Abstract
Background
Circulating extracelluar vesicles (EVs) in epithelial ovarian cancer (EOC) patients emanate from multiple cells. These EVs are emerging as a new type of biomarker as they can be obtained by non-invasive approaches. The aim of this study was to investigate circulating EVs from EOC patients and healthy women to evaluate their biological function and potential as diagnostic biomarkers.
Methods
A quantitative proteomic analysis (iTRAQ) was applied and performed on 10 EOC patients with advanced stage (stage III–IV) and 10 controls. Twenty EOC patients and 20 controls were applied for validation. The candidate proteins were further validated in another 40-paired cohort to investigate their biomarker potential. Coagulation cascades activation was accessed by determining Factor X activity.
Results
Compared with controls, 200 proteins were upregulated and 208 proteins were downregulated in the EOC group. The most significantly involved pathway is complement and coagulation cascades. ApoE multiplexed with EpCAM, plg, serpinC1 and C1q provide optimal diagnostic information for EOC with AUC = 0.913 (95% confidence interval (CI) =0.848–0.957, p < 0.0001). Level of activated Factor X was significantly higher in EOC group than control (5.35 ± 0.14 vs. 3.69 ± 0.29, p < 0.0001).
Conclusions
Our study supports the concept of circulating EVs as a tool for non-invasive diagnosis of ovarian cancer. EVs also play pivotal roles in coagulation process, implying the inherent mechanism of generation of thrombus which often occurred in ovarian cancer patients at late stages.
Similar content being viewed by others
Background
Epithelial ovarian cancer (EOC) is the most lethal cancer among gynecological malignancies [1]. Almost 70% of EOC patients are diagnosed at an advanced stage. Although cytoreductive surgery followed by platinum/taxane-base chemotherapy has significantly improved the overall survival of EOC patients, the 5-year survival rate of EOC patients is approximately 45% [2]. Lack of an effective screening approach for early diagnosis is one of the main reasons for the high mortality. Serum CA125 and ultrasonography are mainstream applied methods accepted clinically in ovarian cancer diagnosis. However, due to the non-specificity of CA125, malignant diseases cannot be distinguished from benign diseases, such as inflammatory situations [3]. Extracellular vesicles (EVs) are small (40-1000 nm) membrane-enclosed microvesicles that play an important role in intercellular communication, involved in multifaceted physiological and pathological activities, including coagulation, angiogenesis, cell survival, modulation of the immune response, and inflammation [2, 4, 5]. Circulating EVs emanate from multiple cells, such as platelets, inflammatory cells, monocytes/macrophages and ovarian cancer cells. As circulating EVs carry complex biological information from their donor cells [6,7,8,9] and can be obtained using non-invasive approaches [2], they are emerging as a new type of cancer biomarker. Some studies have focused on ovarian cancer derived EVs for the potential of serving as biomarkers. Claudin-4 containing exosomes can be detected in the peripheral circulation of ovarian cancer patients, serving as a promising biomarker in ovarian cancer [10]. An exosomal microRNA signature consisting of eight miRNAs (miR-21, miR-141, miR-200a, miR-200c, miR-200b, miR-203, miR-205 and miR-214) was also investigated to be a potential diagnostic tool for ovarian cancer [11]. Besides, proteomic analysis of ovarian cancer cell derived exosomes also proved that exosomal protein present some tissue-specific protein signature which provide a potential source of blood-based protein biomarkers [12]. Despite the progress achieved in several studies regarding exosomal contents in cell lines as diagnostic markers, few studies focused on a systemic proteomic analysis and biological function of serum EVs derived from ovarian cancer patients. Systematic proteomics analysis of serum EVs derived from ovarian cancer patients can not only provide a more comprehensive understanding of EV proteins in clinic, but also lay the foundation of further studies exploring the mechanism of action of EVs in tumorigenesis, metastasis, relapse and so on.
With the development of technology of proteomic analysis, isobaric tags for relative and absolute quantification (iTRAQ) labeling coupled liquid chromatography-mass spectrometry (LC-MS) are newly emerging technologies that provide more information compared with conventional technologies [13]. In this study, we systemically investigated circulating EV proteins in ovarian cancer and healthy states using iTRAQ labeling coupled LC-MS, aiming to identify the differentially expressed proteins and to investigate their biological functions and also the potential of diagnostic biomarkers.
