Abstract
Background
In clinical practice, the detection of biomarkers is mostly based on primary tumors for its convenience in acquisition. However, immune checkpoints may express differently between primary and metastatic tumor. Therefore, we aimed to compare the differential expressions of PD-1, PD-L1 and PD-L2 between the primary and metastatic sites of renal cell carcinoma (RCC).
Methods
Patients diagnosed with RCC by resection or fine needle aspiration of metastasis were included. Immunohistochemistry (IHC) was applied to detect PD-1, PD-L1 and PD-L2 expressions. SPSS 22.0 was applied to conduct Chi-square, consistency tests and Cox’s proportional hazards regression models. GraphPad Prism 6 was used to plot survival curves and R software was used to calculate Predictive accuracy (PA).
Results
In the whole cohort (N = 163), IHC results suggested a higher detection rate of PD-L1 in the metastasis than that of the primary site (χ2 = 4.66, p = 0.03), with a low consistent rate of 32.5%. Among different metastatic tumors, PD-1 was highly expressed in the lung/lymph node (65.3%) and poorly expressed in the brain (10.5%) and visceral metastases (12.5%). PD-L1 was highly expressed in lung/lymph node (37.5%) and the bone metastases (12.2%) on the contrary. In terms of survival analysis, patients with PD-1 expression either in the primary or metastasis had a shorter overall survival (OS) (HR: 1.59, 95% CI 1.08–2.36, p = 0.02). Also, PD-L1 expression in the primary was associated with a shorter OS (HR 2.55, 95% CI 1.06–6.15, p = 0.04). In the multivariate analysis, the predictive accuracy of the whole model for PFS was increased from 0.683 to 0.699 after adding PD-1.
Conclusion
PD-1, PD-L1 and PD-L2 were differentially expressed between primary and metastatic tumors. Histopathological examination of these immune check points in metastatic lesions of mRCC should be noticed, and its accurate diagnosis may be one of the effective ways to realize the individualized treatment.
Similar content being viewed by others
Background
Renal cell carcinoma (RCC) is originated from the epithelium of renal tubules. Clear cell RCC (ccRCC) is the most common type of RCC (82–90%) [1, 2]. Among all RCC patients, nearly 20–30% of them were initially diagnosed as metastatic RCC (mRCC), and 20–40% of patients with localized disease will eventually develop metastatic disease after surgery [3]. RCC is not sensitive to radiotherapy and chemotherapy, and the efficiency of cytokines was limited, especially accompanied by significant adverse events [4,5,6]. Although the development of targeted agents, such as tyrosine kinase inhibitor (TKI) and the drug targeting the mammalian target of rapamycin (mTOR), have dramatically improved the patient’s survival time in the past two decades, mRCC patients still failed to receive satisfactory outcomes [7].
To achieve better outcomes of patients with mRCC, immune check point inhibitors have been successfully introduced into clinical practice. The key procedure of anti-tumor respond is the activation of T cell and tumor cells can inhibit this process through binding its immune checkpoints to receptors expressed on T-cell, thus leading to immune escape. Development of immune checkpoints inhibitors (PD-1 and PD-L1 antibodies) have been approved by the U.S. FDA as the standard second-line treatment for mRCC and even in the first-line for moderate to high risk of mRCC [8, 9]. In addition to RCC, the expression of PD-1/PD-L1 in solid tumors such as melanoma, bladder cancer, hematological malignancies, liver cancer, and non-small cell lung cancer can be used as prognostic markers to predict therapeutic effect of checkpoints inhibitors (CPIs) [10,11,12,13,14,15,16,17,18,19,20]. Although over-expression of PD-L1 in renal cell carcinoma has been shown to be associated with poor pathological features and prognosis [21, 22] its expression in primary tumors failed to predict whether patients can benefit from inhibiting PD-1/PD-L1 axis [11, 13]. In fact, 18% of RCC patients with PD-L1 negative also benefited from CPIs treatment [11], and there were still a large proportion of PD-L1 positive patients did not response to the treatment [16]. Explanations to the discrepancy between the trial results and expectation involve many possibilities. Among them, the heterogeneity of the tumor may play an important role, especially the heterogeneity between the primary and metastatic tumors.
