Abstract
Background
Combined anthracycline-trastuzumab chemotherapy has been associated with LV dysfunction. We aimed to assess early changes in left ventricular (LV) and right ventricular (RV) mechanics associated with combined anthracycline-trastuzumab treatment for breast cancer. As well as explore whether early changes in 2-dimensional (2D)–speckle tracking echocardiography (STE) could predict later chemotherapy-induced cardiotoxicity.
Methods
Sixty-six patients with breast cancer who received anthracycline-trastuzumab treatment were included (mean [±SD] age, 52 [9] years). Echocardiograms were available for analysis with 2D-STE at the following time points: pretreatment (T0), first cycle (T1), and second cycle (T2) of combined chemotherapy. All patients had a normal pretreatment LV ejection fraction (LVEF). Cardiotoxicity was defined as a decrease in LVEF of at least 10 percentage points from baseline on follow-up echocardiography.
Results
Cardiotoxicity developed in 13 of the 66 patients (20%). The mean (±SD) LVEF at T0 was 66% (±6); at T1 60% (±7); and at T2, 54% (±6). For the 53 patients without cardiotoxicity, the LVEF was 65% (±4%) at T0, 63% (±5%) at T1, and 62% (±4) at T2. Global longitudinal strain (GLS) at T1 was the strongest indicator of subsequent cardiotoxicity (area under the curve, 0.85; cutoff value, − 14.06; sensitivity, 91%; specificity, 83%; P = .003). Compared with baseline (T0), left ventricular longitudinal strain, LV circumferential strain, circumferential peak systolic strain rate (SR), circumferential peak early diastolic SR, right ventricular longitudinal strain, and longitudinal peak systolic SR at T1 and T2 were reduced significantly in patients with cardiotoxicity (P < .05).
Conclusions
Anthracycline-trastuzumab treatment leads to early deterioration of LV GLS, circumferential strain, and systolic SR. Right ventricular GLS and SR were also affected. Early changes in GLS are good predictors of subsequent development of anthracycline-trastuzumab–induced cardiotoxicity.
Similar content being viewed by others
Background
Cardiotoxicity due to combined chemotherapy is a leading cause of morbidity and mortality for survivors of breast cancer [1,2,3,4], and the survival rate for patients who subsequently develop heart failure is as low as 25% at 5 years [5]. Early cardiotoxicity may be silent, yet its prompt diagnosis is important for patients with early structural heart changes but no signs or symptoms of heart failure (stage B heart failure [American Heart Association/American College of Cardiology]) [6].
Combined chemotherapeutic agents, such as anthracycline and trastuzumab have increased survival rates for patients with HER 2 positive breast cancer, and combination therapy has thus become a well-established therapeutic approach [7]. However, anthracyclines may generate dose-dependent left ventricular (LV) dysfunction, which is associated with poor prognosis [8]. In addition, trastuzumab results in cardiac dysfunction in 3% to 5% of treated patients [7, 8]. Potential cardiotoxicity of therapy, focused on early detection of minor LV myocardial dysfunction and early intervention, should be considered for patients undergoing cancer therapy with these agents [1, 6, 9]. Early recognition and appropriate therapy can improve outcomes and decrease morbidity, mortality, and progression to clinical heart failure [10].
Monitoring for early signs of cardiotoxicity during a patient’s treatment with chemotherapy can be done by using strain measurements [11]. Two-dimensional–speckle tracking echocardiography (2D-STE) is a promising technique that can evaluate cardiac mechanics in the 3 domains of contractility [12,13,14,15,16]. A growing body of literature supports the use of myocardial deformation parameters to detect early myocardial injury (stage B heart failure) and to forecast ventricular dysfunction in patients receiving cancer therapy [16,17,18,19,20,21,22,23]. Because very limited data exist about the early cardiotoxicity of chemotherapy in patients taking both anthracycline and trastuzumab [22] or about right ventricular (RV) mechanics in patients who receive cancer therapy, we designed a study aimed to detect early changes in LV and RV mechanics and to determine if 2D-STE could predict preclinical cardiotoxicity from anthracycline-trastuzumab treatment after the first and second cycle of chemotherapy in patients with breast cancer.
