) assessed the gallbladder cancer mortality with or without DM; (4) reported the information of hazard ratios (HRs), relative risks (RRs), or standard mortality ratios (SMRs). The exclusion criteria were as follows: (1) case-control or cross-sectional design; (2) unavailable data.
Data extraction
Two authors independently extracted all data from publications using the same criteria. The following data were included: the first author’s name, publication year, country, sample size, the number of male or female participants, mean age at baseline, average follow-up duration, diabetes assessment, and adjusted factors.
Statistical analysis
We used Reviewer Manager 5.3 in this meta-analysis to analyze the data. The pooled HRs with 95% CIs were calculated as the effect estimates for the relationship between DM and gallbladder cancer mortality. The fixed-model was used when the heterogeneity was low, while the random-model was used when the heterogeneity was high. Owing to the low gallbladder cancer mortality in general population, the RRs, SMR were considered the similar estimates of the HRs [25]. Statistical heterogeneity among studies was assessed by the I2 and Q statistics. Both I2 > 50% and P value< 0.1 were regarded as high heterogeneity. We conducted subgroup analysis to evaluate the potential sources of heterogeneity from country, follow-up duration, diabetes assessment, and adjusted factors (including BMI, smoking, and education). A sensitivity analysis was performed by removing each study from the overall analysis to investigate the influence of a single study. We used funnel plots, Begg and Egger tests to assess publication bias. P value< 0.05 was viewed as a significant level. The statistical analyses were performed with Stata software (version 12.0).
Results
Study selection
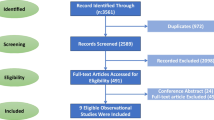
Detailed study selection process was described in Fig. 1. From the initial search, we searched and identified 561 records. Two authors independently assessed the search outputs based on the primary research title or abstract. Three hundred forty-seven articles were discarded for the sake of duplication. One hundred seventy-five articles were excluded based on title or abstract. Then we read the full-text of the remaining paper. We further removed 14 studies that enrolled single-arm DM patients. Fifteen of the 25 remaining studies were subsequently removed due to lack of eligible data. Finally, a total of 10 studies were included in the meta-analysis [26,27,28,29,30,31,32,33,34,35].
Study characteristics
The baseline characteristics of the included studies were listed in Table 1. A total of 5,522,636 participants were included in all 10 studies. Two studies were conducted in the USA, two in the UK, three in the Asia, one in Australia, and two were international conducted studies. The average follow-up duration ranged from 2 to 18 years. Diabetes assessment methods included self-report, medical record, WHO diagnostic criteria, and read code classification. Eight studies reported the relationship between DM and gallbladder cancer mortality, while four studies assessed the different gallbladder cancer mortality in male and female DM patients.
The quality assessment results were shown in Figs. 2 and 3. All of the studies applied random sequence generation and allocation concealment. No attrition bias and reporting bias were reported. Two of all studies completed blinding of outcome assessment. Only one study reported performance bias.
DM and gallbladder cancer mortality
Eight studies focused on the relationship between diabetes mellitus and gallbladder cancer mortality. We merged the data of these studies and found that pre-existing diabetes had a high correlation with the mortality of gallbladder cancer compared with non-DM participants (HR = 1.10; 95% CI: 1.06–1.14; P < 0.00001; Fig. 4). A fix-effects model was applied owing to low heterogeneity (I2 = 0%; P = 0.95). The sensitivity analysis results indicated that the summary HR ranged from 1.09 (95%CI: 1.06–1.13) when excluding study from Chen 2017 to 1.12 (95%CI: 1.07–1.17) when excluding study from Currie 2012 [31, 33].
Subgroup analysis were conducted according to country, follow-up duration, diabetes assessment, and adjustment for confounding factors, including BMI, smoking, and education. All of the results were demonstrated in Table 2. However, no evidence indicated that there were significant differences between subgroups based on factors above.
DM and gallbladder cancer mortality in men and women
A total of four studies estimated the difference of gallbladder cancer mortality between male and female DM patients. The analysis was conducted to see if female DM patients had a higher risk of gallbladder cancer mortality then male patients. The pooled analysis results demonstrated that no significant differences had existed between DM men and women (RR = 1.08, 95%CI = 0.57–2.04, P = 0.80; Fig. 5.). A random-effect model was applied due to high heterogeneity (P = 0.0007, I2 = 82%).
Publication bias
The symmetric funnel plots indicated a potential low publication bias (Fig. 6). Moreover, Egger test (P = 0.371) and Begg test (P = 0.845) showed no significant evidence of publication bias.