In clinical practice, the detection of biomarkers is mostly based on primary tumors due to its convenience in acquisition. However, the tumor microenvironment may have changed at the time of disease progression and treatment administration (e.g., CPIs). Therefore, the value of assessing the primary tumor has been challenged for its poverty in providing full detailed or more accurate biological information in guiding treatment by targeted agents or CPIs.
Therefore, we firstly assessed and analyzed the differential expressions of several key immune checkpoints (PD-1, PD-L1/2) between the primary and metastatic tumors of RCC in Asian patients, and also aimed to analyze the relationship between the differential expressions and clinicopathologic parameters, then to estimate its significance in predicting patient’s survival outcomes.
Methods
Patients and samples
We included patients diagnosed with mRCC in West China Hospital, Sichuan University from 2009.1 to 2016.11. Inclusion and exclusion criteria were described as in our previous study [23]. This study was approved by the Ethics Committee of West China Hospital, Sichuan University and conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The pathological diagnosis was retrieved and confirmed by two experienced genitourinary pathologists independently (Ni Chen and Xiaoxue Yin). Clinical and pathological data were recorded in detail. The clinical outcomes included PFS and OS, and the definition of PFS and OS was described as in our previous study [23]. At the cut-off point, the median follow-up time was about 36 months (16–70 months).
Immunohistochemistry (IHC) was applied to detect PD-1, PD-L1 and PD-L2 expressions. IHC staining was performed by using anti-PD-1 monoclonal antibody (Abcam, clone number: ab52587), anti-PD-L1 monoclonal antibody (Zhongshan Golden Bridge, clone number: ZM-0170) and anti-PD-L2 monoclonal antibody (Abcam, clone number: ab200377) at a 1:200 dilution, respectively. Positive signal of PD-1, PD-L1 and PD-L2 were on the cell membrane. We evaluated staining intensities based on a scale as previously described [23]. The results were recorded as the following categories: staining intensity of null (0), weak (1+), moderate (2+) and strong (3+). Positivity was defined as the positive signal detected on > 5% tumor-infiltrating T cells (PD-1) or tumor cells (PD-L1 and PD-L2) with staining intensity ≥1+. Two experienced genitourinary pathologists (Ni Chen and Xiaoxue Yin) independently assessed all IHC staining results.
Data analysis
We calculated mean and SD for continuous parameters, and proportions for categorical parameters. Chi-square and non-parametric test, conducted by SPSS 22.0, were applied to analyze the statistical difference. For the analysis of the consistency of expression between the primary and metastatic tumors, Kappa test was adopted for the evaluation by conducting SPSS 22.0, and the agreement was classified into the following four levels: 1) slight agreement: Kappa≤0.2; 2) fair agreement: 0.4 < Kappa≤0.6; 3) substantial agreement: 0.6 < Kappa≤0.8; 4) almost perfect agreement: Kappa > 0.8. For the analysis of PFS and OS, we used GraphPad Prism 6 to plot Kaplan-Meier survival curves and SPSS 22.0 to conduct Cox’s proportional hazards regression models. In addition, R software was used to calculate Predictive accuracy (PA). P < 0.05 was considered significant in all results.
Results
Baseline data
From January, 2009 to November, 2016, A total of 163 patients from West China Hospital, Sichuan University were included (107 male, 56 female), with an average age of 53.9 years. Eighty-three of them were paired (with both primary and metastatic samples), and the other 80 were only metastatic specimen (Additional file 1: Table S1).