Methods
Subjects and serum sample collection
EOC serum samples (1.5 ml) were obtained from the tissue banks of Peking Union Medical College Hospital (PUMHC, Beijing) and Fudan University Shanghai Cancer Center (FUSCC Shanghai). All samples were obtained before surgery from patients without any prior treatment. All EOC patients (n = 70) were diagnosed at an advanced stage (stage III–IV) after primary cytoreductive surgery, and all of them were pathologically confirmed. Healthy controls (n = 70) were age-matched female volunteers with no cancer detected. For each group, 10 samples were used for proteomic analysis and 20 samples were used for Elisa validation of the proteomic results. Another cohort of 40-paired samples was prepared for the validation of the biomarker potential of candidate proteins. All serum samples were stored at − 80 °C. Informed consent was obtained from all participants, and this study was approved by the Ethical Committees of Peking Union Medical College Hospital and Fudan University Shanghai Cancer Center.
Circulating extracellular vesicle isolation and identification
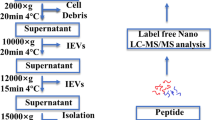
Circulating EVs were isolated using ExoQuick®, a commercial exosome precipitation reagent (Systems BioSciences, Inc. Mountain View CA), following the manufacturer’s protocol [14]. In brief, serum samples from individual patients and controls were centrifuged at 12,000×g for 10 min at 4 °C. The supernatant was then filtered through a 0.22-μm filter (MillPore, Billerica, MA, USA). Four volumes of supernatant was incubated with one volume of ExoQuick® buffer for 30 min at 4 °C. The mixture was centrifuged at 1500×g for 30 min at 4 °C. The flow-through was collected and resuspended the pellets in 200 μl of 1 × PBS and stored at − 20 °C.
Electron microscopy (EM), western blotting and nanoparticle tracking analysis (NTA) were applied for EVs characterization using a previously established method [12, 15]. For EM, about 50 μl of prepared EVs were loaded to Formvar carbon-coated 200-mesh copper grids and dried out. Then the absorbed exosome was negatively stained with 3% phosphotungstic acid and dried at room temperature. Next a transmission electron microscope (Olympus Software Imaging Solutions) was applied for observation at 120.0 kV and images were captured by a digital camera. Size and concentration of isolated extracellular vesicles were quantified by a NanoSight NS500 instrument (NanoSight, Amesbury, UK) using a previously established method [15]. Isolated serum extracellular vesicles were diluted into concentration from 2 × 108 to 2 × 109/ml. NanoSight software was stetted as follows: detection threshold, 9–10; blur, auto; and minimum expected particle size, 10 nm, and all of these settings were kept constant among all samples. Particle size and concentration were analyzed by the equipped NTA 2.0 software. For western blotting, two commonly used markers, ALIX and TSG101 (ProteinTech group, polyclonal, rabbit), were used [12]. Thirty microliters of isolated EV protein were loaded on 12% SDS-PAGE gels. Separated proteins were transferred to a polyvinylidene fluoride (PVDF) membrane, and then the PVDF membrane was blocked with 5% milk in 1× tris-buffered saline with Tween (TBST) (1 × 140 TBS with 0.05% Tween 20) for 1 h at room temperature. Next the membrane was incubated in primary antibodies at 4 °C overnight. Then the membrane was washed in TBST and incubated with horse radish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. An enhanced chemiluminescence (ECL) system (Thermo) was used to detect the blots.
iTRAQ-LC-MS/MS analysis
Protein digestion and iTRAQ labeling
The prepared proteins were reduced with 10 mM DTT at 56 °C for 1 h and with 55 mM IAM in the dark at room temperature for 1 h. After adding 400-μl precooled acetone at − 20 °C for 3 h, the samples were centrifuged at 20,000×g for 30 min at 4 °C. After discarding the supernatant, the deposit was resuspended with 300 μl buffer (50% TEAB, 0.1% SDS). The prepared proteins were run on a short 10% SDS-PAGE gel and the gel was stained with Coomassie Blue G-250. EV protein lysate samples were 100 μg, and equal each sample volume with TEAB containing 1% SDS. Trypsin (3.3 μg) was added and incubated at 37 °C overnight. The digestion solution was lyophilized with 30 μl TEAB.
The peptides were labeled with an 8-plex iTRAQ Multiplex Kit according to the manufacturer’s instructions (AB Sciex, Foster City, CA, USA) [16]. The normal control and EOC groups were individually labeled. Then the labeled samples were mixed equally and dried by vacuum centrifugation.