Methods
Study population
For this retrospective study, we enrolled women newly diagnosed with breast cancer between December 1, 2004, and June 1, 2012, who were treated with an anthracycline (doxorubicin and epirubicin), or a HER 2 inhibitor (trastuzumab), or both. Though the majority of the study population (40/66; 61%) received adjuvant therapy, a substantial number (26/66; 39%); received neoadjuvant therapy. Of the total cohort, one woman was found to have stage IV disease at diagnosis. Twenty three (35%) had stage III disease and 6 of those had inflammatory breast cancer. None of the study population received concurrent anthracyclines and trastuzumab. The average age was 52 (±9), mean anthracycline dose of 252 (±45) mg/m2 and trastuzumab was administered for an average of 11 (±2.7) months in 3 week cycles and a mean dose of 5.4 (±2.7) gr. All patients had to have a normal pretreatment (T0) LV ejection fraction (LVEF), had to have been followed up for 1 year, as well as an echocardiogram at baseline. Patients who met the inclusion criteria and had images of adequate quality, as well as follow-up, were included (N = 66). Because of lack of consensus, we followed the definition of cardiotoxicity used in the clinical trials for combined chemotherapy in HER 2 positive patients, where it was defined as an absolute decrease in LVEF of 10 or more percentage points from baseline echocardiogram [24, 25]. Traditional cardiovascular risk factors, such as age, hypertension, diabetes mellitus, hyperlipidemia, family history of premature coronary artery disease, and smoking status were also considered in the analyses [26,27,28]. The study was approved by the Mayo Clinic Institutional Review Board, and written informed consent was obtained.
Imaging acquisition and speckle tracking analysis
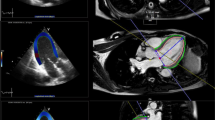
To be included, patients needed to have at least 3 standard echocardiographic examinations: at baseline (T0); from the start of chemotherapy to first echocardiogram (T1) and from the start of chemotherapy to the second echocardiogram (T2) following the cardio oncology clinic protocol [19]. Echocardiographic examinations were performed using a GE Vivid 7 system (General Electric Company) with an M4S transducer (1.5-4.3 MHz). Mean frame rates were 55 Hz for grayscale imaging. Studies were performed and reported according to the guidelines of the American Society of Echocardiography [29]. LVEF was calculated using the biplane Simpson method [13]. Digital images were saved for subsequent, blinded off-line analysis using the Syngo Velocity Vector Imaging software, version 3.5 and 2D-STE analyses were performed.
For all patients, the region of interest analyzed was adjusted to cover at least 90% of the myocardial wall thickness for myocardial strain and SR. LV longitudinal parameters were measured from the apical 4-chamber, 2-chamber, and 3-chamber views, and the myocardium was divided into 6 segments per view. Care was taken to ensure that the long axis of the ventricle was perpendicular to the plane of the mitral annulus in the LV apical views. Circumferential and radial parameters were measured using the parasternal short-axis plane at the midventricular level. RV longitudinal parameters were measured from the apical 4-chamber view. Global and segmental myocardial deformation parameters, including strain (S), peak systolic strain rate (SRs), and peak early diastolic strain rate (SRe) were measured for each patient.
Reproducibility
To determine intraobserver variability, echocardiograms from 20 randomly assigned patients were reanalyzed by the same observer (X.H.) 2 months after the initial analysis. For interobserver variability, the same patients and the same cardiac cycles were analyzed by a second observer (Z.Y.).
Statistical analysis
Continuous data are presented as mean (SD) and categorical data as frequencies (percentages). Deformation parameters were compared among T0, T1, and T2 using 1-way ANOVA and paired t tests. Differences among age subgroups were assessed using the Tukey-Kramer multiple comparisons test. The first(T1) and second time point(T2) were used to construct a receiver operating characteristic (ROC) curve, which was used to predict cardiotoxicity. The best cutoff value was defined as the point with the highest sum of sensitivity and specificity. Univariate and multivariate logistic regression analyses were used to determine predictors of a significant decrease in LVEF. Intraobserver variability, interobserver variability, and intraclass correlation coefficients (ICCs) with 95% CIs were calculated to evaluate test reliability [30, 31]. All statistical analyses were performed using SAS software, version 9.3 (SAS Institute Inc). A P value <.05 was considered statistically significant.
Results
Patient characteristics
Sixty-six patients who completed anthracycline-trastuzumab treatment for breast cancer were included in the study (mean [±SD] age, 52 [±9] years; median [range] age, 51 [34-72] years). The patients’ clinical characteristics are summarized in Table 1. Of the 66 patients, 13 (20%) had cardiotoxicity defined by a decrease in LVEF of 10 or more percentage points from baseline [24, 25]. Forty-six percent of those patients developed cardiotoxicity at T1 and 54% at T2. Both groups were followed up for the same amount of time. The median cumulative doses of anthracycline-trastuzumab are shown in Table 1. The patients LVEF in whom cardiotoxicity developed vs. patients LVEF with no cardiotoxicity is shown in Table 2. Thirty-one patients had baseline cardiac risk factors, including hypertension, 13 patients; diabetes mellitus, 3 patients; hyperlipidemia, 12 patients; family history of premature coronary artery disease, 4 patients; and smoking history, 13 patients. There was no difference in cardiotoxicity for patients with more than or less than 3 risk factors. In addition, 50 patients (76%) began radiotherapy 5.3 (2.2) months after the start of chemotherapy.
