Abstract
Background
Indole-3-carbinol, derived from Cruciferous vegetables is an estrogen receptor antagonist considered a preventive agent that is naturally present in diet. There are no previous studies on its effects in human inflammatory breast cancer or canine inflammatory mammary cancer that is the most aggressive type of breast cancer.
Methods
The aim of this study was to analyze the effect of indole-3-carbinol on a SCID mice xenograft model of canine inflammatory mammary cancer, using equivalent human oral dose as a preventive therapy in humans for 3 weeks.
Results
Indole-3-carbinol treatment decreased tumor proliferation and increased apoptosis, although tumor embolization and liver metastasis were observed in some animals. There was a characteristic subpopulation of lipid-rich cells and increased contents of select steroid hormones in tumor homogenates and serum.
Conclusions
Our data reveal for the first time that the ingestion of indole-3-carbinol, as administered, diminishes proliferation and increases apoptosis of tumor cells in an experimental model of inflammatory breast cancer, although this effect could not be enough to avoid the appearance of tumor embolization and metastasis. Future clinical trials will be needed to clarify the usefulness of indole-3-carbinol in this cancer and to understand the molecular mechanisms involved.
Similar content being viewed by others
Background
The relationship between cancer and nutrition has been largely studied; epidemiologic studies indicate that consumption of vegetables containing dietary phytochemicals reduces the risk of developing cancer [1]. Dietary phytochemicals are a wide variety of biologically active compounds found in plants that contain anti-tumor and anti-inflammatory properties [2,3,4,5]. Many of these substances block tumor growth and inhibit metastasis in animal models [6, 7]. Studies on synergistic effects of different phytochemicals might contribute to establish potential chemopreventive strategies [8, 9]. Indole-3-carbinol (I3C), a natural phytochemical found in cruciferous vegetables (i.e. broccoli, cabbage or cauliflower), is considered a potential anticancer agent that prevents the development of certain types of tumors by activating tumor suppressor genes, genes involved in apoptosis and detoxification [2, 10,11,12,13,14]. Some in vitro and in vivo studies in breast cancer [15,16,17,18,19,20] and other neoplasia types such as colorectal cancer, prostate cancer, ovarian cancer, cervix carcinoma and hepatocarcinoma indicated that I3C suppresses cell proliferation and induces apoptosis [17, 21,22,23,24,25].
Several in vitro studies with breast cancer cells showed that I3C acts by blocking estrogen receptors among other mechanisms [10, 17, 26]. Thus, some authors showed that I3C might be useful as supplement of tamoxifen preventing or treating estrogen-dependent tumors [27, 28]. There are no data on the effect of I3C in human inflammatory breast cancer (IBC) or in spontaneous and experimental canine inflammatory mammary cancer (IMC). Inflammatory breast cancer and IMC are considered as a special clinic-pathological entity and the most malignant type of breast cancer both in humans and dogs, with a fulminant clinical course and an extremely poor survival rate [29,30,31,32,33,34]. Inflammatory breast cancer has been proposed as a natural model to study the human disease [29, 35, 36]. Our group has published the establishment and validation of a xenograft model of canine IMC in mice [37]. Spontaneous and experimental canine IMC have been associated with high levels of steroid hormones in tumor homogenates, suggesting a potential autocrine/paracrine secretion [37,38,39,40,41].
The aim of this study was to analyze the hypothetical antitumor effect of I3C administration on a SCID mouse xenograft model of canine inflammatory mammary cancer using an equivalent dose to that used as a preventive anti-cancer agent in humans.
Methods
Animals and xenograft establishment
The protocol was approved by the Committee of the Universidad Complutense of Madrid, and Animal Protection Area of the Community of Madrid, Spain (Ref. Protocol Number: PROEX 31/15). Twenty-four non-ovariectomized female SCID mice (BALB/cJHan®Hsd-Prkdcscid, Harlan Laboratories Models, S. L.), 6–8 weeks of age and weighing between 20 and 22 g were used. The animals were housed in a flexible-film isolator (Racks IVC de Allentown Inc. Panlab Harvard Apparatus) in cages (2–3 animals per cage), each measuring 330 cm2 × 12 cm, in a room with controlled environmental conditions (20 °C to 22 °C; 50 to 55% relative humidity; 10 to 15 air changes per hour; and a 12:12 h light: dark cycle). Pre-sterilized food and water were provided ad libitum. All experimental procedures were performed between 11:30 a.m. and 12:30 a.m. The method of euthanasia used to sacrifice the mice was isoflurane flow rate adjusted to 5% until one minute after breathing stops. Then, cervical dislocation was applied as method of confirmation of euthanasia.
The xenograft was directly established from a 9-year-old female dog with a spontaneous inflammatory mammary carcinoma, following a protocol previously established [37] as follows: Fragments (3 mm × 2 mm of diameter) from canine primary mammary tumor obtained at necropsy were immediately placed in MEM (Minimum Essential Medium) with Earle’s Salts, L-Glutamine and Penicillin/Streptomycin [100×] (PAA Cell Culture Company, BioPath Stores, Cambridge) before they were subcutaneously implanted into the ventral side of 3 female SCID mice. The mice were previously anesthetized with isoflurane (IsoVet 1000 mg/g, B Braun VetCare SA, Barcelona, Spain) at 4% for induction and at 1.5% for maintaining anesthesia. Isoflurane was supplied in a fresh gas flow rate of 0.5 l of oxygen/minute. The mice were monitored for tumor growth and, whenever palpable, the xenograft tumor volumes were measured twice weekly with a caliper-like instrument throughout the experiment, and the tumor size was estimated using the following formula: (L × W2)/2, where W = width and L = length. When the implanted tumors reached approximately 1.0 cm3, they were successively transplanted into three SCID mice (second passage) and from each of these animals to three new animals (third passage, n = 30), in order to verify that the xenograft model was stable and that these tumors did not present histopathological modifications between the consecutive passages. The engraftment efficiency of the second and third passages was 90% (n = 24).