Mass spectrometry
The mixed labeled samples were analyzed by nano LC-MS/MS with a HPLC-RP column (Phenomenex, Luna 5u C18(2), 100 mm × 75 mm). Peptides were loaded on a trapping column and over a 75-μm analytical column at 400 nL/min using a 65-min reverse phase gradient [14]. Resulting peptide and fragmentation spectra were input into software PD (Proteome Discoverer 1.3, Thermo), and analyzed using a Mascot database (Matrix Science, London, UK; version 2.3.0). In this study, 1.2-fold change (upregulation or downregulation) was used as a cut-off for biological significance based on the standard deviation and normalized peptide ratios [17].
Bioinformatics analysis
All differentially expressed proteins were searched in the PANTHER database (http://www.pantherdb.org/) [18]. Protein classification was based on functional annotations. The Ingenuity Pathway Analysis (IPA, Qiagen, USA) database was applied for pathway analysis. The accession numbers of identified proteins were submitted to the IPA with stetted p-values and fold changes. Canonical pathways, biological functions and networks of interconnected proteins were analyzed.
Validation of proteins using ELISA
Twenty samples were used for validation in each EOC group and control group. Candidate protein levels were determined using an ELISA kit from SAB Inc. according to the manufacturer’s instructions.
Factor X chromogenic activity assay
Coagulation was accessed by determining Factor X activity. The Factor X chromogenic activity assay (Abcam, MA, USA) measures the activation of zymogen Factor X to Factor Xa by RVV. Factor Xa as the activator of prothrombin occupies a central position linking the two blood coagulation pathways. The assay was conducted according to manufacturer’s manual. In brief, all reagents, samples and standards were prepared as instructed. EVs were extracted from 400 μL serum. 20 μL of Factor X standard or samples was added into the plate. 40 μL of freshly prepared Assay Mix was then added and mixed well by shaking. The UV absorbance at 405 nm was recorded every 2 min for 10 min by a plate reader (Thermo Fisher, MA, USA). The changes in absorbance per minute and standard concentrations were utilized to generate a standard curve. The unknown sample concentration was determined from the standard curve and multiplied by the dilution factor.
Statistical analysis
All the quantitative measurements were triplicate. Student’s t test and Mann-Whitney U were used for comparison and a p value < 0.05 was considered as a significant difference. AUC curve were performed with SPSS and MedCalc using ROC analysis.
Results
Isolation and identification of circulating extracellular vesicles
Isolated circulating EVs were characterized by EM, western blotting and NTA (Fig. 1). Typical size, shape and protein markers were well defined, which indicated that circulating EVs from both EOC patients and controls were successfully isolated with high quality.
Identification of circulating EVs from EOC and control group by TEM, NTA and WB. a and c show EOC EVs identified by TEM and NTA. b and d shows circulating EVs identified by TEM and NTA from control group. e and f show EVs identified by WB using commonly used biomarkers TSG101 and Alix. Typical shape, size, size distribution and biomarkers of EVs were detected
Differentially expressed proteins and ingenuity pathway analyses
Clinical characteristics of the patients recruited for proteomics analysis were shown in Table 1. Details of clinicopathology data of all those patients were shown in Additional file 2: Table S1. Proteomic analysis of circulating EVs from the EOC group and controls totally yielded 1913 proteins (Additional file 2: Table S1) and 408 significantly differentially expressed proteins (Additional file 3: Table S2). Compared with normal controls, 200 proteins were upregulated and 208 proteins were downregulated in EOC group. Cellular component, biological process and molecular functions of differentially expressed proteins were analyzed (Additional file 1: Figure S1A, B, C). Results indicated that most components were from extracelluar region, and have receptor activity, which was in concordance with the origins of these proteins.
IPA analysis was used to further analyze the functions and interaction among these differently expressed proteins. The disease and biological function analysis revealed that most differentially expressed proteins were involved in inflammatory response, metabolic disease, cardiovascular disease, hematological disease and organism injury and abnormalities (Table 2). According to canonical pathway analysis, five related pathways and three networks were identified. The five pathways comprised the acute phase response signaling pathway, LXR/RXR activation, FXR/RXR activation, the complement system and the coagulation system (Fig. 2). It is generally accepted that gynecological cancers are associated with a high rate of thromboembolism, especially in ovarian cancer. Therefore, special attention was paid to the complement and coagulation pathway for further study.
Three significant networks identified were: Network1, RNA Post-Transcriptional Modification, Cancer, Cell Death and Survival (p-score 51); Network2, Humoral Immune Response, Inflammatory Response, and Hematological Disease (p-score 34); Network3, Cellular Assembly and Organization, Cellular Function and Maintenance, Cell-To-Cell Signaling and Interaction (p-score 34).