LV and RV mechanics at T0, T1, and T2
Echocardiographic follow up was obtained in three time points: baseline (T0) at the time of diagnosis; (T1) from the start of chemotherapy to first echocardiogram (median [interquartile range {IQR}], 2.25 [1.84-2.99] months); and (T2) from the start of chemotherapy to the second echocardiogram (T2) (median [IQR], 5.44 [4.61-6.47] months).
Serial 2D-STE parameters at T0, T1, and T2 are summarized in Fig. 1. Compared with T0, GLS at T1 and T2 and GCS at T1 and T2 were significantly reduced (P < .01 for all). Also significantly reduced were RV GLS at T1, SRs at T1, RV GLS at T2, SRs at T2 and SRe at T1 (P < .001 for all). There was a significant decrease with increasing age in LV GLS both at T1 and T2, in LV longitudinal SRe at T2, and in RV longitudinal SRe at T1 (P < .01 for all). There was no significant decrease in any parameter measured with increasing age (Table 3).
Dynamic changes of LVEF, GLS, GCS, RVLS and GLSRs at different time points (T0-T2). Multi-line graph showing the relationship between LVEF% (purple line), GLS% (blue line), RVLS%(green line), GCS% (red line) and GCSRs (orange line), at three different time points: T0, T1, T2. Notice that there is significant change between T0 and T1 for right ventricular longitudinal strain (RVLS), global circumferential strain (GCS), global longitudinal strain (GLS), and global circumferential systolic strain rate (GCSRs) at T1 that can predict the development of cardiac toxicity at T2
Predicting a decrease in LVEF
Combining both RV GLS at T1 and LV GLS was the strongest predictor of cardiotoxicity (area under the curve [AUC], 0.91; sensitivity, 100%; specificity, 73%; P < .001). LV GLS at T1 (AUC, 0.85; cutoff, − 14.06; sensitivity, 91%; specificity, 83%; P = .003) was also a strong indicator of subsequent cardiotoxicity. Combining LV GLS with longitudinal early diastolic strain rate (LSRe) at T1 (AUC, 0.90; sensitivity, 91%; specificity, 86%; P < .001) and combining GLS with radial early diastolic strain rate (RSRe) at T1 (AUC, 0.88; sensitivity, 91%; specificity, 78%; P < .001) were also strong predictors of subsequent cardiotoxicity (Table 4).
Reproducibility
The intraobserver and interobserver agreement are shown in Table 5. Both measurements of agreement were lowest for radial S, SRs, and SRe values.
Discussion
This study resulted in several main findings. First, to our knowledge, this is the first study that has shown that combining GLS and RV GLS measurements is a strong predictor of cardiotoxicity in patients with breast cancer who receive anthracycline-trastuzumab treatment. Second, after the first few months of chemotherapy, there was a significant decrease in LV GLS, GCS, SRs, and SRe, even in patients receiving under the upper limit of the recommended cardiac safe dose. Third, we observed significant changes in RV mechanics in our patients, with important decreases in RV GLS, SRs, and SRe at T1 and in S and SRs at T2.
Previous studies have also shown that GLS is the optimal marker for detecting subclinical heart failure (type B) [11, 32]. As a result, we recommend that this measurement be incorporated as a marker of stage B heart failure in this population of patients. Anthracyclines are well known antineoplastic agents that have proven to be cardiotoxic in a dose-dependent manner [8]. For example, doxorubicin has a lower risk of congestive heart failure for doses below 450 mg/m2, but it is associated with moderate risk at 550 mg/m2 and high risk at more than 1000 mg/m2 [29, 33]. Our study population received a mean dose of 466 mg/m2, which is below the threshold for moderate risk and slightly above that for low risk. Several studies have shown that when trastuzumab is given alone or combined with anthracyclines it is associated with cardiotoxicity. Previous randomized controlled trials have also shown better survival for patients with HER2-positive breast cancer treated with trastuzumab, a monoclonal antibody that targets the HER2 receptor [15, 24, 34]. For this reason, 2D-STE can help in the detection of subclinical systolic dysfunction in patients given combined chemotherapy, as in our study. These variables, which include S and SR, will allow us to detect early systolic and diastolic dysfunction in order to predict future changes in EF [34, 35].