Expressions of PD-1, PD-L1 and PD-L2 in the whole and paired cohort
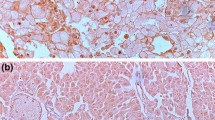
Figure 1a showed negative expression of PD-1 in RCC tumor. In the whole cohort (N = 163), PD-1 was detected in 76 patients (46.6%). PD-1 was mainly expressed on the membrane of tumor infiltrating immune cells (Fig. 1b-d). Figure 1e and i showed negative expressions of PD-L1 and PD-L2 in RCC tumors. PD-L1 and PD-L2 were detected in 53 patients (32.5%) and 43 patients (26.4%), respectively. Both PD-L1 and PD-L2 were mainly expressed on the surface of tumor cell membrane (Fig. 1f-h, Fig. 1j-l).
Immunohistochemical staining against PD-1, PD-L1 and PD-L2. Representative images of FFPE samples immunostained with PD-1 (a-d), PD-L1 (e-h) and (i-l), original magnification: 200×. A, E and I represented negative expression of PD-1, PD-L1 and PD-L2, respectively. B-D, F-H and J-L represented different positive intensities (1+, 2+ and 3+) of PD-1, PD-L1 and PD-L2, respectively
The associations between clinical pathological parameters and expressions in the primary or metastatic tumors of the three immune check points were shown in Table 1. PD-1 positive was associated with metastatic sites of lymph node(p < 0.001), brain(p < 0.001) and viscera(p = 0.02. Expression of PD-L1 was correlated with metastatic sites of lymph node(p = 0.02) and bone(p = 0.01). However, PD-L2 was only associated with nephrectomy status(p = 0.02).
After stratified patients’ specimens into primary tumors and specific metastatic sites, as shown in Table 2, PD-1 was positive in 31.3%(26/83) of primary tumors and 42.0%(73/173) of metastatic tumors (p = 0.10). However, PD-1 expression differences between the primary and metastatic sites of lung/lymph node (65.3%, P < 0.001), brain (10.5%, P < 0.001) and viscera (12.5%, p = 0.01) were found to be statistically significant. For PD-L1, the expression rates were 24.1%(20/83) in primary tumors and 28.7%(50/174) in metastasis (p = 0.44). Yet, the expression differences between the primary and metastatic sites were also observed in lung/lymph node (37.5%, p = 0.03) and bone (12.2%, p = 0.01). When comes to PD-L2, there were no statistical expression differences between the primary (16.9%) and metastasis (23.6%) or any metastatic sites.
In the paired cohort, the concordance rate of PD-1 expression between the primary and metastasis was 57.8% (48/83, Kappa = 0.168, p = 0.09), with no significant expression difference between the primary and metastasis (χ2 = 2.795, p = 0.09). Meanwhile, the concordance rate of PD-L1 expression was only 32.5% (27/83, Kappa = 0.229, p = 0.03), with a significant expression difference between the primary and metastasis (χ2 = 4.664, p = 0.03). For PD-L2, the concordance rate was 73.5% (61/83, Kappa = 0.193, p = 0.07), with no statistical expression difference between the primary and metastasis (χ2 = 3.241, p = 0.07). We further stratified patients into specific metastatic sites, as shown in Additional file 2: Table S2, the significance was unavailable for several metastatic sites due to the limited quantities. However, we could find that PD-1 was differentially expressed between the primary tumor and brain metastasis(χ2 = 7, p = 0.01). The detection rate of PD-L1 was numerically higher in the metastasis of lung/lymph node than that of the primary tumor (38.8% vs. 24.1%), with a borderline statistical significance (χ2 = 3.056, p = 0.08). For PD-L2, the detection rate of bone was higher than that of the primary tumor (χ2 = 4.5, p = 0.03).
Relationship between differential expressions of PD-1, PD-L1&2 and clinical pathological parameters
As shown in Additional file 3: Table S3, the differential expression of PD-1 was only correlated with gender. Compared to female patients (37.9%, 11/29), a higher concordance rate of PD-1 was observed in male patients (68.5%, 37/54). In patients with brain metastasis, the concordance rate was 100%, which was significantly different from those with non-brain metastasis(p = 0.02). As for PD-L1, the concordance rate was 100% in patients treated with cytokine therapy, which was predominantly higher than those who didn’t received the treatment. However, no factor was associated with the differential expression of PD-L2.
