Abstract
Background
We previously observed that T-bet+ tumor-infiltrating T lymphocytes (T-bet+ TILs) in primary breast tumors were associated with adverse clinicopathological features, yet favorable clinical outcome. We identified BRD4 (Bromodomain-Containing Protein 4), a member of the Bromodomain and Extra Terminal domain (BET) family, as a gene that distinguished T-bet+/high and T-bet−/low tumors. In clinical studies, BET inhibitors have been shown to suppress inflammation in various cancers, suggesting a potential link between BRD4 and immune infiltration in cancer. Hence, we examined the BRD4 expression and clinicopathological features of breast cancer.
Methods
The cohort consisted of a prospectively ascertained consecutive series of women with axillary node-negative breast cancer with long follow-up. Gene expression microarray data were used to detect mRNAs differentially expressed between T-bet+/high (n = 6) and T-bet−/low (n = 41) tumors. Tissue microarrays (TMAs) constructed from tumors of 612 women were used to quantify expression of BRD4 by immunohistochemistry, which was analyzed for its association with T-bet+ TILs, Jagged1, clinicopathological features, and disease-free survival.
Results
Microarray analysis indicated that BRD4 mRNA expression was up to 44-fold higher in T-bet+/high tumors compared to T-bet−/low tumors (p = 5.38E-05). Immunohistochemical expression of BRD4 in cancer cells was also shown to be associated with T-bet+ TILs (p = 0.0415) as well as with Jagged1 mRNA and protein expression (p = 0.0171, 0.0010 respectively). BRD4 expression correlated with larger tumor size (p = 0.0049), pre-menopausal status (p = 0.0018), and high Ki-67 proliferative index (p = 0.0009). Women with high tumoral BRD4 expression in the absence of T-bet+ TILs exhibited a significantly poorer outcome (log rank test p = 0.0165) relative to other subgroups.
Conclusions
The association of BRD4 expression with T-bet+ TILs, and T-bet+ TIL-dependent disease-free survival suggests a potential link between BRD4-mediated tumor development and tumor immune surveillance, possibly through BRD4’s regulation of Jagged1 signaling pathways. Further understanding BRD4’s role in different immune contexts may help to identify an appropriate subset of breast cancer patients who may benefit from BET inhibitors without the risk of diminishing the anti-tumoral immune activity.
Similar content being viewed by others
Background
BRD4 (Bromodomain-Containing Protein 4) is a transcriptional epigenetic regulator that plays a crucial role in cancer and inflammatory diseases [1]. It is a member of the BET (Bromodomain and Extra Terminal domain) family that utilizes tandem bromodomains to recognize specific acetylated lysine residues in the N-terminal tails of histone proteins [2]. Upon interaction with chromatin, BRD4 has been shown to promote acetylation-dependent assembly of transcriptional regulator complexes that activate various transcriptional programs, such as those involved in cell proliferation and cell cycle control [3, 4].
Small molecule inhibitors that specifically target BET proteins have been demonstrated to interfere with expression of genes involved in cell growth and apoptosis evasion. Therapeutic benefits of the BET inhibitors have been observed in B-cell lymphoma [5] and acute myeloid leukemia [6, 7], as well as in lung [8], prostate [9], pancreatic [10], colorectal [11] and breast cancers [12]. Interestingly, BET inhibitors have also been shown to have an anti-inflammatory effect in the treatment of various inflammatory diseases and cancer [1, 13, 14], suggesting that BRD4 may have an active role in supporting inflammation.
Numerous studies have shown BRD4 to be important in the promotion of NF-kB-mediated transcription of inflammatory genes [15,16,17], whose functions in cancer initiation and progression have shown to be manifold and complex [18, 19]. Considering the clinical benefits of cancer immunotherapies that have been demonstrated through blockades of immune inhibitory pathways and stimulation of immune effector functions in tumors, investigating the potential link between BRD4 and immune infiltration in cancer may present a novel insight into the regulatory role of BRD4 in tumor immune surveillance.
Breast cancer is a complex and heterogeneous disease. Despite improvements in disease classification using tumor-related prognostic markers, a large disparity of clinical outcomes continues to be seen. This reflects the limitation of utilizing intrinsic tumoral characteristics as the sole determining factors of disease progression. An increasing number of studies have demonstrated that the components of tumor microenvironment, including immune infiltration, interact dynamically with the tumor, and influence clinical outcome. Particularly, infiltration by T lymphocytes has been shown to be associated with a good prognosis in breast cancer patients, and higher response rate to neoadjuvant therapy [20,21,22,23,24,25,26,27].