RV abnormalities may also occur in patients with cancer, although the frequency has not been determined. One study of 37 patients showed a decrease in RV systolic and diastolic indices on echocardiography relatively soon after chemotherapy with anthracyclines; however, most indices were within normal ranges [34]. In our study we observed significant reduction on RV mechanic parameters at both T1 and T2.
Fifty-four of our patients received anthracycline at T1 and anthracycline plus trastuzumab at T2. When compared with the changes from T0 to T1, the differences for T1 to T2 were significant (P<. 05 for all), in favor of improvement rather than further worsening of heart failure. These results suggest that patients with breast cancer can better tolerate the appropriate dose of anthracycline-trastuzumab treatment after early changes in myocardial mechanics occur.
Study limitations and future directions
This study was limited by its retrospective design and small sample size. However, it was able to show that RV mechanical parameters should be studied in a larger population. Our data shows increased risk of cardio toxicity in the older population, however there is not enough power when the data is divided into age groups to draw a definitive conclusion.
Conclusions
Abnormal values of 2D-STE in the presence of a normal EF can predict a future drop in ejection fraction. GLS should be used as a marker of stage B heart failure in patients given a combined anthracycline-trastuzumab regimen, despite treatment with doses in the moderate-risk range. In addition, S and SR provided data that showed early changes in LV myocardial function (stage B heart failure) after less than 3 months of treatment with chemotherapy.
Abbreviations
- 2D-STE:
-
2-dimensional–speckle tracking echocardiography
- AUC:
-
area under curve
- EF:
-
ejection fraction
- GLS:
-
global longitudinal strain
- ICC:
-
intraclass correlation coefficient
- IQR:
-
interquartile range
- LSRe:
-
longitudinal peak early diastolic strain rate
- LSRs:
-
longitudinal peak systolic strain rate
- LV:
-
left ventricle
- LVEF:
-
left ventricular ejection fraction
- ROC:
-
receiver operating characteristic
- RSRe:
-
radial early diastolic strain rate
- RV:
-
right ventricle
- RVLS:
-
right ventricular longitudinal strain
- S:
-
strain
- SR:
-
strain rate
- SRe:
-
early diastolic strain rate
- SRs:
-
peak systolic strain rate
References
Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL. Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J Clin Oncol. 2005;23(34):8597–605.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92.
Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342(15):1077–84.
Negishi K, Negishi T, Haluska BA, Hare JL, Plana JC, Marwick TH. Use of speckle strain to assess left ventricular responses to cardiotoxic chemotherapy and cardioprotection. Eur Heart J Cardiovasc Imaging. 2014;15(3):324–31.
Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3(3):315–22.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. American College of Cardiology Foundation; American Heart Association task force on practice guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013;62(16):e147–239 Epub 2013 Jun 5.
Unitt C, Montazeri K, Tolaney S, Moslehi J. Cardiology patient page: breast cancer chemotherapy and your heart. Circulation. 2014;129(25):e680–2.
Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99(5):365–75.
Xu Y, Herrmann J, Pellikka PA, Ansell SM, Cha SS, Villarraga HR. Early changes in 2D-speckle-tracking echocardiography may predict a decrease in left ventricular ejection fraction in lymphoma patients undergoing anthracyclin chemotherapy: a pilot study. J Clin Exp Oncol. 2015;1:1.
Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation. 2006;113(24):2851–60.
Abdel-Qadir H, Amir E, Thavendiranathan P. Prevention, detection, and management of chemotherapy-related cardiac dysfunction. Can J Cardiol. 2016;32(7):891–9.
Nhola LF, Mulvagh SL, Abdelmoneim SS, Herrmann J, Bordun KA, Premecz S, et al. Is there a change in myocardial mechanical function in patients on vascular endothelial grow factor axis inhibitor therapy for genitourinary and gastrointestinal cancer? [abstract]. J Am Soc Echocardiogr. 2015;28(6):B41–2.
Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61(1):77–84.
Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55(3):213–20.
Wadhwa D, Fallah-Rad N, Grenier D, Krahn M, Fang T, Ahmadie R, et al. Trastuzumab mediated cardiotoxicity in the setting of adjuvant chemotherapy for breast cancer: a retrospective study. Breast Cancer Res Treat. 2009;117(2):357–64.
Kocabay G, Muraru D, Peluso D, Cucchini U, Mihaila S, Padayattil-Jose S, et al. Normal left ventricular mechanics by two-dimensional speckle-tracking echocardiography: reference values in healthy adults. Rev Esp Cardiol (Engl Ed). 2014;67(8):651–8.