Associations of PD-1, PD-L1 and PD-L2 expressions with clinical outcomes
Until follow-up date, 156 of patients (95.7%) showed progression, 95 of patients (58.3%) were dead. The median PFS was 23.0 months (IQR:9.0–46.0), and the median OS was 36.0 month (IQR:16.0–70.0). In 64 patients with TKI therapy, the median PFS and OS were 21.0 and 52.0 months, respectively.
In the whole cohort (N = 163), PD-1 expression either in the primary or metastatic tumor was correlated with PFS (HR 1.59, 95%CI 1.08–2.36, p = 0.02, Fig. 2a), and no statistical significance was found for OS (Fig. 2b). However, no significant association was found between PD-L1 and PD-L2 expression and clinical outcomes. In all metastatic tumors, expressions of PD-1, PD-L1 and PD-L2 were not associated with PFS and OS. In the paired patients (N = 83), PD-L1 expression in primary tumor was numerically associated with PFS (Fig. 2c) and statistically associated with OS (HR 2.55, 95%CI 1.06–6.15, p = 0.04, Fig. 2d). However, its expression in metastatic tumors was not associated with PFS nor OS. PD-1 and PD-L2 had no impact on both PFS and OS in the paired patients.
The association of expressions of immune checkpoints with clinical outcomes in patients with RCC. Representative images of kaplan–Meier survival curves of PD-1 expression either in the primary or metastatic tumors in the whole cohort (a-b), PD-L1 expression in the primary tumor (c-d) and PD-L2 expression in the primary tumor (e-f)
Multivariate analysis of PFS and OS in the whole and paired cohort
In whole cohort, univariate analysis demonstrated that ISUP≥3, nephrectomy, ECOG score, IMDC classification, metastatic status, serum WBC and PD-1 positive either in the primary or metastatic tumor were correlated with PFS (Additional file 4: Table S4). Further multivariate analysis showed that IMDC was an independently predictive factor for PFS, with a predictive accuracy (PA) of 0.683 in whole cohort. After adding expression of PD-1 either in the primary or metastatic tumor into the Cox regression model, the PA was increased to 0.699. In terms of OS, time interval from diagnosis to metastasis was an independent predictive factor (P = 0.02) with PA of 0.747(Table 3).
In paired patients, univariate analysis showed that IMDC was an influencing factor for PFS. IMDC, interval time from diagnosis to metastasis interval, cytokine therapy, HGB, ALP, LDH and serum Na+ level were influencing factors for OS (Additional file 5: Table S5). Expressions of PD-1, PD-L1 and PD-L2 in primary or metastatic sites showed no predictive value for PFS. As for OS, PD-L1 was a risk factor (P = 0.04), however, other checkpoints showed no predictive value. Subsequent multivariate analysis suggested that IMDC was an independently risk factor for PFS in the paired patients, with a PA of this model as 0.676. As for OS, nephrectomy and WBC were independently influencing factors, and the PA of OS model was 0.754. After adding PD-L1 expression in primary tumor, the PA was increased to 0.757 (Table 4).
Discussion
In the present study, we firstly compared expressions of several checkpoints (PD-1, PD-L1/2) in primary RCC and their metastases among Asian population. We also evaluated the correlations between clinicopathological parameters and discordant expressions of PD-1, PD-L1 and PD-L2. Furthermore, the value of expression in metastases of these immune checkpoints in predicting patient’s prognosis was assessed.