In two independent cohorts of women with familial breast cancer [28] and axillary node-negative (ANN) breast cancer [29], we have observed that T-bet+ tumor-infiltrating T lymphocytes (T-bet+ TILs) were associated with adverse clinicopathological features such as large tumor size, high grade, mutant p53, ER negativity, CK5 positivity, EGFR positivity, and basal molecular subtype [29, 30]. Despite being associated with an aggressive tumor phenotype, patients with a high level of T-bet+ TILs in their tumors had a favorable clinical outcome [29, 30]. T-bet is an immune-specific member of the T box family of transcription factors that is essential for differentiation of type 1 helper (Th1) T lymphocytes, as well as production of IFNy in CD4+ Th1 T lymphocytes and CD8+ cytotoxic T lymphocytes – subsets of immune cells that promote anti-tumoral inflammatory response [31, 32].
To examine how T-bet+ TILs may be associated with tumor development, we further investigated gene expression differences associated with T-bet+ TILs, and assessed their clinicopathological implications. Here we show that tumoral BRD4 expression is associated with T-bet+ TILs, relatively aggressive clinicopathological features, and a poor disease-free outcome in breast cancer.
Methods
Patient cohort
The patient cohort was composed of a prospectively ascertained consecutive series of women with axillary lymph-node negative (ANN) breast cancer, who were enrolled at eight Toronto hospitals from September 1987 to October 1996 as previously described [30, 33]. The clinicopathological features of the cohort have been reported previously [34], and disease-free survival (DFS) and overall survival (OS) data have also been collected with minimum follow-up time of 56 months after surgery and median follow-up time of 100 months. Written informed consent was obtained from all study participants. Approval of the study protocol was obtained from the Research Ethics Board of Mount Sinai Hospital (#01–0313-U) and the University Health Network (#02–0881-C).
Definition of intrinsic subtypes
Molecular subtypes for tumors were defined based on previous publications [35,36,37]. HER2 subtype consisted of tumors positive for HER2 overexpression. Luminal subtype included tumors that were negative for HER2 overexpression and positive for ER. Basal subtype included tumors that were negative for HER2 overexpression and ER, and positive for CK5 and/or EGFR. The luminal subtype was subsequently distinguished into luminal A and luminal B based on PgR, p53 status and Ki-67 labeling index. Tumors with a Ki-67 labeling index of ≥14% and were negative for PgR or positive for mutant p53 were assigned to the luminal B subgroup [37].
Quantitation of T-bet+ TILs using tissue microarrays
Tissue microarrays (TMAs) constructed from formalin-fixed, paraffin-embedded (FFPE) tumor blocks were examined by an expert breast pathologist (AMM) to quantitate for T-bet+ TILs and other immunohistochemical markers as described previously [29].
Gene expression
Data from gene expression microarray profiling performed previously in our laboratory were statistically analyzed. The mRNA expression profiling was conducted on 19 k arrays (18,981 cDNA/EST clones) manufactured by the University Health Network Microarray Center at the Ontario Cancer Institute (https://www.pmgenomics.ca/arrays/index.htm). Tumor and reference cDNAs (5μg) were indirectly labeled using aminoallyl nucleotide analogs with Cy3 and Cy5 fluorescent tags respectively. Of the 137 flash-frozen ANN tumors analyzed for mRNA expression, 47 tumors had available IHC data for T-bet+ TILs, in which six were T-bet+/high and 41 were T-bet−/low. Supervised statistical analyses and hierarchical clustering were conducted on the gene clones using BRB ArrayTools software (http://linus.nci.nih.gov/BRB-ArrayTools.html).