Treatment groups
Animals of third passage were randomly assigned to an I3C treatment/control group (n = 12/12). All mice from the two groups were treated by oral gavage: the control group, administered with 200 μl of distilled water/polyethylene glycol (Panreac Quim. S.A, Barcelona, Spain) (6:4 ratio, n = 12/24) and the I3C group (administered with 200 μl of distilled water/polyethylene glycol containing 150 mg I3C/kg/day) (Indole-3-carbinol, Sigma-Aldrich Co., Madrid, Spain), (n = 12/24). The dose was chosen from previous studies [42, 43]. Mice were given the doses through an oral feeding tube (18G x ½”) for 3 weeks (the first dose was given 7 days after the xenografts, when tumors were palpable), following a dosage of 5 consecutive days of administration and 2 days of rest. The operator was blinded to the treatment group assignment of each animal at all times.
Histopathology and immunohistochemistry
Fragments from the treated tumors were fixed in neutral formalin and then embedded in paraffin for tumor histopathology. The samples were histologically diagnosed on HE-stained sections following the histological classification of canine mammary tumors [44]. Histology and immunohistochemistry of samples were evaluated by an experienced veterinary pathologist.
Immunohistochemistry for Ki-67, caspase-3 and estrogen receptor (ER) was performed on deparaffinised sections and using the streptavidin–biotin–complex peroxidase method or detection kits. High-temperature antigen retrieval with 10 mM citrate buffer pH = 6.0 was performed. Table 1 shows the primary antibodies and developing systems used. In the case of caspase-3, the slides were incubated with streptavidin-HRP conjugated anti-caspase-3 antibody (TermoFisher Scientific ref. 43–4323, dilution 1/4000) for 30 min at room temperature (RT) and developed with a chromogen solution containing 3, 3′-diaminobenzidine tetrachloride (DAB). Thereafter, the slides were counterstained with hematoxylin. Washes and dilutions were made in Tris-buffered saline (pH = 7.4) for each marker; corresponding positive and negative control slides were performed.
Tumor proliferation index (PI) was determined by counting Ki-67 positive and negative nuclei in 8–10 selected High Power Fields (HPF) with the highest percentage of labeling (minimum 1000 cells). Every immunostained nucleus was considered positive regardless of the intensity of stain. The PI or proportion of positive neoplastic cells in each sample was calculated as previously described [44].
Caspase-3 immunostaining was performed to analyze the presence of apoptotic bodies in the xenograft tissue and it was semi-quantitatively assessed by the intensity of immunoexpression, which was evaluated as low (+), moderate (++), or intense (+++), by the percentage of positive cells (apoptotic index).
Finally, the expression of ER was considered positive when more than 10% of the cells within the tumors were positive independently of the intensity of the staining [29].
Steroid hormone concentrations in serum samples and tumor homogenates
At the time of necropsy of the control and experimental SCID mice, 1 ml of blood was obtained by cardiac puncture using a 25G needle from each mouse. Blood samples were collected in special tubes (microtube Serum-Gel Clotting Activator, Sarstedt, Nümbrecht, Germany) and spun down in a refrigerated centrifuge (Hettich Zentrifugen Universal 320 R, Germany) at 4 °C and a speed of 1200 x g (RCF) for 20 min. Serum was harvested and stored at − 20 °C until assayed for the presence of hormones. Frozen tissue specimens of approximately 0.1–0.5 g from individual mice were homogenized in 5 ml of PBS (pH = 7.2) and the homogenate centrifuged at 1200 x g, for 20 min at 4 °C. The supernatants were harvested and stored at − 80 °C until hormone assays [45].
Levels of estrone sulphate (E1SO4), estradiol (E2), androstenedione (A4), testosterone (T), dehydroepiandrosterone (DHEA) and progesterone (P4) in tumor homogenates and serum samples were assayed by amplified Enzyme-Immunoassay (EIA) previously validated in our laboratory [45].
Statistical analysis
Statistical analyses were performed by the IBM SPSS Statistics, 19.0 for Windows (Chicago, IL, USA). The relationship between continuous variables (plasma and tumoral steroid hormone levels, Ki-67 and caspase index) and categorical variables (pathological parameters such as ulceration, sebaceous hyperplasia, dermal emboli, tumor emboli, degenerated emboli, thrombosis, lipid-rich subpopulation, distant metastases, liver metastasis and estrogen receptor alpha) was established using the analysis of variance (ANOVA) followed by appropriate post hoc tests for similar variances (Duncan Test) or different ones (Games-Howell test). The relationship between continuous variables was assessed using the Pearson correlation. The association between categorical variables was analyzed using Pearson’s chi-squared test (χ2). Differences were considered significant at p-value < 0.05.
Results
Macroscopic growth and metastases
A stable serial transplantable xenograft was successfully established with a constant and rapid growth in all mice. At the end of the experiment, in 4 weeks, the tumors had an approximate size of 0.9–1.2 cm3. The size and weight of the xenograft tumors in I3C treated and untreated mice (controls) did not show significant differences (p > 0.05).