It has been demonstrated that PD-L1 expression was positively associated with efficacy of immune checkpoint inhibitors among malignant tumors [24,25,26], however, the situation was not the same in RCC [11]. Because of its inherent heterogeneity, tumors of RCC were potentially characterized as discrepant in expressions of immune checkpoints among different sites of tumors, especially the primary and metastases. Several studies have shown that gene expression profiles and biomarkers were differentially expressed between the primary and metastatic tumors, such as breast cancer, gastric cancer, etc. [27,28,29,30]. Similarly, an identical phenomenon was observed in RCC [31,32,33]. The differential expression suggested that the evaluation of PD-L1 expression in metastasis might give a more accurate prediction of curative effect of PD-1/PD-L1 inhibitors [34, 35]. Although Callea et al. had analyzed the expression of PD-L1 in 56 patients with matched primary and metastatic tumors, they reached the conclusion that PD-L1 was non-differentially expressed between primary and metastatic tumors. Anyway, they did found that PD-L1 was differentially expressed in the same tumor—the higher the nuclear grade was, the more evident expression of PD-L1 was observed, suggesting that PD-L1 expression was possibly required to be evaluated in metastases in order to more accurately predict the therapeutic effect of immune checkpoint inhibitors [35]. However, the study included a limited number of cases and only analyzed the expression of PD-L1. In another study, Giraldo et al. enrolled 135 primary RCC tumors and 51 lung metastases derived from RCC to analyze the differential expressions of PD-1, PD-L1, PD-L2 and LAG-3. Their results showed that when PD-1 was combined with PD-L1 or PD-L2, the expression of PD-1 in metastasis, but not the primary, was an independent risk factor for OS, suggesting that the differential expressions of immune checkpoints between the primary and metastatic tumors were correlated with patients’ prognosis [36]. Since this study only included a limited number of lung metastasis and these metastases were not matched with the primary tumors, the differential expressions of immune checkpoints between the primary and metastatic tumors still need to be further verified.
Until now, studies reporting on differential expressions of immune checkpoints in RCC were in shortage, especially no study reported data of Asian patients. Immune check points might differentially expressed within an individual or among individuals for tumor heterogeneity. Therefore, we firstly detected and compared the differential expressions of several immune checkpoints (PD-1, PD-1/2 and TIM-3) between primary and metastatic tumors in Chinese RCC patients, hopefully, to provide a laboratory basis for predicting the efficacy of immunotherapy in patents with mRCC and ultimately offer some insights into the realization of individualized treatment. Therefore, based on results of the present study, we concluded that the expression differences of PD-1, PD-L1&2 between primary and metastatic tumor were significant, which was different from the previous report of Western population [35]. The underlying reason for the difference was not clear. However, it might be attributed to the regional difference. Regional or racial difference could be one of the multi-factors in contributing to heterogeneity and it has also been proved that these differences have evident influence on the incidence, risk and prognosis of RCC [37]. What’s more, the population included in our study had a lower rate of T stage≥3 than that of Callea et al. reported. In addition to this, the detection rates of PD-1, PD-L1 and PD-L2 were different from other studies. In the present study, the overall detection rates were 46.6% (PD-1), 32.5% (PD-L1) and 26.4% (PD-L2), respectively. Notably, the detection rates of these checkpoints in the primary tumor were 31.1% (PD-1), 24.1% (PD-L1), 16.9% (PD-L2), respectively. And the reported detection rates of PD-1, PD-L1 and PD-L2 in the primary tumor were 56.6% [38], 13.0–66.3% [21, 22, 39, 40], and 21.0% [41] respectively. Besides, in our study, the detection rates of PD-1, PD-L1 and PD-L2 in the metastasis were 42.0, 28.7 and 23.6% respectively, while the reported data of other studies were 25.5% [22], 10.0–75.0% [36, 42] and 29.0% [36], respectively. Except for racial or regional differences, several other concerned factors might contribute to the above differences could be different detection method, efficiency of the primary antibody and evaluation criterion of positivity. Another noteworthy point was that, it has been reported that PD-1 was related to T stage, nuclear grade, sarcomatous differentiation and necrosis, and PD-L1 expression was related to T stage, nuclear grade, necrosis and ECOG [21, 39, 43]. The present study failed to reach similar results, which might be reason of small number of included patients and disease status (metastatic or advanced RCC).