Immunohistochemical staining and analysis of BRD4
Immunohistochemical (IHC) staining was performed to examine BRD4 protein expression and localization using polyclonal anti-human BRD4 (HPA061646, Sigma Aldrich) published on the public protein database, The Human Protein Atlas project (https://www.proteinatlas.org/ENSG00000141867-BRD4/antibody). After optimizing the BRD4 antibody for IHC staining on a series of control normal and breast tumor tissues, the BRD4 protein expression was assessed on the TMAs from the previously described cohort of women with ANN breast cancer [30, 33, 34]. The automated BenchMark XT system (Ventana Medical Systems, Inc., Tucson, AZ) was used to perform the IHC staining. The slides were pre-treated with CC1 (Tris-based EDTA buffer, pH 8.0) (Ventana), and incubated with the BRD4 antibody at a 1:300 dilution. Complete pathological report and the level of T-bet+ TILs were available for each tumor in this study.
Immunohistochemically-stained sections were examined for nuclear BRD4 expression, and quantitated using the Allred scoring method [38] by a pathologist with subspecialty training in breast pathology (FT). The score consisted of two components: 1) the average intensity of BRD4 staining (negative: 0; weak: 1; medium: 2; and strong: 3), and 2) the percentage of BRD4-stained nuclei (none: 0; < 1%: 1; 1–10%: 2; 11–33%: 3; 34–66%: 4; and 67–100%: 5). The sum of the two component scores is the overall score with possible values of 0 or 2–8. Due to the lack of validated cut-offs for BRD4 in breast cancer, an arbitrary cut-off score of 6 was decided by assessing nuclear BRD4 expression levels in breast cancer cases that were available in The Human Protein Atlas project.
Statistical analysis
Genes were ranked based on the fold-difference in expression between T-bet+/high and T-bet−/low tumors as determined by SAM (Significance Analysis of Microarrays) moderated t-test. Chi square test and Fisher exact test were used to analyze the BRD4 marker associations with T-bet TILs, Jagged1, clinicopathologic variables, IHC markers (markers used to define intrinsic subtype), and intrinsic subtype. Clinicopathological variables used in the analyses were selected based on previous studies performed in this cohort [33, 34, 37, 39]. The association of DFS with BRD4 and T-bet marker statuses was examined with log rank test and presented as Kaplan-Meier survival curves.
A P value significance criterion of < 0.05 was applied for the tests. Statistical analyses of associations were performed using SAS 9.1 software (SAS Institute, Inc.). Survival curves were plotted using R statistical software, version 2.15.0 (http://r-project.org/).
Results
Association of BRD4 mRNA expression in breast cancer with T-bet+ TILs
The mRNA expression differences associated with T-bet+ TIL status were examined by interrogating gene expression microarray data that consisted of 6 T-bet+/high and 41 T-bet−/low breast tumors (Supplementary Material 1 and 2). The top 100 differentially expressed mRNAs (p < 0.005) were ranked by Significance of Microarray (SAM), and are presented in a heat map (Fig. 1). One of the top differentially expressed genes associated with T-bet+ TILs (Supplementary Material 3) chosen for further study was BRD4 (p = 5.38E-05, FDR = 43.6%), a gene of interest for its potential immune modulatory role in tumors via promotion of NF-kB-mediated inflammation. BRD4 expression in T-bet+/high tumors was up to 44-fold higher than that in T-bet−/low tumors.
Protein expression and localization of tumoral BRD4
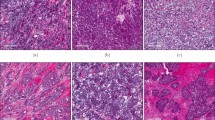
Immunohistochemistry was performed on TMAs to examine the differential protein expression of BRD4 (Fig. 2). Tumoral BRD4 expression that was assigned an Allred score of 6 or higher was considered to be BRD4 positive in this study. Overall, BRD4 positivity was observed in 76.6% of tumors (n = 469/612).
Association between tumoral BRD4, T-bet+ TILs, and Jagged1
A number of studies have indicated BRD4 to be an upstream regulator of Jagged1 – a ligand that has been shown to participate in various signaling pathways with effects on both intrinsic tumorigenic functions and immune functions. Therefore, we have examined Jagged1 mRNA and protein expression that previously had been quantitated by in situ hybridization (ISH) and IHC respectively in the ANN cohort [40]. BRD4 positive tumors were associated with T-bet+ TILs (p = 0.0415) (Table 1), as well as with Jagged1 mRNA (p = 0.0171) (Table 2) and protein (p = 0.0010) (Table 3) expression. Moreover, Jagged1 mRNA-positive tumors were associated with T-bet+ TILs (p = 0.0091) (Table 4).