Fine NM, Shah AA, Han IY, Yu Y, Hsiao JF, Koshino Y, et al. Left and right ventricular strain and strain rate measurement in normal adults using velocity vector imaging: an assessment of reference values and intersystem agreement. Int J Cardiovasc Imaging. 2013;29(3):571–80.
Marwick TH, Leano RL, Brown J, Sun JP, Hoffmann R, Lysyansky P, et al. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging. 2009;2(1):80–4.
Duncan AE, Alfirevic A, Sessler DI, Popovic ZB, Thomas JD. Perioperative assessment of myocardial deformation. Anesth Analg. 2014;118(3):525–44.
Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2(5):356–64.
Baratta S, Damiano MA, Marchese ML, Trucco JI, Rizzo MM, Bernok F, et al. Serum markers, conventional Doppler echocardiography and two-dimensional systolic strain in the diagnosis of chemotherapy-induced myocardial toxicity. Argentine J Cardiol. 2013;81(2):139–46.
Mavinkurve-Groothuis AM, Marcus KA, Pourier M, Loonen J, Feuth T, Hoogerbrugge PM, et al. Myocardial 2D strain echocardiography and cardiac biomarkers in children during and shortly after anthracycline therapy for acute lymphoblastic leukaemia (ALL): a prospective study. Eur Heart J Cardiovasc Imaging. 2013;14(6):562–9.
Mornos C, Petrescu L. Early detection of anthracycline-mediated cardiotoxicity: the value of considering both global longitudinal left ventricular strain and twist. Can J Physiol Pharmacol. 2013;91(8):601–7.
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–83.
Verma S, Lavasani S, Mackey J, Pritchard K, Clemons M, Dent S, et al. Optimizing the management of HER2-positive early breast cancer: the clinical reality. Curr Oncol. 2010;17:20–33.
Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr. 2013;26(5):493–8.
Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57(22):2263–70.
Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clin Proc. 2014;89(9):1287–306.
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911–39.
Yu HK, Yu W, Cheuk DK, Wong SJ, Chan GC, Cheung YF. New three-dimensional speckle-tracking echocardiography identifies global impairment of left ventricular mechanics with a high sensitivity in childhood cancer survivors. J Am Soc Echocardiogr. 2013;26(8):846–52.
Khouri MG, Douglas PS, Mackey JR, Martin M, Scott JM, Scherrer-Crosbie M, et al. Cancer therapy-induced cardiac toxicity in early breast cancer: addressing the unresolved issues. Circulation. 2012;126(23):2749–63.
Eschenhagen T, Force T, Ewer MS, de Keulenaer GW, Suter TM, Anker SD, et al. Cardiovascular side effects of cancer therapies: a position statement from the heart failure Association of the European Society of cardiology. Eur J Heart Fail. 2011;13(1):1–10.
Steingart RM, Bakris GL, Chen HX, Chen MH, Force T, Ivy SP, et al. Management of cardiac toxicity in patients receiving vascular endothelial growth factor signaling pathway inhibitors. Am Heart J. 2012;163(2):156–63.
Tanindi A, Demirci U, Tacoy G, Buyukberber S, Alsancak Y, Coskun U, et al. Assessment of right ventricular functions during cancer chemotherapy. Eur J Echocardiogr. 2011;12(11):834–40.
Barros-Gomes S, Williams B, Nhola LF, Grogan M, Maalouf JF, Dispenzieri A, et al. Prognosis of light chain amyloidosis with preserved LVEF: added value of 2D speckle-tracking echocardiography to the current prognostic staging system. JACC Cardiovasc Imaging. 2017;10(4):398–407 Epub 2016 Sep 14.
Acknowledgements
Not Applicable
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
HV and ZY proposed study design, participated in data analysis, interpretation and critically revised manuscript. HX collected data and collaborated in writing manuscript. MA participated in data analysis and drafting and writing of manuscript. NS, PP, and JH were involved in drafting of manuscript and critically revised it. SC performed data analysis and interpretation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Mayo Clinic Institutional Review Board approved the study, and patients gave a written consented to analysis and review of their medical records.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Arciniegas Calle, M.C., Sandhu, N.P., Xia, H. et al. Two-dimensional speckle tracking echocardiography predicts early subclinical cardiotoxicity associated with anthracycline-trastuzumab chemotherapy in patients with breast cancer. BMC Cancer 18, 1037 (2018). https://doi.org/10.1186/s12885-018-4935-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-018-4935-z