Twenty-three focused molecules, including serpin C1 and C1q in Network 2 and another 23 proteins from Network3 were selected for further analysis.
Biomarker potential of candidate biomarkers and promote coagulation activation
Clinical characteristics of patients with epithelial ovarian cancer recruited for ELISA was presented in Table 3. Four overexpressed proteins present in the EOC group, including EpCAM, C1q, ApoE and Plasminogen (plg) were chosen as the candidate markers for the validation of diagnosis evaluation. Serpin C1 was selected because it was significantly downregulated in the EOC group. Besides, one study suggested that ApoE is associated with intraluminal vesicles (ILV) within endosomes and remains associated with ILVs when they are secreted as EVs [19]. Moreover, plasminogen (plg) was proved to be a favorable biomarker for prediction of survival in advanced high-grade serous ovarian cancer [20]. Epithelial cellular adhesion molecule (EpCAM) was also proposed as a cancer-related factor in other malignancies. ELISA assay was applied for the quantification of protein levels in validation cohort. The expression profile of these four proteins in ELISA resembles that in proteomic analysis, showing a similar trend. The expression levels of EpCAM, plg, ApoE, serpinC1 and C1q in EOC group were 119.83 ng/ml, 58,127.48 ng/ml, 3716.77 ng/ml, 54,949.01 ng/ml and 254.41 ng/ml respectively; while the expression levels of EpCAM, plg, ApoE, serpinC1 and C1q in the control group were 112.65 ng/ml, 43,634.99 ng/ml, 3232.29 ng/ml, 97,900.40 ng/ml and 129.72 ng/ml. Expression levels of the five biomarkers in the EOC group and the control group were significantly different (all p < 0.05) (Fig. 3). These results were consistent with the results obtained by the proteomic analysis.
Activation of Factor X to Factor Xa was higher in EOC group than control (5.35 ± 0.14 vs. 3.69 ± 0.29, p < 0.0001) (Fig. 4). AUC curve of single biomarker of ApoE multiplexed with EpCAM, plg, serpinC1 and C1q were presented in Fig. 5. Multivariable logistic regression confirmed that ApoE multiplexed with EpCAM, plg, serpinC1 and C1q provide optimal diagnostic information for EOC with AUC = 0.913, (95% confidence interval (CI) =0.848–0.957, p < 0.0001) (Fig. 6).
Discussion
Lack of highly specific and sensitive serum biomarkers is a major problem in early detection of ovarian cancer. The most commonly used biomarkers in ovarian cancer is CA125 with low specificity [3]. Tumor-derived EVs are emerging as a new type of cancer biomarker [2]. Some studies focused on exosomal proteins [10] or exosomal microRNAs [11] as diagnostic biomarkers. Compared with conventional specimens, EV biomarkers provide a non-invasive approach and higher specificity and sensitivity known as “liquid biopsy” [21]. In this study, we systemically studied serum EVs proteins and their biological functions in both ovarian cancer patients and healthy women. A commercially-available exosome precipitation reagent was applied for isolation because of its high efficiency. EM, NTA and western blotting were used for identification of isolated EVs. Typical shape, size and biomarkers were confirmed by those methods indicating that high quality and purity serum EVs were successfully obtained, which is the foundation for our subsequent systemic proteomic analysis. Using iTRAQ labeling coupled LC-MS provide more precise quantification, and finally 408 significantly differentially expressed proteins were identified and their biological functions were investigated. Canonical pathway analysis identified five related pathway and we paid special attention to the complement system and the coagulation system. Proteins involved in the two systems namely plg, C1q and serpinC1 were selected for validation. Besides, ovarian cancer tissue specific protein EpCAM and ApoE were also verified. Validation results were consistent with the results obtained by the proteomic analysis, and these also proved the reliability of our proteomic results. Furthermore, we confirmed serum EVs promote coagulation by using a Factor X chromogenic activity assay. ApoE multiplexed with EpCAM, plg, serpinC1 and C1q provide optimal diagnostic information for EOC.