Tumoral BRD4 expression and clinicopathologic and molecular parameters
Tumors exhibiting high levels of BRD4 expression (BRD4+/high) were more likely to be larger (p = 0.0049), and were associated with pre-menopausal status (p = 0.0018) (Table 5). BRD4+/high tumors were also associated with a high proliferative index as determined by Ki-67 expression (p = 0.0009) (Table 6).
Complete data to generate molecular subtypes was available for 375 tumors (Table 7). Molecular subtypes did not differ significantly between BRD4+/high and BRD4−/low tumors. However, a trend towards an overall difference among the subtypes was observed.
Prognostic relevance of tumoral BRD4 expression in the context of T-bet+ TILs
Disease-free survival (DFS) among all four subgroups (T-bet+/high, BRD4+/high; T-bet+/high, BRD4−/low; T-bet−/low, BRD4+/high; T-bet−/low, BRD4−/low) was analyzed. While the overall difference of DFS among the four groups was not significant, T-bet−/low, BRD4+/high trended towards higher recurrence rate than other groups (log rank test p = 0.0967) (Fig. 3).
Based on this observation, DFS between the T-bet−/low, BRD4+/high group and the combination of other groups was statistically compared, in which patients with T-bet−/low, BRD4+/high tumors were shown to have a significantly a poorer DFS (log rank test p = 0.0165) (Fig. 4). Compared to the other subgroups combined, the T-bet−/low, BRD4+/high group was associated with reduced DFS in univariate analysis (LR test p = 0.0207, RR = 2.55, 95% CI, 1.15–5.62) (Table 8). This association was retained in multivariate analysis that included traditional clinicopathological parameters and HER2 (LR test p = 0.0103, RR = 2.91, 95% CI, 1.29–6.59) (Table 8).
Kaplan-Meier disease-free survival of BRD4+/high, T-bet−/low ANN patients (Red) in comparison to the rest of the subgroups (i.e. T-bet−/low, BRD4−/low; T-bet+/high, BRD4+/high; T-bet+/high, BRD4−/low) (Green): The first number in the parenthesis denotes the number of patients, and the second number denotes the number of recurrences in the corresponding group
Discussion
In this prospectively accrued cohort of women with ANN breast cancer, we examined the relationship between BRD4 and T-bet+ TILs, and evaluated associations of BRD4 expression with Jagged1, clinicopathological features, and clinical outcomes.
We have demonstrated that BRD4 positivity (Allred score of 6 or higher) is significantly associated with T-bet+ TILs, which are a subset of T cells that we have previously determined to be associated with a good outcome in breast cancer patients, despite being associated with adverse clinicopathological features. This suggests a potential link between BRD4-associated tumor progression and the inflammatory lymphocytic infiltrate in breast tumors. BRD4 has been implicated in a number of studies for its role in promoting inflammation [13, 14, 41] notably via activating NF-kB-regulated pathways in cancer cells [17]. NF-kB is a major transcription factor involved in regulating immune and inflammatory responses, and in influencing cancer progression [42, 43]. In particular, NF-kB is crucial in mediating the synthesis of proinflammatory cytokines, such as TNF-α, IL-1, IL-6, and IL-8 [44], which suggests that BRD4 may be an upstream regulator of inflammatory immune response in tumors. Consequently, BRD4 inhibitors, such as JQ1 and I-BET, have been demonstrated to be effective suppressors of inflammation in treating various cancers and inflammatory diseases [13, 14, 41].
Furthermore, BRD4 was associated with pre-menopausal status, large tumor size, and high Ki-67 expression, which are characteristics that are generally associated with a basal subtype. Multiple studies have demonstrated that prognosis of basal breast cancer is positively associated with expression of immune response genes [45,46,47,48]. Although no significant overall difference among intrinsic subtypes was observed between BRD4+/high and BRD4−/low tumors, the association of BRD4 expression with features related to the basal subtype reinforces the idea that the association of BRD4 with immunogenic tumors is potentially through its pro-inflammatory functions.