Immediately after sacrifice, complete necropsy was performed in each case. Common target organs for IMC such as bone, lung, liver, brain or distant lymph nodes were extensively examined. At necropsy, one xenotrasplanted control mouse presented pulmonary and mesenteric metastases (1/12, 8.3% of the control group) and one out of 12 (8.3%) I3C treated xenografts had pulmonary and mesenteric metastases. Four animals in the I3C group showed metastasis in the liver (4/12, 33.3%, p = 0.028). All metastases were histologically confirmed.
Histopathological findings
Primary canine IMC and control xenografts
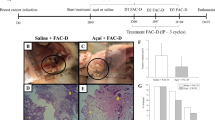
According to the clinical characteristics and the histological invasion of dermal lymphatic vessels by tumor/neoplastic emboli (Fig. 1a), the primary canine mammary tumor was diagnosed as an inflammatory mammary cancer originated by a highly undifferentiated anaplastic/solid mammary carcinoma grade III with scattered lipid-rich cells. Control xenografts reproduced the histological features of the primary canine mammary tumor. Hence, emboli at peripheral lymphatic and blood vessels, as well as inside the tumor were observed (2/12, 16.7%) (Fig. 1b). The control xenografts were highly infiltrative in the dermis and striated muscle and were partially surrounded by a fibro-myxoid tissue. Neoplastic cells displayed a solid pattern together with areas of isolated cells; the stroma was scant. Scattered tumor cells showed a lipid-rich cytoplasm. The neoplastic cells exhibited a high pleomorphism with marked cell and nuclear atypia, and an elevated mitotic index (anaplastic cells). Binucleated and multinucleated cells, as well as atypical mitoses were frequently found. Large intratumor areas of necrosis, dermal lymphangiectasias, and severe edema in the dermis were also present.
Representative histological features. a IMC with neoplastic emboli in superficial dermal lymphatic vessels and infiltration of carcinomatous cells in the female dog origin of the xenografts. b IMC with tumor emboli in SCID mouse (BALB/cJHan®Hsd-Prkdcscid) xenograft control group. c IMC with tumor emboli in dermis in SCID mouse xenograft I3C group (p = 0.012, compared with control group), and non-dermal tumor emboli in I3C mice (p = 0.035). d IMC with abundant lipid-rich cells in SCID mouse xenograft I3C group (× 20), (× 40) (p = 0.001). e IMC with liver metastasis in SCID mouse xenograft I3C group (× 2), (× 20) (p = 0.028). f and g IMC showing caspase-3 positive immunolabeling in a low number of cells in SCID mouse xenograft control group versus I3C group where positive immunolabeling in numerous cells (× 4), (× 20) (p < 0.001). h IMC showing positive Ki-67 immunolabeling in numerous cells in xenograft control group, and i lower number of Ki-67 positive cells in the I3C group SCID mouse xenograft (× 20) (p < 0.001). Analysis of variance followed by appropriate post hoc tests for similar variances (Duncan Test) or different ones (Games Howell test) was used. IMC inflammatory mammary carcinoma, I3C indole-3-carbinol
Histological and immunohistochemical findings in the I3C treated xenografts
Histological and immunohistochemical findings of both experimental groups are depicted in Table 2. Indole-3-carbinol treatment diminished proliferation and increased apoptosis of tumor cells although embolization of neoplastic cells (7/12), liver metastasis and presence of tumor cells showing a lipid-rich cytoplasm (8/12) (Figs. 1c-i) together with hyperplasia of dermal sebaceous glands (10/12) were also observed in some animals. This lipid-rich cell subpopulation frequently presented degeneration (8/12) and necrosis.
Primary canine tumor and all xenografts samples were negative for ER immunostaining. The positive control was canine uterus.
Determination of steroid hormones
The content of steroid hormones in serum and tumor homogenates of both control and I3C xenograft groups are shown in Table 3. Significantly higher levels of estrone sulphate (p = 0.009), estradiol (p < 0.001) and androstenedione (p = 0.049) and lower level of testosterone (p = 0.048) in I3C xenograft homogenates compared with the control group were found. Serum progesterone (p = 0.003) and testosterone levels (p = 0.022) were also higher in I3C group.
Discussion
Several experimental studies have shown that I3C possesses preventive anti-cancer [15, 17, 22] and disrupting estrogen signalling properties [10, 46,47,48]. Indole-3-carbinol is considered a potential agent in the prevention and treatment of hormone-dependent breast tumors [2, 21]. Thus, it is freely available in the stores and supermarkets as a dietary phytochemical (non as an approved pharmaceutical drug) for preventing cancer, diminishing premenstrual syndrome and perimenopause-related disturbances. Nevertheless, the effect of I3C on patients with neoplasms, especially breast cancer is poorly documented [20, 49]. To the best of our knowledge, this is the first study regarding the effects of I3C treatment on experimental or spontaneous IMC. Both IBC and canine IMC are considered a special and very aggressive type of breast cancer due to its particular/unique biological, molecular, pathological, genetic and clinical signature features [30, 32,33,34]. In the present study, I3C treatment on a xenograft model of canine IMC reduced tumor growth and increased apoptosis, although metastasis and alterations in the peripheral levels of steroid hormones were also observed in some animals.