In the present study, PD-1, PD-L1, and PD-L2 were differentially expressed between the primary and metastatic tumors. All of them had a higher expression rate in metastasis than that of the primary tumor. These checkpoints had poor concordance rate between the primary and metastatic tumors, suggesting that the assessment of primary tumor was insufficient to accurately predict patient’s treatment outcomes and prognosis. What’s more, PD-1, PD-L1, and PD-L2 had differential expressions in various metastasis. Therefore, the treatment outcomes might be correlated with different metastatic sites. Since both PD-1 and PD-L1 had higher expression rate in lung/lymph node, it seems that patients with metastasis in lung and lymph node were potential targets for immune therapy.
Limitations of the present study were: PD-L1/2 expressions were: 1) not assessed in tumor infiltrating immune cells; 2) sample size was limited, especially the number of paired patients; 3) different types of specimens of tumors might have influences on the results of IHC. However, we still have reached several conclusions: First, checkpoint detection of the primary tumor in mRCC might not provide enough information for predicting treatment effect, prognosis and making clinical decisions. Next, biopsy and resection specimens of metastases could provide more accurate evidence. In addition, the treatment effect of mRCC might be correlated with different metastatic sites. Patients with lung and/or lymph node metastases were potential candidates for immunotherapy.
Conclusions
The expression differences between the primary and metastatic tumor of PD-1, PD-L1&2 were significant. The value of assessing immune checkpoints in the primary tumor is limited and the efficacy of immunotherapy might be associated with the site of metastasis. Therefore, histopathological evaluation of metastatic sites is worth of notice, and its accurate diagnosis might be one of the effective ways to realize the individualized treatment.
Abbreviations
- BMI:
-
Body Mass Index
- ECOG:
-
Eastern Cooperative Oncology Group
- IHC:
-
Immunohistochemistry
- IMDC:
-
International Metastatic Renal-Cell Carcinoma Database Consortium
- ISUP:
-
International Society of Urological Pathology
- mRCC:
-
Metastatic Renal Cell Carcinoma
- OS:
-
Overall Survival
- PA:
-
Predicted Accuracy
- PD-1:
-
Programmed Death-1
- PD-L1:
-
Programmed Death-Ligand 1
- PD-L2:
-
Programmed Death-Ligand 2
- PFS:
-
Progression-Free Survival
- RCC:
-
Renal Cell Carcinoma
References
Kovacs G, Akhtar M, Beckwith BJ, Bugert P, Cooper CS, Delahunt B, Eble JN, Fleming S, Ljungberg B, Medeiros LJ, et al. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183(2):131–3.
Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373(9669):1119–32.
Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30(4):843–52.
Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13(3):688–96.
McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, Kirkwood JM, Gordon MS, Sosman JA, Ernstoff MS, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23(1):133–41.
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–90.
Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, Mackenzie M, Wood L, Donskov F, Tan MH, et al. External validation and comparison with other models of the international metastatic renal-cell carcinoma database consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141–8.
Xu JX, Maher VE, Zhang L, Tang S, Sridhara R, Ibrahim A, Kim G, Pazdur R. FDA Approval Summary: Nivolumab in Advanced Renal Cell Carcinoma After Anti-Angiogenic Therapy and Exploratory Predictive Biomarker Analysis. Oncologist. 2017;22(3):311-317.
Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthelemy P, Porta C, George S, et al. Nivolumab plus Ipilimumab versus Sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54.
Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S, Logan TF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33(13):1430–7.
Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116(7):1757–66.
Choueiri TK, Fishman MN, Escudier BJ, Kim JJ, Kluger HM, Stadler WM, Perez-Gracia JL, McNeel DG, Curti BD, Harrison MR, et al. Immunomodulatory activity of nivolumab in previously treated and untreated metastatic renal cell carcinoma (mRCC): biomarker-based results from a randomized clinical trial. J Clin Oncol. 2014;32 5s((suppl; abstr 5012)).