Women with T-bet−/low, BRD4+/high tumors had worse disease-free survival in comparison to the other women. One explanation may lay in the paradoxical roles of inflammation in cancer that is dependent on the immune composition of the tumor. The poor clinical outcome associated with the BRD4+/high group in the absence of T-bet+ TILs suggests that BRD4 may promote tumor progression through upregulation of chronic inflammatory pathways marked by the production of proinflammatory cytokines such as IL-1α, IL-1β, and IL-6. On the other hand, the relatively favorable outcome that is associated with T-bet+/high tumors despite having high BRD4 expression may indicate a dynamic immune interplay, in which the BRD4-mediated production of proinflammatory cytokines in the presence of tumor-specific T-bet+ TILs may reinforce an anti-tumor immune response. The context-specific role of inflammation in tumor development has been previously demonstrated in mouse models of myeloma and B-cell lymphoma [49]. In the latter study, increased local levels of both proinflammatory cytokines (IL-1α, IL-1β and IL-6) and Th1-associated cytokines (INFγ, IL-2 and IL-12) were shown to be consistently correlated with a successful tumor immune response mounted by tumor-specific CD4+ T cells. Hence, in a T-bet+ TIL-mediated tumor microenvironment, BRD4-mediated NF-kB activation, and subsequent proinflammatory cytokine production may contribute to tumor suppression as the pro-inflammatory cytokines have shown to be important in recruiting circulating leukocytes and activating CD4+ T cell functions.
Another explanation may lay in BRD4’s role in the upregulation of Jagged1 expression [2], which was observed to be associated with BRD4 positivity and T-bet+ TILs in this study. Jagged1 is one of the canonical ligands for the Notch receptor family [50, 51] that serves a multifaceted and highly context-dependent function in regular tissue development and cancer progression. The binding of Jagged1 to Notch1 or Notch3 receptors initiates their activation that involves proteolysis by γ-secretase and release of Notch intracellular domain (NICD). NICD translocates to the nucleus and associates with a transcription complex to regulate expression of target genes. In tumors, the paracrine Jagged1-Notch interaction between cancer cells has been shown to promote proliferation, epithelial-mesenchymal transition, angiogenesis, and metastasis [51]. A recent study demonstrated that BRD4 was the upstream regulator of Jagged1 expression and Notch1 signaling, and played an important role in sustaining breast cancer migration and invasion [2]. In patients, BRD4 and Jagged1 expression has been shown to correlate with the presence of distant metastases [2].
Based on the positive associations observed between BRD4 expression, Jagged1 expression, and T-bet+ TILs, Jagged1, through BRD4 regulation, may also be important in mediating tumor-immune cell interaction. Jagged1-mediated activation of Notch signaling has been shown to promote persistence of immature myeloid cells [52] and immunosuppressive IL-10 production [53], which are characteristics possessed by myeloid-deprived suppressor cells (MDSCs). A recent study by Sierra et al. has shown that humanized anti-Jagged1/2 suppressed tumor growth, decreased the accumulation and tolerogenic activity of MDSCs in tumors, and inhibited the expression of immunosuppressive factors, iNOS and arginase, which in turn, promoted CD8+ T cell infiltration into tumors, and improved the in vivo efficacy of T-cell based immunotherapy [54]. Hence, BRD4+/high tumors in the absence of T-bet+ TILs may exhibit BRD4-mediated upregulation of Jagged1 that may induce Jagged-1-Notch1-mediated accumulation and activation of MDSCs, and suppress the infiltration and anti-tumor activity of T-bet+ T cells.
In the presence of T-bet+ TILs, however, Jagged1 may promote anti-tumoral immune response as its expression has shown to be vital in co-stimulation and regulation of Th1 cells through binding of their cell surface receptor, CD46 (membrane cofactor protein, MCP) [55]. The latter study has shown that disturbance of Jagged1-CD46 crosstalk impeded IFNγ secretion in Th1 cells, and CD4+ T cells from patients with Jagged1 mutation (Alagille Syndrome) or CD46 deficiency failed to mount appropriate Th1 responses in vitro and in vivo. This finding, in addition to the positive association between Jagged1 and T-bet+ TILs observed in this study, suggests that in BRD4+/high, T-bet+/high tumors, BRD4-mediated upregulation of Jagged1 may reinforce the anti-tumoral activity of T-bet+ TILs, and facilitate disease-free survival of patients with breast cancer.