Tumor proliferation index has been associated with poor prognosis in human [49] and canine mammary cancer [50]. Our xenografts from the I3C group had lower proliferation index compared with control group, indicating a loss of proliferative capacity after I3C administration. In accordance with our results, several reports using breast cancer cell lines [3, 18, 19, 51, 52] and human breast cancer cell-derived tumor xenografts, have reported an antiproliferative effect of I3C [20, 49]. The antiproliferative property of I3C has been associated to changes in cell signaling, specially disrupting estrogen responsiveness [19, 22, 47, 48]. Indole-3-carbinol could have also a role in the induction of specific carcinogen detoxifying enzymes, such as CYP1A [3, 17, 53], and in cell adhesion, dissemination and invasion of human breast cancer cells [2].
In the present study, the lack of ulceration and increased apoptosis in treated I3C tumors is in accordance with a reduction of tumor growth and with other previous in vitro and in vivo studies in human breast cancer [22, 51, 53, 54], probably through pro-apoptotic and anti-proliferative mechanisms [12, 19, 55, 56]. In addition, it has been reported that I3C promotes apoptosis of breast cancer cells by activating caspase-3 and -9, among other mechanisms [4, 22, 54]. Accordingly, our study showed a high expression of caspase-3 in I3C treated xenografts. Both IBC and IMC are typically angiogenic, lymphangiogenic and lymphangiocentric, being the presence of massive neoplastic emboli in dermal vessels, their main histological feature [29, 35, 57, 58]. The presence of dermal neoplastic emboli in experimental mice IBC/IMC xenotransplant models has been documented but it is not seen in all the samples [37, 57,58,59,60,61]. It is known that the presence of emboli within the dermal lymphatic vessels in IMC, contributes to the rapid development of metastasis [62,63,64], and is responsible for the morbidity of this disease. In spite of the antiproliferative and apoptosic effect, emboli in dermal lymphatic vessels and liver metastasis were observed in some animals. Therefore, the effect of I3C could not be enough to avoid the appearance of tumor embolization and metastasis.
The appearance of metastases indicates that this biomodel is appropriate for the study of metastatic tumors in general and inflammatory mammary cancer in particular, since both IBC and IMC are very aggressive tumors with an extremely high metastatic potential. The capacity of I3C to inhibit cell adhesion, migration and invasion of non-inflammatory ER- breast cancer cell has been previously reported [2]. However, in this study, metastases were observed in some animals. A potential limitation of the current study is the scarce number of animals used. More studies should be performed to evaluate the capacity of I3C to inhibit the appearance of metastasis in this cancer.
The presence of thrombosis and some necrotic/degenerated neoplastic cells inside the emboli has been previously documented in IMC xenografts and suggests a partial destruction of metastatic cells, reducing the possibility of establishing distant metastasis [37]. In our study, only the control group showed thrombosis, which could suggest a reduced defense mechanism in I3C treated IMC xenografts. The possible host defense mechanisms in these xenografts remain to be elucidated.
The effect of I3C treatment on estrogens metabolism has been previously indicated [10, 27, 28, 53, 65]; nevertheless, to the best of our knowledge, this is the first time that the possible effects of this indole on other steroid hormones and general steroidogenesis have been studied. According to previous studies, the alteration of estrogen metabolism by I3C can be mediated by cytochrome P450 [66, 67] or the activation of aryl hydrocarbon receptor (AhR)-mediated pathways [68,69,70].
Histopathological evidence of lipid droplets in neoplastic cells has been described in spontaneous and experimental canine IMC, and it has been shown that could be due to steroid secretion [29, 37, 40]. The frequent presence of scattered lipid-rich cells in our murine model has been previously described in another IMC xenograft model [37]. In the present study I3C treatment increased significantly the number of intracellular lipid droplets that appeared forming large areas of lipid-rich cells, which were not present in the control group tumors. According to previous studies, these lipid-rich cells could contain large amounts of steroids that could be locally secreted [37, 38, 40]. The steroid secretion by IBC and IMC cell lines has been recently indicated [71]. The presence of large areas containing a lipid-rich cell subpopulation is in accordance with previous observations and explains the high amounts of steroids found in tumor homogenates of I3C treated xenografts of the present study. According to our results, several steroid hormones (estradiol, estrone sulphate and androstenedione) were significantly increased in I3C treated tumor homogenates while testosterone was diminished. Interestingly, testosterone was significantly increased in the sera of I3C treated mice. Further studies should elucidate if these changes in steroid hormone content are related to a major production of estrogens in situ (via aromatase or sulphatase enzymes).
The mechanisms causing hormonal variations after I3C administration are uncertain. The documented anti-estrogen effect of I3C through ER should be questioned in this particular case, since the model is ERα negative [3, 28, 46, 65]. As pointed out before, I3C can alter the estrogen metabolism through other ER-independent pathways [15, 27, 47, 53]. The role of other receptors such as ERβ should be considered and elucidated in future studies. The relevance of ERβ in the pathogenesis of IMC has been suggested previously [39]. Our study suggests that both E1SO4 and E2 can be locally synthesized, especially after I3C administration. Following the aromatase or sulphatase pathways, androgens (especially testosterone that is diminished in tissue) could be transformed in high tissue E1SO4 and E2 concentrations. Estrone sulphate could be a reservoir for the synthesis of active estrogens in the mammary tumors, including the canine IMC [39, 40, 72]. Previous studies indicated that I3C effect on breast cancer cells favors estradiol 2-hydroxylation [46, 73]. Although there are no previous studies on intratumoral E2 levels in I3C treated cells, such the activation of estradiol 2-hydroxylation should induce a low level of E2 [19, 74] but an increase of E2 and E1SO4 concentrations in tumor homogenates was observed. This particular finding has to be compared in non-inflammatory breast cancer xenografts to elucidate if the high amounts of E2 found in the present study are due to the particular characteristics of IMC. In addition to our results refer to intratumoral levels, as stated above.