ApoE is an ovarian cancer tissue specific protein which has been recently identified as a potential biomarker in ovarian cancer [22, 23]. It is frequently detected in ovarian serous carcinomas, and is also a prognostic marker in ovarian cancer patients [22]. It has been demonstrated that ApoE expression is elevated both in ovarian cancer cells [12] and in ovarian cancer tumor fluids [24]. Beside, ApoE is required for cell proliferation and survival in ovarian cancer [22]. Ovarian cancer cell derived exosomes also overexpress ApoE [12]. EpCAM is also considered as an ovarian cancer tissue specific protein which is used for isolation of ovarian cancer derived exosomes [11, 25]. By using a 3D novel engineered ExoProfile chip, diagnostic power of seven markers (EGFR, HER2, CA125, FRα, CD24, EpCAM, and CD9 plus CD63) were evaluated with AUC = 1 in ovarian cancer derived exosomes [26]. Although only 15 patients were enrolled in this study, results showed a promising prospect of diagnostic potential of circulating exosomes. Serum PLG was once detected in a rat model using iTRAQ technique, and potential as biomarker was investigated [27]. It was also demonstrated as a favorable biomarker for prediction of survival in advanced high-grade serous ovarian cancer [20]. In our study, both of exosomal ApoE, EpCAM and Plg were detected and verified, and this proved that ovarian cancer tissue associated proteins are expressed on serum EVs and this is the foundation for further investigation of their biomarker potential.
It is generally accepted that gynecological cancers are associated with a high rate of thromboembolism, especially in ovarian cancer [28, 29]. Therefore, we paid special attention to the complement and coagulation pathway. It has long been known that EVs play a role as regulators of the coagulation system in cancer [30]. Tissue factor (TF), which is expressed on non-vascular cells, is the main activator of the coagulation cascade. TF is largely expressed on monocyte/macrophage-derived microvesicles, including exosomes [31]. SerpinC1 is a inhibitor of TF [32], and as the expression level of serpinC1 was downregulated in the EOC group, the TF-dependent coagulation pathway was promoted. As coagulation factor X is a central component of the coagulation cascade, factor Xa as the activator of prothrombin occupies a central position linking the two blood coagulation pathways, we accessed coagulation by determining Factor X activity. Level of activated Factor X was significantly higher in EOC group than control, and this demonstrated that EOC derived circulating EVs promote coagulation. Complement C1Q is the defining component of the classical pathway as it forms the C1Q/C1RC1S complex [33]. A previous study demonstrated that malignant cell-derived EVs activated the complement system [34]. Our results showed differentially expressed circulating EV proteins were involved in complement system activation, and all EV proteins that were involved in the complement cascade were elevated in the EOC group. Compared with the control group, the expression levels of C1Q in EOC EVs were significantly elevated. All of these demonstrated that EOC circulating EVs from multiple cells play an important role in complement system activation. And those results might give information for the management and treatment of ovarian cancer patients.
Receiver operating characteristic (ROC) curve analysis indicated that the area under the curve (AUC) for EpCAM, plg, serpinC1 and C1q was statically significant. Multivariable logistic regression confirmed that ApoE multiplexed with EpCAM, plg, serpinC1 and C1q provide optimal diagnostic information for differentiating the EOC and control group with AUC = 0.913 (95% confidence interval (CI) =0.848–0.957, p < 0.0001). These results demonstrated that the panel of EV biomarkers might be more promising in ovarian cancer diagnosis than the individual biomarker.
In early ovarian cancer detection, biomarkers based on high-throughput technologies of proteomics have shown promise prospect in the past decades [3]. Specimen ranged from various body fluids, including utero-tubal lavage [35], tumor fluids [24], plasma [36] and even cell or circulating EVs [12, 37]. Compare with biomarkers detected in traditional specimens, exosomal biomarkers is more specific and sensitive due to their excellent stability [25]. Marcisauskas et al. [38] verified one biomarker panel with nine proteins in cystfluid and serum, and the biomarker panel achieved ROC AUC 0.96 and 0.57 respectively. Enroth et al. [36] identified a high-accuracy 11 plasma protein biomarker signature for ovarian cancer with an AUC 0.94. In our study, biomarker potential of a panel of five EV proteins was verified with an AUC 0.913. Compared with those studies, our serum EV protein biomarker panel performed better as we only enrolled 5 proteins and more noninvasive compared with cystfluid. Biomarker potential of exosomal Claudin-4 and microRNAs were also investigated, and our study provides a more comprehensive understanding of EV proteins in vivo which can provide more precise information for further study.