Conclusion
Tumoral BRD4 expression in breast cancer is significantly associated with T-bet+ TILs, clinicopathological features, and a poor disease-free survival in the absence of T-bet+ TILs. On the other hand, the favorable clinical outcome associated with BRD4 expression in tumors with high levels of T-bet+ TILs may reinforce the T-bet+ TIL-driven tumor immune surveillance. The context-specific association of BRD4 expression with disease-free survival based on the presence of T-bet+ TILs suggests that while the anti-inflammatory treatments against cancer, such as BET inhibitors, may be beneficial in reducing chronic inflammation, they may also reduce the tumor-suppressive, T-bet+ TIL-mediated inflammatory immune response. Hence, deeper understanding of BRD4’s immune modulatory roles in different immune contexts may be important in accurately administering BET inhibitors to patients without the risk of dampening the ongoing anti-tumor immune response.
Abbreviations
- ANN:
-
Axillary node-negative
- BET:
-
Bromodomain and extra terminal domain
- BRD4:
-
Bromodomain-containing protein 4
- CK5:
-
Cytokeratin 5
- DFS:
-
Disease-free survival
- EGFR:
-
Epidermal growth factor receptor
- ER:
-
Estrogen receptor
- FFPE:
-
Formalin-fixed, paraffin-embedded
- HER2:
-
Human epidermal growth factor receptor 2
- IFNγ:
-
Interferon-gamma
- IL:
-
Interleukin
- Ki-67:
-
Marker of proliferation Ki-67
- NF-kB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- OS:
-
Overall survival
- SAM:
-
Significance analysis of microarrays
- T-bet:
-
T box transcription factor
- Th1:
-
T helper 1
- TILs:
-
Tumor-infiltrating lymphocytes
- TMAs:
-
Tissue microarrays
- TNFα:
-
Tumor necrosis factor-alpha
References
Filippakopoulos P, Knapp S. Targeting bromodomains: epigenetic readers of lysine acetylation. Nat Rev Drug Discov. 2014;13(5):337–56.
Andrieu G, Tran AH, Strissel KJ, Denis GV. BRD4 regulates breast Cancer dissemination through Jagged1/Notch1 signaling. Cancer Res. 2016;76(22):6555–67.
Dey A, Nishiyama A, Karpova T, McNally J, Ozato K. Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription. Mol Biol Cell. 2009;20(23):4899–909.
Maruyama T, Farina A, Dey A, Cheong J, Bermudez VP, Tamura T, Sciortino S, Shuman J, Hurwitz J, Ozato K. A mammalian bromodomain protein, brd4, interacts with replication factor C and inhibits progression to S phase. Mol Cell Biol. 2002;22(18):6509–20.
Ceribelli M, Kelly PN, Shaffer AL, Wright GW, Xiao W, Yang Y, Mathews Griner LA, Guha R, Shinn P, Keller JM, et al. Blockade of oncogenic IkappaB kinase activity in diffuse large B-cell lymphoma by bromodomain and extraterminal domain protein inhibitors. Proc Natl Acad Sci U S A. 2014;111(31):11365–70.
Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478(7370):524–8.
Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, Robson SC, Chung CW, Hopf C, Savitski MM, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478(7370):529–33.
Shimamura T, Chen Z, Soucheray M, Carretero J, Kikuchi E, Tchaicha JH, Gao Y, Cheng KA, Cohoon TJ, Qi J, et al. Efficacy of BET bromodomain inhibition in Kras-mutant non-small cell lung cancer. Clin Cancer Res. 2013;19(22):6183–92.
Asangani IA, Dommeti VL, Wang X, Malik R, Cieslik M, Yang R, Escara-Wilke J, Wilder-Romans K, Dhanireddy S, Engelke C, et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510(7504):278–82.
Garcia PL, Miller AL, Kreitzburg KM, Council LN, Gamblin TL, Christein JD, Heslin MJ, Arnoletti JP, Richardson JH, Chen D, et al. The BET bromodomain inhibitor JQ1 suppresses growth of pancreatic ductal adenocarcinoma in patient-derived xenograft models. Oncogene. 2016;35(7):833–45.
Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373–80.
Shu S, Lin CY, He HH, Witwicki RM, Tabassum DP, Roberts JM, Janiszewska M, Huh SJ, Liang Y, Ryan J, et al. Response and resistance to BET bromodomain inhibitors in triple-negative breast cancer. Nature. 2016;529(7586):413–7.
Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung CW, Chandwani R, Marazzi I, Wilson P, Coste H, et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468(7327):1119–23.
Oliver SS, Denu JM. Disrupting the reader of histone language. Angew Chem Int Ed Engl. 2011;50(26):5801–3.