The higher concentration of steroids and longer half-life suggests the formation of biologically active estrogens in tumor tissue [40]. In order to proliferate, it is possible that tumor increased estradiol levels from the E1SO4 reservoir. In our results, serum P4 concentrations increased in I3C group, probably due to a lower expression of progesterone receptors by I3C, and consequently, higher serum P4 levels [10, 65, 75]. As occurs with estrogens and progesterone a local synthesis of androgens by IBC and IMC tumor cell lines, has also been confirmed [71]. The role of androgens in breast cancer is not clear. Some studies suggest an antiproliferative effect [76], but others note an increased proliferation in breast cancer cell lines [77, 78]. The decrease of testosterone levels in I3C-treated group could be due to the inhibitory effect of I3C on the androgen receptors transcription [66]. Similar to E2, a higher content of intratumoral A4 in I3C group could be due to an increased local synthesis and to the binding of I3C to androgen receptors, increasing A4 free levels in tissue homogenates. However, intratumoral T concentrations decreased in I3C group when compared with control group, probably by the I3C-associated stimulatory effect on the aromatase enzyme [79] which stimulates T to E2 conversion.
Conclusions
Our data reveal for the first time that the ingestion of indole-3-carbinol, as administered, diminishes proliferation and increases apoptosis of tumor cells in an experimental model of inflammatory breast cancer, although this effect could not be enough to avoid the appearance of tumor embolization and metastasis. Additionally, stimulated steroid hormones levels and a characteristic lipid-rich cell population were presented. These results could be attributable to the special characteristics of this particular cancer. Future clinical trials and studies will be needed to clarify the usefulness of indole-3-carbinol in this cancer and to understand the molecular mechanisms involved.
Abbreviations
- A4:
-
Androstenedione
- AhR:
-
Aryl hydrocarbon receptor
- DAB:
-
3, 3′-diaminobenzidine tetrachloride
- DHEA:
-
Dehydroepiandrosterone
- E1SO4 :
-
Estrone sulphate
- E2:
-
Estradiol
- EIA:
-
Enzyme-Immunoassay
- ER:
-
Estrogen receptor
- HPF:
-
High Power Fields
- I3C:
-
Indole-3-carbinol
- IBC:
-
Inflammatory breast cancer
- IMC:
-
Canine inflammatory mammary cancer
- MEM:
-
Minimum Essential Medium
- P4:
-
Progesterone
- PI:
-
Proliferation index
- RCF:
-
Relative Centrifugal Force or G-force
- RT:
-
Room Temperature
- T:
-
Testosterone
References
McCullough ML, Giovannucci EL. Diet and cancer prevention. Oncogene. 2004;23:6349–64.
Meng Q, Qi M, Chen DZ, Yuan R, Goldberg ID, Rosen EM, et al. Suppression of breast cancer invasion and migration mediated by indole-3-carbinol: associated with up-regulation of BRCA1 and Ecadherin/ catenin complexes. J Mol Med. 2000;78:155–65.
Meng Q, Goldberg ID, Rosen EM, Fan S. Inhibitory effects of indole-3-carbinol on invasion and migration in human breast cancer cells. Breast Cancer Res Treat. 2000;63:147–52.
Rahman KM, Aranha O, Glazyrin A, Chinni SR, Sarkar FH. Translocation of Bax to mitochondria induces apoptotic cell death in indole-3-carbinol (I3C) treated breast cancer cells. Oncogene. 2000;19:5764–71.
Chinni SR, Li YW, Upadhyay S, Koppolu PK, Sarkar FH. Indole3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene. 2001;20:2927–36.
Bradford PG, Awad AB. Phytosterols as anticancer compounds. Mol Nutr Food Res. 2007;51:161–70.
Meadows GG. Diet, nutrients, phytochemicals, and cancer metastasis suppressor genes. Cancer Metastasis Rev. 2012;31:441–54.
Nakamura Y, Yogosawa S, Izutani Y, Watanabe H, Otsuji E, Sakai T. A combination of indol-3-carbinol and genistein synergistically induces apoptosis in human colon cancer HT-29 cells by inhibiting Akt phosphorylation and progression of autophagy. Mol Cancer. 2009;8:100.
de Santi M, Galluzzi L, Duranti A, Magnani M, Brandi G. The Indole-3-carbinol cyclic tetrameric derivative CTet synergizes with cisplatin and doxorubicin in triple-negative breast cancer cell lines. Anticancer Res. 2013;33:1867–72.
Meng Q, Yuan F, Goldberg ID, Rosen EM, Auborn K, Fan S. Indole-3-carbinol is a negative regulator of estrogen receptor-a signaling in human tumor cells. J Nutr. 2000;130:2927–31.
Carter TH, Liu K, Jr RW, Chen D, Qi M, Fan S, et al. Diindolylmethane alters gene expression in human keratinocytes. J Nutr. 2002;132:3314–24.
Chinni SR, Sarkar FH. Akt inactivation is a key event in indole-3-carbinol-induced apoptosis in PC-3 cells. Clin Cancer Res. 2002;8:1228–36.
Rahman KW, Li Y, Wang Z, Sarkar SH, Sarkar FH. Gene expression profiling revealed survivin as a target of 3,3′-diindolylmethane-induced cell growth inhibition and apoptosis in breast cancer cells. Cancer Res. 2006;66:4952–60.