What type of “liquid fraction” of blood should be performed for analytical study is a constant debate. As serum is free of clotting proteins, cells and platelets, it is considered as the gold standard in many applications [39]. In our published data [37], biomarker potential and biological functions of plasma EV proteins were also investigated. Compared with serum EV proteins, 57 differentially expressed proteins were also detected in plasma EV proteins and most of them were involved in blood coagulation pathway and plasminogen activating pathway. By using different proteomic approaches and different blood fraction, we found that differentially expressed proteins are overlap in plasma and serum EVs, and most of them were involved in coagulation system. This demonstrated that circulating EVs play an important and universal role in coagulation in ovarian cancer. In this study, we established a protein database for serum EVs of ovarian cancer which is many differences as well as similarities compared with plasma EVs.
There was some limitations of our study, such as the small sample size for validation. What’s more, in this study, all the enrolled patients were diagnosed at advanced stage, and CA125 levels of all patients were elevated, we didn’t compare the performance of CA125 with this panel of biomarkers. Because more than 70% patients are diagnosed at advanced stage, we are enrolling more patients at early stage especially those with normal CA125 level to compare the performance of CA125 with our panel of biomarkers. Besides, we are enrolling more patients diagnosed as other benign conditions to validate the specificity of these biomarkers.
Conclusion
We identified circulating EVs as a potential tool for non-invasive diagnosis of ovarian cancer which confirms the conception of circulating EVs as liquid biopsy biomarker in cancer. EVs also play pivotal roles in coagulation process, implying the inherent mechanism of generation of thrombus which often occurred in ovarian cancer patients at late stages. Our study shed light on the comprehensive profiling of EVs proteins in ovarian cancer, providing diverse aspects for further evaluation.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- EM:
-
Electron microscopy
- EOC:
-
Epithelial ovarian cancer
- EVs:
-
Extracelluar vesicles
- FUSCC:
-
Fudan University Shanghai Cancer Center
- HRP:
-
Horse radish peroxidase
ECL
Enhanced chemiluminescence
- IPA:
-
Ingenuity pathway analysis
- iTRAQ:
-
Quantitative proteomic analysis
- LC-MS:
-
Liquid chromatography-mass spectrometry
- NTA:
-
Nanoparticle tracking analysis
- PD:
-
Proteome discoverer
- plg:
-
Plasminogen
- PUMHC:
-
Peking Union Medical College Hospital
- PVDF:
-
Polyvinylidene fluoride
- TBST:
-
Tris-buffered saline with Tween
- ILV:
-
Intraluminal vesicles
- EpCAM:
-
Epithelial cellular adhesion molecule
- AUC:
-
Under the curve
- ROC:
-
Receiver operating characteristic
- TF:
-
Tissue factor
- PSGL-1:
-
P-selectin glycoprotein ligand-1
- PMV:
-
Platelet-derived microvesicles
- TLRs:
-
Toll-like receptors
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
Dorayappan KDP, Wallbillich JJ, Cohn DE, Selvendiran K. The biological significance and clinical applications of exosomes in ovarian cancer. Gynecol Oncol. 2016;142(1):199–205.
Lowry KP, Lee SI. Imaging and screening of ovarian Cancer. Radiol Clin N Am. 2017;55(6):1251–9.
Aharon A, Tamari T, Brenner B. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb Haemost. 2008;100(5):878–85.
Bretz NP, Ridinger J, Rupp AK, Rimbach K, Keller S, Rupp C, Marme F, Umansky L, Umansky V, Eigenbrod T, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via toll-like receptor signaling. J Biol Chem. 2013;288(51):36691–702.
Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166(12):7309–18.
Ekstrom K, Valadi H, Sjostrand M, Malmhall C, Bossios A, Eldh M, Lotvall J. Characterization of mRNA and microRNA in human mast cell-derived exosomes and their transfer to other mast cells and blood CD34 progenitor cells. J Extracellular Vesicles. 2012;1:18389-10.
Gezer U, Ozgur E, Cetinkaya M, Isin M, Dalay N. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biol Int. 2014;38(9):1076–9.
Tetta C, Ghigo E, Silengo L, Deregibus MC, Camussi G. Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine. 2013;44(1):11–9.
Li J, Sherman-Baust CA, Tsai-Turton M, Bristow RE, Roden RB, Morin PJ. Claudin-containing exosomes in the peripheral circulation of women with ovarian cancer. BMC Cancer. 2009;9:244.
Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110(1):13–21.
Liang B, Peng P, Chen S, Li L, Zhang M, Cao D, Yang J, Li H, Gui T, Li X, et al. Characterization and proteomic analysis of ovarian cancer-derived exosomes. J Proteome. 2013;80:171–82.
Moulder R, Bhosale SD, Goodlett DR, Lahesmaa R. Analysis of the plasma proteome using iTRAQ and TMT-based isobaric labeling. Mass Spectrom Rev. 2018;37(5):583–606.