Huang B, Yang XD, Zhou MM, Ozato K, Chen LF. Brd4 coactivates transcriptional activation of NF-kappaB via specific binding to acetylated RelA. Mol Cell Biol. 2009;29(5):1375–87.
Xu Y, Vakoc CR. Brd4 is on the move during inflammation. Trends Cell Biol. 2014;24(11):615–6.
Zou Z, Huang B, Wu X, Zhang H, Qi J, Bradner J, Nair S, Chen LF. Brd4 maintains constitutively active NF-kappaB in cancer cells by binding to acetylated RelA. Oncogene. 2014;33(18):2395–404.
Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer. 2013;12:86.
Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12(8):715–23.
Lee AH, Gillett CE, Ryder K, Fentiman IS, Miles DW, Millis RR. Different patterns of inflammation and prognosis in invasive carcinoma of the breast. Histopathology. 2006;48(6):692–701.
Disis ML. Immune regulation of cancer. J Clin Oncol. 2010;28(29):4531–8.
Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8(3):151–60.
Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–13.
Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949–55.
Kohrt HE, Nouri N, Nowels K, Johnson D, Holmes S, Lee PP. Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Med. 2005;2(9):e284.
Rakha EA, Aleskandarany M, El-Sayed ME, Blamey RW, Elston CW, Ellis IO, Lee AH. The prognostic significance of inflammation and medullary histological type in invasive carcinoma of the breast. Eur J Cancer. 2009;45(10):1780–7.
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–7.
John EM, Hopper JL, Beck JC, Knight JA, Neuhausen SL, Senie RT, Ziogas A, Andrulis IL, Anton-Culver H, Boyd N, et al. The breast Cancer family registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res. 2004;6(4):R375–89.
Mulligan AM, Pinnaduwage D, Tchatchou S, Bull SB, Andrulis IL. Validation of Intratumoral T-bet+ lymphoid cells as predictors of disease-free survival in breast Cancer. Cancer Immunol Res. 2016;4(1):41–8.
Mulligan AM, Raitman I, Feeley L, Pinnaduwage D, Nguyen LT, O'Malley FP, Ohashi PS, Andrulis IL. Tumoral lymphocytic infiltration and expression of the chemokine CXCL10 in breast cancers from the Ontario familial breast Cancer registry. Clin Cancer Res. 2013;19(2):336–46.
Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–69.
Szabo CI, King MC. Inherited breast and ovarian cancer. Hum Mol Genet. 1995;4:1811–7.
Andrulis IL, Bull SB, Blackstein ME, Sutherland D, Mak C, Sidlofsky S, Pritzker KP, Hartwick RW, Hanna W, Lickley L, et al. Neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. Toronto breast Cancer study group. J Clin Oncol. 1998;16(4):1340–9.
Mulligan AM, Pinnaduwage D, Bull SB, O'Malley FP, Andrulis IL. Prognostic effect of basal-like breast cancers is time dependent: evidence from tissue microarray studies on a lymph node-negative cohort. Clin Cancer Res. 2008;14(13):4168–74.
Kornegoor R, Verschuur-Maes AH, Buerger H, Hogenes MC, de Bruin PC, Oudejans JJ, van der Groep P, Hinrichs B, van Diest PJ. Molecular subtyping of male breast cancer by immunohistochemistry. Mod Pathol. 2012;25(3):398–404.
Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–7.
Feeley LP, Mulligan AM, Pinnaduwage D, Bull SB, Andrulis IL. Distinguishing luminal breast cancer subtypes by Ki67, progesterone receptor or TP53 status provides prognostic information. Mod Pathol. 2014;27(4):554–61.
Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11(2):155–68.
Bull SB, Ozcelik H, Pinnaduwage D, Blackstein ME, Sutherland DA, Pritchard KI, Tzontcheva AT, Sidlofsky S, Hanna WM, Qizilbash AH, et al. The combination of p53 mutation and neu/erbB-2 amplification is associated with poor survival in node-negative breast cancer. J Clin Oncol. 2004;22(1):86–96.
Reedijk M, Pinnaduwage D, Dickson BC, Mulligan AM, Zhang H, Bull SB, O'Malley FP, Egan SE, Andrulis IL. JAG1 expression is associated with a basal phenotype and recurrence in lymph node-negative breast cancer. Breast Cancer Res Treat. 2008;111(3):439–48.
Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, et al. Selective inhibition of BET bromodomains. Nature. 2010;468(7327):1067–73.
Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25(51):6817–30.
Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2(4):301–10.
Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11.
Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol. 2007;8(8):R157.
Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, van de Vijver MJ. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9(5):R65.
Rody A, Holtrich U, Pusztai L, Liedtke C, Gaetje R, Ruckhaeberle E, Solbach C, Hanker L, Ahr A, Metzler D, et al. T-cell metagene predicts a favorable prognosis in estrogen receptor-negative and HER2-positive breast cancers. Breast Cancer Res. 2009;11(2):R15.
Teschendorff AE, Caldas C. A robust classifier of high predictive value to identify good prognosis patients in ER-negative breast cancer. Breast Cancer Res. 2008;10(4):R73.
Haabeth OA, Lorvik KB, Hammarstrom C, Donaldson IM, Haraldsen G, Bogen B, Corthay A. Inflammation driven by tumor-specific Th1 cells protects against B-cell cancer. Nat Commun. 2011;2:240.
Kopan R, Ilagan MX. The canonical notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137(2):216–33.
Li D, Masiero M, Banham AH, Harris AL. The notch ligand JAGGED1 as a target for anti-tumor therapy. Front Oncol. 2014;4:254.
Cheng P, Nefedova Y, Corzo CA, Gabrilovich DI. Regulation of dendritic-cell differentiation by bone marrow stroma via different notch ligands. Blood. 2007;109(2):507–15.
Bao Y, Wu X, Chen J, Hu X, Zeng F, Cheng J, Jin H, Lin X, Chen LF. Brd4 modulates the innate immune response through Mnk2-eIF4E pathway-dependent translational control of IkappaBalpha. Proc Natl Acad Sci U S A. 2017;114(20):E3993–4001.
Sierra RA, Trillo-Tinoco J, Mohamed E, Yu L, Achyut BR, Arbab A, Bradford JW, Osborne BA, Miele L, Rodriguez PC. Anti-jagged immunotherapy inhibits MDSCs and overcomes tumor-induced tolerance. Cancer Res. 2017;77(20):5628–38.
Le Friec G, Sheppard D, Whiteman P, Karsten CM, Shamoun SA, Laing A, Bugeon L, Dallman MJ, Melchionna T, Chillakuri C, et al. The CD46-Jagged1 interaction is critical for human TH1 immunity. Nat Immunol. 2012;13(12):1213–21.
Acknowledgements
We thank the study participants, Drs. Michael Reedijk and Sean Egan, and the members of the Andrulis lab for helpful discussions.
Funding
This research was supported in part by a grant from the Canadian Institutes of Health Research #MOP-93715 (ILA, SBB), Syd Cooper Program for the Prevention of Cancer Progression (ILA), and The Richard Venn and Carol Mitchell Graduate Studentship in Women’s Health Research 2014–2015 (ML). ILA holds the Anne and Max Tanenbaum Chair in Molecular Medicine at Mount Sinai Hospital and the University of Toronto. The funding agencies were not involved in in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
ILA and SBB were involved in the original study design; ML was involved in molecular analysis; ML, JB, and JMSB were involved in immunochemistry; DP performed the statistical analysis; FT and AMM performed the pathology review; ML, FT, DP, JB, JMSB, AMM, SBB and ILA were involved in the manuscript preparation. All of the authors contributed to the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from all study participants. The study was approved by the Research Ethics Board of Mount Sinai Hospital, Toronto, ON, Canada (#01–0313-U), and the University Health Network, Toronto, ON, Canada (#02–0881-C). Specific consent for retrieving the specimen TMA blocks was obtained as part of the previous studies involving the use of this cohort.
Consent for publication
Not applicable.
Competing interests
Lee, and Drs. Tayyari, Pinnaduwage, Bayani, Bartlett, Mulligan, Bull, Andrulis have no competing interests to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lee, M., Tayyari, F., Pinnaduwage, D. et al. Tumoral BRD4 expression in lymph node-negative breast cancer: association with T-bet+ tumor-infiltrating lymphocytes and disease-free survival. BMC Cancer 18, 750 (2018). https://doi.org/10.1186/s12885-018-4653-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-018-4653-6