Fan S, Meng Q, Auborn K, Carter T, Rosen EM. BRCA1 and BRCA2 as molecular targets for phytochemicals indole-3-carbinol and genistein in breast and prostate cancer cells. Brit J Cancer. 2006;94:407–26.
Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4:1201–15.
Rahman KM, Sarkar FH, Banerjee S, Wang Z, Liao DJ, Hong X, et al. Therapeutic intervention of experimental breast cancer bone metastasis by indole-3-carbinol in SCID-human mouse model. Mol Cancer Ther. 2006;5:2747–56.
Weng JR, Tsai CH, Kulp SK, Chen CS. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. 2008;262:153–63.
Moiseeva EP, Heukers R, Manson MM. EGFR and Src are involved in indole-3- carbinol-induced death and cell cycle arrest of human breast cancer cells. Carcinogenesis. 2007;28:435–45.
Marconett CN, Sundar SN, Tseng M, Tin AS, Tran KQ, Mahuron KM, et al. Indole-3-carbinol downregulation of telomerase gene expression requires the inhibition of estrogen receptor-alpha and Sp1 transcription factor interactions within the hTERT promoter and mediates the G1 cell cycle arrest of human breast cancer cells. Carcinogenesis. 2011;32:1315–23.
Aronchik I, Bjeldanes LF, Firestone GL. Direct inhibition of elastase activity by indole-3-carbinol triggers a CD40-TRAF regulatory cascade that disrupts NF-κB transcriptional activity in human breast cancer cells. Cancer Res. 2010;70:4961–71.
Bonnesen C, Eggleston IM, Hayes JD. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001;61:6120–30.
Ahmad A, Sakr WA, Rahman KM. Anticancer properties of indole compounds: mechanism of apoptosis induction and role in chemotherapy. Curr Drug Targets. 2010;11:652–66.
Taylor-Harding B, Agadjanian H, Nassanian H, Kwon S, Guo X, Miller C, et al. Indole-3-carbinol synergistically sensitises ovarian cancer cells to bortezomib treatment. Brit J Cancer. 2012;106:333–43.
Rahman KW, Sarkar FH. Inhibition of nuclear translocation of nuclear factor-kappaB contributes to 3,3′-diindolylmethane-induced apoptosis in breast cancer cells. Cancer Res. 2005;65:364–71.
Rahman KW, Banerjee S, Ali S, Ahmad A, Wang Z, Kong D, et al. 3,3′-diindolylmethane enhances taxotere-induced apoptosis in hormonerefractory prostate cancer cells through survivin downregulation. Cancer Res. 2009;69:4468–75.
Firestone GL, Bjeldanes LF. Indole-3-carbinol and 3-3′-diindolylmethane antiproliferative signaling pathways control cell-cycle gene transcription in human breast cancer cells by regulating promoter-Sp1 transcription factor interactions. J Nutr 2003;133 Suppl 7:2448S–2455S.
Cover CM, Hsieh SJ, Tran SH, Hallden G, Kim GS, Bjeldanes LF, et al. Indole-3-carbinol inhibits the expression of cyclin-dependent kinase-6 and induces a G1 cell cycle arrest of human breast cancer cells independent of estrogen receptor signaling. J Biol Chem. 1998;273:3838–47.
Gao X, Petroff BK, Oluola O, Georg G, Terranova PF, Rozman KK. Endocrine disruption by indole-3-carbinol and tamoxifen: blockage of ovulation. Toxicol Appl Pharm. 2002;183:179–88.
Peña L, Perez-Alenza MD, Rodriguez-Bertos A, Nieto A. Canine inflammatory mammary carcinoma: histopathology, immunohistochemistry and clinical implications of 21 cases. Breast Cancer Res Treat. 2003;78:141–8.
Pérez-Alenza MD, Tabanera E, Peña L. Inflammatory mammary carcinoma in dogs: 33 cases (1995-1999). J Am Vet Med Assoc. 2001;219:1110–4.
Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst. 2005;97:966–75.
Walshe JM, Swain SM. Clinical aspects of inflammatory breast cancer. Breast Dis. 2005-2006;22:35–44.
Levine PH, Veneroso C. The epidemiology of inflammatory breast cancer. Semin Oncol. 2008;35:11–6.
Woodward WA. Inflammatory breast cancer: unique biological and therapeutic considerations. Lancet Oncol. 2015;16:e568–76.
Clemente M, Sánchez-Archidona AR, Sardón D, Díez L, Martín-Ruiz A, Cáceres S, al e. Different role of COX-2 and angiogenesis in canine inflammatory and non-inflammatory mammary cancer. Vet J. 2013;197:427–32.
de Andrés PJ, Illera JC, Cáceres S, Díez L, Pérez-Alenza MD, Peña L. Increased levels of interleukins 8 and 10 as findings of canine inflammatory mammary cancer. Vet Immunol Immunopathol. 2013;152:245–51.
Camacho L, Peña L, González Gil A, Cáceres S, Díez L, Illera JC. Establishment and characterization of a canine xenograft model of inflammatory mammary carcinoma. Res Vet Sci. 2013;95:1068–75.
Peña L, Silván G, Pérez-Alenza MD, Nieto A, Illera JC. Steroide hormone profile of canine mammary carcinoma: a preliminary study. J Steroid Biochem Mol Biol. 2003;84:211–6.
Illera JC, Pérez-Alenza MD, Nieto A, Jiménez MA, Silván G, Dunner S, et al. Steroids and receptor in canine mammary cancer. Steroids. 2006;71:541–8.