Drake CGMD, Sznol M, Choueiri TK, Kluger HM, Powderly JD, et al. Survival, safety, and response duration results of nivolumab (anti-PD-1; BMS-936558; ONO-4538) in a phase I trial in patients with previously treated metastatic renal cell carcinoma (mRCC): long-term patient follow-up. J Clin Oncol. 2013:31 Available from: http://meetinglibrary.asco.org/content/113579-132. Cited 31 Jul 2014.
Grosso JHC, Inzunza D, Cardona DM, Simon JS, Gupta AK, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538). J Clin Oncol. 2013:31 Available from: http://meetinglibrary.asco.org/content/113904-132. Cited 2014 Jul 18.
McDermott DFSM, Sosman JA, Soria J-C. Immune correlates and long term follow up of a phase Ia study of MPDL3280A, an engineered PD-L1 antibody, in patients with metastatic renal cell carcinoma (mRCC). Ann Oncol. 2014;25(suppl_4):iv280.
Hsu MC, Hsiao JR, Chang KC, Wu YH, Su IJ, Jin YT, Chang Y. Increase of programmed death-1-expressing intratumoral CD8 T cells predicts a poor prognosis for nasopharyngeal carcinoma. Mod Pathol. 2010;23(10):1393–403.
Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15(3):971–9.
Miyoshi H, Kiyasu J, Kato T, Yoshida N, Shimono J, Yokoyama S, Taniguchi H, Sasaki Y, Kurita D, Kawamoto K, et al. PD-L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T-cell leukemia/lymphoma. Blood. 2016;128(10):1374–81.
Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56(8):1173–82.
Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–5.
Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101(49):17174–9.
Zhang X, Yin X, Zhang H, Sun G, Yang Y, Chen J, Shu K, Zhao J, Zhao P, Chen N, et al. Differential expression of TIM-3 between primary and metastatic sites in renal cell carcinoma. BMC Cancer. 2019;19(1):49-60.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28.
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30.
Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, Zhao X, Martinez AJ, Wang W, Gibney G, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311–8.
Inari H, Suganuma N, Kawachi K, Yoshida T, Yamanaka T, Nakamura Y, Yoshihara M, Nakayama H, Yamanaka A, Masudo K, et al. Expression of enhancer of zeste homolog 2 correlates with survival outcome in patients with metastatic breast cancer: exploratory study using primary and paired metastatic lesions. BMC Cancer. 2017;17(1):160.
Hao X, Sun B, Hu L, Lahdesmaki H, Dunmire V, Feng Y, Zhang SW, Wang H, Wu C, Wang H, et al. Differential gene and protein expression in primary breast malignancies and their lymph node metastases as revealed by combined cDNA microarray and tissue microarray analysis. Cancer. 2004;100(6):1110–22.
Xie HL, Li ZY, Gan RL, Li XJ, Zhang QL, Hui M, Zhou XT. Differential gene and protein expression in primary gastric carcinomas and their lymph node metastases as revealed by combined cDNA microarray and tissue microarray analysis. J Dig Dis. 2010;11(3):167–75.
Wang L, Zhu JS, Song MQ, Chen GQ, Chen JL. Comparison of gene expression profiles between primary tumor and metastatic lesions in gastric cancer patients using laser microdissection and cDNA microarray. World J Gastroenterol. 2006;12(43):6949–54.
Ho TH, Serie DJ, Parasramka M, Cheville JC, Bot BM, Tan W, Wang L, Joseph RW, Hilton T, Leibovich BC et al. Differential gene expression profiling of matched primary renal cell carcinoma and metastases reveals upregulation of extracellular matrix genes. Ann Oncol. 2016;28(3):604-610.
Rausch S, Beermann J, Scharpf M, Hennenlotter J, Fend F, Stenzl A, Schollenberger D, Bedke J, Kruck S. Differential expression and clinical relevance of MUC1 in renal cell carcinoma metastasis. World J Urol. 2016;34(12):1635–41.