Moyron RB, Gonda A, Selleck MJ, Luo-Owen X, Catalano RD, O'Callahan T, Garberoglio C, Turay D, Wall NR. Differential protein expression in exosomal samples taken from trauma patients. Proteomics Clin Appl. 2017;11:9–10.
Zhang W, Yang J, Cao D, You Y, Shen K, Peng P. Regulation of exosomes released from normal ovarian epithelial cells and ovarian cancer cells. Tumour Biol. 2016;37(12):15763–71.
Qi Y, Xu F, Chen L, Li Y, Xu Z, Zhang Y, Wei W, Su N, Zhang T, Fan F, et al. Quantitative proteomics reveals FLNC as a potential progression marker for the development of hepatocellular carcinoma. Oncotarget. 2016;7(42):68242–52.
Yang S, Chen L, Chan DW, Li QK, Zhang H. Protein signatures of molecular pathways in non-small cell lung carcinoma (NSCLC): comparison of glycoproteomics and global proteomics. Clin Proteomics. 2017;14:31.
Li C, Guo Z, Zhao R, Sun W, Xie M. Proteomic analysis of liver proteins in a rat model of chronic restraint stress-induced depression. Biomed Res Int. 2017;2017:7508316.
van Niel G, Bergam P, Di Cicco A, Hurbain I, Lo Cicero A, Dingli F, Palmulli R, Fort C, Potier MC, Schurgers LJ, et al. Apolipoprotein E regulates amyloid formation within endosomes of pigment cells. Cell Rep. 2015;13(1):43–51.
Zhao S, Dorn J, Napieralski R, Walch A, Diersch S, Kotzsch M, Ahmed N, Hooper JD, Kiechle M, Schmitt M, et al. Plasmin (ogen) serves as a favorable biomarker for prediction of survival in advanced high-grade serous ovarian cancer. Biol Chem. 2017;398(7):765–73.
Zhang W, Peng P, Kuang Y, Yang J, Cao D, You Y, Shen K. Characterization of exosomes derived from ovarian cancer cells and normal ovarian epithelial cells by nanoparticle tracking analysis. Tumour Biol. 2016;37(3):4213–21.
Chen YC, Pohl G, Wang TL, Morin PJ, Risberg B, Kristensen GB, Yu A, Davidson B, Shih Ie M. Apolipoprotein E is required for cell proliferation and survival in ovarian cancer. Cancer Res. 2005;65(1):331–7.
Podzielinski I, Saunders BA, Kimbler KD, Branscum AJ, Fung ET, DePriest PD, van Nagell JR, Ueland FR, Baron AT. Apolipoprotein concentrations are elevated in malignant ovarian cyst fluids suggesting that lipoprotein metabolism is dysregulated in epithelial ovarian cancer. Cancer Investig. 2013;31(4):258–72.
Poersch A, Grassi ML, Carvalho VP, Lanfredi GP, Palma CS, Greene LJ, de Sousa CB, Carrara HHA, Candido Dos Reis FJ, Faca VM. A proteomic signature of ovarian cancer tumor fluid identified by highthroughput and verified by targeted proteomics. J Proteome. 2016;145:226–36.
Dorayappan KDP, Gardner ML, Hisey CL, Zingarelli RA, Smith BQ, Lightfoot MDS, Gogna R, Flannery MM, Hays J, Hansford DJ, et al. A microfluidic Chip enables isolation of exosomes and establishment of their protein profiles and associated signaling pathways in ovarian Cancer. Cancer Res. 2019;79(13):3503–13.
Zhang P, Zhou X, Zeng Y. Multiplexed immunophenotyping of circulating exosomes on nano-engineered ExoProfile chip towards early diagnosis of cancer. Chem Sci. 2019;10(21):5495–504.
Huang Y, Zhang X, Jiang W, Wang Y, Jin H, Liu X, Xu C. Discovery of serum biomarkers implicated in the onset and progression of serous ovarian cancer in a rat model using iTRAQ technique. Eur J Obstet Gynecol Reprod Biol. 2012;165(1):96–103.
Tas F, Kilic L, Bilgin E, Keskin S, Sen F, Ciftci R, Yildiz I, Yasasever V. Clinical and prognostic significance of coagulation assays in advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2013;23(2):276–81.
Ye S, Zhang W, Yang J, Cao D, Huang H, Wu M, Lang J, Shen K. Pattern of venous thromboembolism occurrence in gynecologic malignancy: incidence, timing, and distribution a 10-year retrospective single-institutional study. Medicine. 2015;94(50):e2316.