Sánchez-Archidona AR, Jiménez MA, Pérez-Alenza D, Silván G, Illera JC, et al. Steroid pathway and oestrone sulphate production in canine inflammatory mammary carcinoma. J Steroid Biochem Mol Biol. 2007;104:93–9.
Queiroga FL, Pérez-Alenza MD, Silván G, Peña L, Lopes CS, Illera JC. Crosstalk between GH/IGF-I axis and steroid hormones (progesterone, 17beta-estradiol) in canine mammary tumors. J Steroid Biochem Mol Biol. 2008;110:76–82.
Exon JH, South EH. Dietary indole-3-carbinol alters immune functions in rats. J Toxicol Environ Health A. 2000;59:271–9.
Rogan EG. The natural chemopreventive compound indole-3-carbinol: state of the science. In Vivo. 2006;20(2):221–8.
Goldschmidt M, Peña L, Rasotto R, Zappulli V. Classification and grading of canine mammary tumors. Vet Pathol. 2011;48:117–31.
Illera JC, Silván G, Munro CJ, Lorenzo PL, Illera MJ, Liu IKM, et al. Amplified androstenedione enzymeimmunoassay for the diagnosis of cryptorchidism in the male horse: comparison with testosterone and estrone sulphate methods. J Steroid Biochem Mol Biol. 2003;84:377–82.
Ashok BT, Chen YG, Liu X, Garikapaty VP, Seplowitz R, Tschorn J, et al. Multiple molecular targets of indole-3-carbinol, a chemopreventive anti-estrogen in breast cancer. Eur J Cancer Prev. 2002;11(Suppl 2):S86–93.
Firestone GL, Sundar SN. Minireview: modulation of hormone receptor signaling by dietary anticancer indoles. Mol Endocrinol. 2009;23:1940–7.
Marconett CN, Singhal AK, Sundar SN, Firestone GL. Indole-3-carbinol disrupts estrogen receptor-alpha dependent expression of insulin-like growth factor-1 receptor and insulin receptor substrate-1 and proliferation of human breast cancer cells. Mol Cell Endocrinol. 2012;363:74–84.
Tin AS, Park AH, Sundar SN, Firestone GL. Essential role of the cancer stem/progenitor cell marker nucleostemin for indole-3-carbinol anti-proliferative responsiveness in human breast cancer cells. BMC Biol. 2014;12:72.
Peña L, Nieto A, Pérez-Alenza MD, Cuesta P, Castaño M. Immunohistochemical detection of Ki-67 and PCNA in canine mammary tumors: relationship to clinical and pathological variables. J Vet Diagn Investig. 1998;10:237–46.
Stuart-Harris R, Caldas C, Pinder SE, Pharoah P. Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 95 studies in 32,825 patients. Breast. 2008;17:323–34.
Hong C, Firestone GL, Bjeldanes LF. Bcl-2 family-mediated apoptotic effects of 3,3′-diindolylmethane (DIM) in human breast cancer cells. Biochem Pharmacol. 2002;63:1085–97.
Rahman KM, Aranha O, Sarkar FH. Indole-3-carbinol (I3C) induces apoptosis in tumorigenic but not in nontumorigenic breast epithelial cells. Nutr Cancer. 2005;10:236–43.
Brandi G, Fraternale A, Lucarini S, Paiardini M, De Santi M, Cervasi B, et al. Antitumoral activity of indole-3-carbinol cyclic tri- and tetrameric derivatives mixture in human breast cancer cells: in vitro and in vivo studies. Anti Cancer Agents Med Chem. 2013;13:654–62.
Howells LM, Gallacher-Horley B, Houghton CE, Manson MM, Hudson EA. Indole-3-carbinol inhibits protein kinase B/Akt and induces apoptosis in the human breast tumor cell line MDA MB468 but not in the nontumorigenic HBL100 line. Mol Cancer Ther. 2002;1:1161–72.
Maruthanila VL, Poornima J, Mirunalini S. Attenuation of carcinogenesis and the mechanism underlying by the influence of Indole-3-carbinol and its metabolite 3,3′-Diindolylmethane: a therapeutic marvel. Adv Pharmacol Sci. 2014;2014:832161.
Charafe-Jauffret E, Tarpin C, Patrice V, Bertucci F. Defining the molecular biology of inflammatory breast cancer. Semin Oncol. 2008;35:41–50.
Resetkova E. Pathologic aspects of inflammatory breast carcinoma: part 1. Histomorphology and differential diagnosis. Semin Oncol. 2008;35:25–32.
Xiao Y, Ye Y, Yearsley K, Jones S, Barsky SH. The lymphovascular embolus of inflammatory breast cancer expresses a stem cell-like phenotype. Am J Pathol. 2008;173:561–74.
Fernandez SV, Robertson FM, Pei J, Aburto-Chumpitaz L, Mu Z, Chu K, al e. Inflammatory breast cancer (IBC): clues for targeted therapies. Breast Cancer Res Treat. 2013;140:23–33.
Camacho L, Peña L, Gil AG, Martín-Ruiz A, Dunner S, Illera JC. Immunohistochemical vascular factor expression in canine inflammatory mammary carcinoma. Vet Pathol. 2014;51:737–48.
Kleer CG, van Golen KL, Merajver SD. Molecular biology of breast cancer metastasis. Inflammatory breast cancer: clinical syndrome and molecular determinants. Breast Cancer Res. 2000;2:423–9.