Discussion
This meta-analysis of cohort studies provided comprehensive evidence that the diabetes mellitus had an impact on the survival of patients with gallbladder cancer. Our results suggested that diabetes patients had a higher mortality rate of gallbladder cancer compared with non-diabetes patients. And the results were independent of country, follow-up duration, diabetes assessment, BMI, smoking, or education. Though previous analysis had indicated that DM women were more likely to develop gallbladder cancer than DM men due to sex hormones [36], we found no obvious differences between male and female diabetes patients in gallbladder cancer mortality. However, the results remained to be tested due to lack of eligible data.
Several physiological mechanisms might account for the increase of gallbladder cancer mortality in DM patients. A growing number of studies have found that overweight, obesity, metabolic syndrome, and insulin resistance were closely related to the increase of gallbladder disease [37,38,39]. Hyperinsulinemia was also a phenomenon commonly existed in DM patients. Excess insulin directly or indirectly regulated the activity of insulin-like growth factor-1 (IGF-1), which was an important cytokine that influenced the development and progression of cancer [40]. Both in vitro and in vivo researches have proved that up-regulation of IGF-1 contributed to the proliferation of bile duct cancer cells and the inhibition of apoptosis [41, 42]. In addition, diabetes impaired the function of gallbladder emptying. The gallbladder smooth muscle cells of DM patients have reduced sensitivity to cholecystokinin. Meanwhile, the number of cholecystokinin receptors on the gallbladder wall in DM patients was also reduced [43]. These physiological mechanisms were consistent with the increased risk of biliary tract cancer [44].
To our knowledge, our meta-analysis was the first study focused on the impact of DM on the survival of patients with gallbladder cancer. Previous study has proved that diabetes might increase the risk of gallbladder diseases [45]. One meta-analysis has proved the association between DM and the increased GBC risk [24]. However, the meta-analysis included both case-control studies and cohort studies, which might somehow increase the overall heterogeneity. Furthermore, the majority of the included cohort studies focused on the gallbladder cancer incidence rather than mortality. Our analysis attempted to find an optimal prognostic indicator that would increase the GBC mortality. In addition, a subgroup analysis was conducted to see the difference of GBC mortality in male and female DM patients.
The present meta-analyses had some strengths, including prospective design of cohort studies, eligible data from large sample size, detailed subgroup analyses, and low heterogeneity. Our findings provided an important message for patients with comorbid DM and gallbladder cancer that preventing the progression of diabetes might increase the survival from gallbladder cancer.
There were several potential limitations in our study. First, residual confounding could not be ignored. Compared with non-DM participants, DM patients often had less healthy lifestyles, including higher rate of obesity, less physically activity, and more likely to smoke and drink. Though most of the included studies have adjusted these factors and our subgroup analysis showed no obvious heterogeneity between subgroups, we could not completely exclude the influence of these factors. Second, most studies did not tell the differences between type 1 and type 2 DM, though the majority of individuals were type 2 survivals. Older individuals were more likely to develop type 2 DM, while type 1 DM was a more common type in younger individuals. As a result of incomplete initial data on distinguishing this difference, some degree of inaccuracy of results was inevitable. Third, the number of eligible literatures remained low, which might have some influence on the final conclusion. The results of the difference of gallbladder cancer mortality between male and female patients remained open to question due to the lack of data and a high heterogeneity. Forth, the effect of medicine had not taken into account in the researches. Many studies have indicated that metformin, a commonly used diabetic medication, could retard the development of some cancers. None of the included researches have made adjustments for the use of diabetic medication. Last but not least, the multiplicity might exist in this analysis. The multiplicity attributed to a number of factors. On one hand, the subjects came from various backgrounds. Different rate, country, and age aggravated the multiplicity. On the other hand, the subjects from different studies might have an overlap.
Conclusion
In conclusion, this meta-analysis suggested that diabetes patients had a higher mortality of gallbladder cancer. More relevant studies were needed to certify this association and tell the difference between men and women.
Availability of data and materials
All data generated in this analysis are available from the corresponding author.
Abbreviations
- HRs:
-
Hazard risks
- RRs:
-
Relative risks
- CIs:
-
Confidence intervals
- DM:
-
Diabetes mellitus
- ORs:
-
Odd ratios
- SMRs:
-
Standard mortality ratios
- BMI:
-
Body mass index
- IGF-1:
-
Insulin-like growth factor-1
- ERFC:
-
Emerging Risk Factors Collaboration
- T1DM:
-
Type 1 Diabetes Mellitus
- T2DM:
-
Type 2 Diabetes Mellitus
- WHO:
-
World Health Organization
- NSAIDs:
-
Nonsteroidal Anti-inflammatory Drugs
References
Wernberg JA, Lucarelli DD. Gallbladder cancer. Surg Clin North Am. 2014;94(2):343–60.
Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BJ, Youssef BM, Klimstra D, Blumgart LH. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234(4):507–17 discussion 517-509.
Levy AD, Murakata LA, Rohrmann CA Jr. Gallbladder carcinoma: radiologic-pathologic correlation. Radiographics. 2001;21(2):295–314 questionnaire, 549–255.
Konstantinidis IT, Deshpande V, Genevay M, Berger D, Fernandez-del Castillo C, Tanabe KK, Zheng H, Lauwers GY, Ferrone CR. Trends in presentation and survival for gallbladder cancer during a period of more than 4 decades: a single-institution experience. Arch Surg. 2009;144(5):441–7 Discussion 447.
Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67(3):253–6.
Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. Jama. 2011;306(7):737–45.
Dietrich K, Demidenko E, Schned A, Zens MS, Heaney J, Karagas MR. Parity, early menopause and the incidence of bladder cancer in women: a case-control study and meta-analysis. Eur J Cancer. 2011;47(4):592–9.
Stern MC, Lin J, Figueroa JD, Kelsey KT, Kiltie AE, Yuan JM, Matullo G, Fletcher T, Benhamou S, Taylor JA, et al. Polymorphisms in DNA repair genes, smoking, and bladder cancer risk: findings from the international consortium of bladder cancer. Cancer Res. 2009;69(17):6857–64.
Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109.
Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, D'Angelica M, Dematteo RP, Blumgart LH, O'Reilly EM. Gallbladder cancer (GBC): 10-year experience at memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol. 2008;98(7):485–9.
Khan T, Yang J, Wozniak G. Trends in medical expenditures prior to diabetes diagnosis: the early Burden of diabetes. Popul Health Manag. 2020;0:1–6.
Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50.
Lee JY, Jeon I, Lee JM, Yoon JM, Park SM. Diabetes mellitus as an independent risk factor for lung cancer: a meta-analysis of observational studies. Eur J Cancer. 2013;49(10):2411–23.
Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen Y, Li G, Wang L. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130(7):1639–48.
Huang W, Ren H, Ben Q, Cai Q, Zhu W, Li Z. Risk of esophageal cancer in diabetes mellitus: a meta-analysis of observational studies. Cancer Causes Control. 2012;23(2):263–72.
Ge Z, Ben Q, Qian J, Wang Y, Li Y. Diabetes mellitus and risk of gastric cancer: a systematic review and meta-analysis of observational studies. Eur J Gastroenterol Hepatol. 2011;23(12):1127–35.
Jiang Y, Ben Q, Shen H, Lu W, Zhang Y, Zhu J. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2011;26(11):863–76.
Chen L, Li H, Gu L, Ma X, Li X, Gao Y, Zhang Y, Shen D, Fan Y, Wang B, et al. The impact of diabetes mellitus on renal cell carcinoma prognosis: a meta-analysis of cohort studies. Medicine. 2015;94(26):e1055.
Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121(4):856–62.
Castillo JJ, Mull N, Reagan JL, Nemr S, Mitri J. Increased incidence of non-Hodgkin lymphoma, leukemia, and myeloma in patients with diabetes mellitus type 2: a meta-analysis of observational studies. Blood. 2012;119(21):4845–50.
Lee JY, Jeon I, Kim JW, Song YS, Yoon JM, Park SM. Diabetes mellitus and ovarian cancer risk: a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer. 2013;23(3):402–12.
Jian Gang P, Mo L, Lu Y, Runqi L, Xing Z. Diabetes mellitus and the risk of prostate cancer: an update and cumulative meta-analysis. Endocr Res. 2015;40(1):54–61.
Zhu Z, Zhang X, Shen Z, Zhong S, Wang X, Lu Y, Xu C. Diabetes mellitus and risk of bladder cancer: a meta-analysis of cohort studies. PLoS One. 2013;8(2):e56662.
Gu J, Yan S, Wang B, Shen F, Cao H, Fan J, Wang Y. Type 2 diabetes mellitus and risk of gallbladder cancer: a systematic review and meta-analysis of observational studies. Diabetes Metab Res Rev. 2016;32(1):63–72.
Zhu Z, Wang X, Shen Z, Lu Y, Zhong S, Xu C. Risk of bladder cancer in patients with diabetes mellitus: an updated meta-analysis of 36 observational studies. BMC Cancer. 2013;13:310.
Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159(12):1160–7.
Yagyu K, Lin Y, Obata Y, Kikuchi S, Ishibashi T, Kurosawa M, Inaba Y, Tamakoshi A. Bowel movement frequency, medical history and the risk of gallbladder cancer death: a cohort study in Japan. Cancer Sci. 2004;95(8):674–8.
Swerdlow AJ, Laing SP, Qiao Z, Slater SD, Burden AC, Botha JL, Waugh NR, Morris AD, Gatling W, Gale EA, et al. Cancer incidence and mortality in patients with insulin-treated diabetes: a UK cohort study. Br J Cancer. 2005;92(11):2070–5.
Lam EK, Batty GD, Huxley RR, Martiniuk AL, Barzi F, Lam TH, Lawes CM, Giles GG, Welborn T, Ueshima H, et al. Associations of diabetes mellitus with site-specific cancer mortality in the Asia-Pacific region. Ann Oncol. 2011;22(3):730–8.
Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–41.
Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35(2):299–304.
Harding JL, Shaw JE, Peeters A, Cartensen B, Magliano DJ. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care. 2015;38(2):264–70.
Chen Y, Wu F, Saito E, Lin Y, Song M, Luu HN, Gupta PC, Sawada N, Tamakoshi A, Shu X-O, et al. Association between type 2 diabetes and risk of cancer mortality: a pooled analysis of over 771,000 individuals in the Asia cohort consortium. Diabetologia. 2017;60(6):1022–32.
Tseng CH, Chong CK, Tseng CP, Chan TT. Age-related risk of mortality from bladder cancer in diabetic patients: a 12-year follow-up of a national cohort in Taiwan. Ann Med. 2009;41(5):371–9.
Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care. 2012;35(9):1835–44.
Gabbi C, Kim HJ, Barros R, Korach-Andre M, Warner M, Gustafsson JA. Estrogen-dependent gallbladder carcinogenesis in LXRbeta−/− female mice. Proc Natl Acad Sci U S A. 2010;107(33):14763–8.
Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89(3):309–19.
Mendez-Sanchez N, Chavez-Tapia NC, Motola-Kuba D, Sanchez-Lara K, Ponciano-Rodriguez G, Baptista H, Ramos MH, Uribe M. Metabolic syndrome as a risk factor for gallstone disease. World J Gastroenterol. 2005;11(11):1653–7.
Mendez-Sanchez N, Bermejo-Martinez LB, Vinals Y, Chavez-Tapia NC, Vander Graff I, Ponciano-Rodriguez G, Ramos MH, Uribe M. Serum leptin levels and insulin resistance are associated with gallstone disease in overweight subjects. World J Gastroenterol. 2005;11(39):6182–7.
Phillips LS, Harp JB, Goldstein S, Klein J, Pao CI. Regulation and action of insulin-like growth factors at the cellular level. Proc Nutr Soc. 1990;49(3):451–8.
Cai HH, Sun YM, Bai JF, Shi Y, Zhao HL, Miao Y. Relationship between the GH-IGFs axis and the proliferation of bile duct cancer cell line QBC939 in vitro. Hepatobiliary Pancreat Dis Int. 2008;7(1):76–81.
Alvaro D, Barbaro B, Franchitto A, Onori P, Glaser SS, Alpini G, Francis H, Marucci L, Sterpetti P, Ginanni-Corradini S, et al. Estrogens and insulin-like growth factor 1 modulate neoplastic cell growth in human cholangiocarcinoma. Am J Pathol. 2006;169(3):877–88.
Pazzi P, Scagliarini R, Gamberini S, Pezzoli A. Review article: gall-bladder motor function in diabetes mellitus. Aliment Pharmacol Ther. 2000;14(Suppl 2):62–5.
Hsing AW, Gao YT, Han TQ, Rashid A, Sakoda LC, Wang BS, Shen MC, Zhang BH, Niwa S, Chen J, et al. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer. 2007;97(11):1577–82.
Aune D, Vatten LJ. Diabetes mellitus and the risk of gallbladder disease: a systematic review and meta-analysis of prospective studies. J Diabetes Complicat. 2016;30(2):368–73.
Acknowledgements
Not applicable.
Declarations
There is no conflict of interests.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
CJ and ZYW collected and analyzed all the included data. XF designed this study and drafted the manuscript. All of the authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jing, C., Wang, Z. & Fu, X. Effect of diabetes mellitus on survival in patients with gallbladder Cancer: a systematic review and meta-analysis. BMC Cancer 20, 689 (2020). https://doi.org/10.1186/s12885-020-07139-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-020-07139-y