Ozcan A, de la Roza G, Ro JY, Shen SS, Truong LD. PAX2 and PAX8 expression in primary and metastatic renal tumors: a comprehensive comparison. Arch Pathol Lab Med. 2012;136(12):1541–51.
Pinato DJ, Shiner RJ, White SD, Black JR, Trivedi P, Stebbing J, Sharma R, Mauri FA. Intra-tumoral heterogeneity in the expression of programmed-death (PD) ligands in isogeneic primary and metastatic lung cancer: implications for immunotherapy. Oncoimmunology. 2016;5(9):e1213934.
Callea M, Albiges L, Gupta M, Cheng SC, Genega EM, Fay AP, Song J, Carvo I, Bhatt RS, Atkins MB, et al. Differential expression of PD-L1 between primary and metastatic sites in clear-cell renal cell carcinoma. Cancer Immunol Res. 2015;3(10):1158–64.
Giraldo NA, Becht E, Pages F, Skliris G, Verkarre V, Vano Y, Mejean A, Saint-Aubert N, Lacroix L, Natario I, et al. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell Cancer. Clin Cancer Res. 2015;21(13):3031–40.
Sims JN, Yedjou CG. Racial Disparities and Preventive Measures to Renal Cell Carcinoma. 2018;15(6):1089-1102.
Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13(6):1757–61.
Choueiri TK, Fay AP, Gray KP, Callea M, Ho TH, Albiges L, Bellmunt J, Song J, Carvo I, Lampron M, et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann Oncol. 2014;25(11):2178–84.
Iacovelli R, Nole F, Verri E, Renne G, Paglino C, Santoni M, Cossu Rocca M, Giglione P, Aurilio G, Cullura D, et al. Prognostic role of PD-L1 expression in renal cell carcinoma. A systematic review and meta-analysis. Target Oncol. 2016;11(2):143–8.
Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–74.
Bellmunt J, Mullane SA, Werner L, Fay AP, Callea M, Leow JJ, Taplin ME, Choueiri TK, Hodi FS, Freeman GJ, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol. 2015;26(4):812–7.
Frigola X, Inman BA, Lohse CM, Krco CJ, Cheville JC, Thompson RH, Leibovich B, Blute ML, Dong H, Kwon ED. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17(7):1915–23.
Acknowledgements
The authors would like to thank Ms. Junya Tan for giving technical support in immunohistological staining.
Funding
This work was supported by Natural Science Foundation of China (NSFC 81402110 and 81672547), Science and Technology Support Program of Sichuan Province (2015SZ0230–3) and 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (No.0040205301E21). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The authors declare that materials described in the manuscript, including all relevant raw data, will be available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Contributions
XZ, PS and HZeng designed the experiments, XZ, XY and HZhang performed the experiments. XZ, GX, JC, XZhu, JG, PZ and NC collected clinicopathological data. XZ, GS, XY and YY analyzed the results. XZhang and YY generated the data, prepared the figures and Tables. XT, PS and JW wrote the manuscript. All authors have reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The protocol of the present study, involving human clinical samples, was approved by the Ethics Committee of West China Hospital, Sichuan University. Written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Baseline clinicopathological parameters of included patients. (DOCX 16 kb)
Additional file 2:
Table S2. Differential expressions of PD-1,PD-L1 and PD-L2 between the primary and metastatic tumors in the paired cohort. (DOCX 16 kb)
Additional file 3:
Table S3. Relationship between the differential expressions of PD-1, PD-L1 and PD-L2 and clinicopathological parameters in the paired cohort. (DOCX 20 kb)
Additional file 4:
Table S4. Unvariate analysis of PFS and OS in all patients. (DOCX 19 kb)
Additional file 5:
Table S5. Unvariate analysis of PFS and OS in paired patients. (DOCX 18 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhang, X., Yin, X., Zhang, H. et al. Differential expressions of PD-1, PD-L1 and PD-L2 between primary and metastatic sites in renal cell carcinoma. BMC Cancer 19, 360 (2019). https://doi.org/10.1186/s12885-019-5578-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-019-5578-4