Rak J. Microparticles in cancer. Semin Thromb Hemost. 2010;36(8):888–906.
Boriachek K, Islam MN, Moller A, Salomon C, Nguyen NT, Hossain MSA, Yamauchi Y, Shiddiky MJA. Biological Functions and Current Advances in Isolation and Detection Strategies for Exosome Nanovesicles. Small. 2018;14(6).
Rao LV, Nordfang O, Hoang AD, Pendurthi UR. Mechanism of antithrombin III inhibition of factor VIIa/tissue factor activity on cell surfaces. Comparison with tissue factor pathway inhibitor/factor Xa-induced inhibition of factor VIIa/tissue factor activity. Blood. 1995;85(1):121–9.
Lu J, Kishore U. C1 complex: an adaptable proteolytic module for complement and non-complement functions. Front Immunol. 2017;8:592.
Whitehead B, Wu L, Hvam ML, Aslan H, Dong M, Dyrskjot L, Ostenfeld MS, Moghimi SM, Howard KA. Tumour exosomes display differential mechanical and complement activation properties dependent on malignant state: implications in endothelial leakiness. J Extracellular Vesicles. 2015;4:29685.
Barnabas GD, Bahar-Shany K, Sapoznik S, Helpman L, Kadan Y, Beiner M, Weitzner O, Arbib N, Korach J, Perri T, et al. Microvesicle proteomic profiling of uterine liquid biopsy for ovarian Cancer early detection. Mol Cell Proteomics. 2019;18(5):865–75.
Enroth S, Berggrund M, Lycke M, Broberg J, Lundberg M, Assarsson E, Olovsson M, Stalberg K, Sundfeldt K, Gyllensten U. High throughput proteomics identifies a high-accuracy 11 plasma protein biomarker signature for ovarian cancer. Communications Biol. 2019;2:221.
Zhang W, Ou X, Wu X. Proteomics profiling of plasma exosomes in epithelial ovarian cancer: a potential role in the coagulation cascade, diagnosis and prognosis. Int J Oncol. 2019;54(5):1719–33.
Marcisauskas S, Ulfenborg B, Kristjansdottir B, Waldemarson S, Sundfeldt K. Univariate and classification analysis reveals potential diagnostic biomarkers for early stage ovarian cancer type 1 and type 2. J Proteome. 2019;196:57–68.
Pietrowska M, Wlosowicz A, Gawin M, Widlak P. MS-based proteomic analysis of serum and plasma: problem of high abundant components and lights and shadows of albumin removal. Adv Exp Med Biol. 2019;1073:57–76.
Acknowledgements
Special thanks were given to Dr. Zhi Yang for his prompt assistance in this study.
Funding
Wei Zhang is supported by the National Natural Science Foundation of China (81602270).
National Human Genetic Resources Sharing Service Platform (2005DKA21300).
National Key R&D Program of China (2017YFC1311004, 2016YFC1201701).
Shanghai R&D Public Service Platform Project (12DZ2295100).
Author information
Authors and Affiliations
Contributions
Conception and Design: WZ; PP; KS;XHW, Data Acquisition: WZ; PP; XXO, Data analysis and interpretation: WZ; PP;XHW, Drafting the article: WZ; PP, Revising it critically for important intellectual content: WZ; PP; KS, Final approval of the version to be published: WZ; PP; KS;XHW, Agreement to be accountable for all aspects of the work: XHW, Read and approved the manuscript: WZ; PP; XXO; KS; XHW. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional, and consent were informed and obtained in written. This study approved by the Ethics Committee of Fudan University Shanghai Cancer Center (050432–4-1805C) and carried out in accordance with the approved guidelines. All patients had signed informed consent for donating their samples to tissue bank of Fudan University Shanghai Cancer Center.
Consent for publication
Not applicable.
Competing interests
The author(s) declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1A-C.
Cellular component (A), biological process (B) and molecular functions(C) of differentially expressed proteins.
Additional file 2: Table S1.
Total protein identified by proteomic profiling.
Additional file 3: Table S2.
Differential protein identified by proteomic profiling.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhang, W., Peng, P., Ou, X. et al. Ovarian cancer circulating extracelluar vesicles promote coagulation and have a potential in diagnosis: an iTRAQ based proteomic analysis. BMC Cancer 19, 1095 (2019). https://doi.org/10.1186/s12885-019-6176-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-019-6176-1