Alpaugh ML, Tomlinson JS, Kasraeian S, Barsky SH. Cooperative role of E-cadherin and sialyl-Lewis X/A-deficient MUC1 in the passive dissemination of tumor emboli in inflammatory breast carcinoma. Oncogene. 2002;21:3631–43.
Lehman HL, Dashner EJ, Lucey M, Vermeulen P, Dirix L, Van Laere S, et al. Modeling and characterization of inflammatory breast cancer emboli grown in vitro. Int J Cancer. 2013;132:2283–94.
Sundar SN, Kerekatte V, Equinozio CN, Doan VB, Bjeldanes LF, Firestone GL. Indole-3-carbinol selectively uncouples expression and activity of estrogen receptor subtypes in human breast cancer cells. Mol Endocrinol. 2006;20:3070–82.
Le HT, Schaldach CM, Firestone GL, Bjeldanes JF. Plant derived 3,30-diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J Biol Chem. 2003;278:21136–45.
Sanderson JT, Slobbe L, Lansbergen GW, Safe S, van den Berg M. 2,3,7,8- Tetrachlorodibenzo-p-dioxin and diindolylmethanes differentially induce cytochrome P450 1A1, 1B1, and 19 in H295R human adrenocortical carcinoma cells. Toxicol Sci. 2001;61:40–8.
Ociepa-Zawal M, Rubis B, Lacinski M, Trzeciak WH. The effect of indole-3-carbinol on the expression of CYP1A1, CYP1B1 and AhR genes and proliferation of MCF-7 cells. Acta Biochim Pol. 2007;54:113–7.
Szaefer H, Licznerska B, Krajka-Kuźniak V, Bartoszek A, Baer-Dubowska W. Modulation of CYP1A1, CYP1A2 and CYP1B1 expression by cabbage juices and indoles in human breast cell lines. Nutr Cancer. 2012;64:879–88.
Yamamoto R, Shimamoto K, Ishii Y, Kimura M, Fujii Y, Morita R, et al. Involvement of PTEN/Akt signaling and oxidative stress on indole-3-carbinol (I3C)-induced hepatocarcinogenesis in rats. Exp Toxicol Pathol. 2013;65:845–52.
Illera JC, Caceres S, Peña L, de Andres PJ, Monsalve B, Illera MJ, et al. Steroid hormone secretion in inflammatory breast cancer cell lines. Horm Mol Biol Clin Investig. 2015;24:137–45.
Pasqualini JR, Chetrite G, Nestour EL. Control and expression of oestrone sulphatase activities in human breast cancer. J Endocrinol. 1996;150(Suppl):S99–105.
Michnovicz JJ, Adlercreutz H, Bradlow HL. Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J Nat Cancer Inst. 1997;89:718–23.
Meng Q, Yuan F, Goldberg ID, Rosen EM, Auborn K, Fan S. Indole-3-carbinol is a negative regulator of estrogen receptor-alpha signaling in human tumor cells. J Nutr. 2000;130:2927–31.
Safe S, Kim K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J Mol Endocrinol. 2008;41(5):263–75.
Poulin R, Baker D, Labrie F. Androgens inhibit basal and estrogen-induced cell proliferation in the ZR-75-1 human breast cancer cell line. Breast Cancer Res Treat. 1988;12:213–25.
Birrell SN, Hall RE, Tilley WD. Role of the androgen receptor in human breast cancer. J Mammary Gland Biol Neoplasia. 1998;3:95–103.
Liao DJ, Dickson RB. Roles of androgens in the development, growth and carcinogenesis of the mammary gland. J Steroid Biochem Mol Biol. 2002;80:175–89.
De Santi M, Carloni E, Galluzzi L, Diotallevi A, Lucarini S, Magnani M, et al. Inhibition of testosterone aromatization by the Indole-3-carbinol derivative CTet in CYP19A1-overexpressing MCF-7 breast Cancer cells. Anti Cancer Agents Med Chem. 2015;15:896–904.
Acknowledgements
We are grateful to Pedro Aranda for the histological technical assistance and to Carmen Pilar Garcia for animal’s care.
Funding
This research was supported by the Spanish Ministry of Science and Education (research project SAF2009–10572). The funding body did not contribute in any of the following: design of the study, data collection, data analysis, interpretation of data, or in writing the manuscript.
Availability of data and materials
All data analyzed for this study are included in this article. Supplementary information files are available upon request to the corresponding author.
Author information
Authors and Affiliations
Contributions
LP and JCI conceived of the study. LP, AG and JCI participated in its design and coordination, interpretation of results and helped to draft the manuscript. AM generated the animal model and AM, LDC and SC carried out the experimental study and collected all data. LP, JCI, AG and AM wrote the manuscript. SC and AM carried out the hormonal assays and LDC the immunohistochemical techniques. All authors read and approved the final manuscript, and all of them agree that they are accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval
All procedures were carried out according to European and Spanish legislative and regulatory guidelines (European convention ETS 1 2 3, on the use and protection of vertebrate mammals in experimentation and for other scientific purposes and Spanish Law 32/2007, and R.D. 1201/2005 on the protection and use of animals in scientific research). The protocol was approved by the Committee of the Universidad Complutense of Madrid, and Animal Protection Area of the Community of Madrid, Spain (Ref. Protocol Number: PROEX 31/15).
Competing interests
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Martín-Ruiz, A., Peña, L., González-Gil, A. et al. Effects of indole-3-carbinol on steroid hormone profile and tumor progression in a mice model of canine inflammatory mammarycancer. BMC Cancer 18, 626 (2018). https://doi.org/10.1186/s12885-018-4518-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-018-4518-z