Abstract
Background
The purpose of this study was to develop an effective nomogram capable of estimating the individual survival outcomes of patients with hepatocellular carcinoma (HCC), and compare the predictive accuracy and discriminative ability with other staging systems.
Methods
The nomogram was established based on a retrospective study of 661 patients newly diagnosed with HCC at the Beijing Ditan Hospital (Beijing, China), Capital Medical University, between October 2008 and July 2012. The predictive accuracy and discriminative ability of the previously developed nomogram were assessed by C-index and calibration curves, and were compared to seven current commonly used staging systems. The results were validated, using a bootstrap approach to correct for bias, in a prospective study of 220 patients consecutively enrolled between August 2012 and March 2013.
Results
Multivariate analysis of the primary cohort for survival analysis identified the independent factors to be aspartate aminotransferase, ɣ-glutamyl transpeptidase, white blood cell count, neutrophil-to-lymphocyte ratio, prothrombin activity, α-fetoprotein, tumor number and size, lymph node metastasis, and portal vein involvement, which were all included to build the nomogram. The calibration curve for predicting the probability of survival showed consistency between the nomogram and the actual observation. The C-index of the nomogram was 0.81 (95% confidence interval, 0.79–0.82), which was statistically better than that of the Tumor, Node, Metastasis staging (0.71), Barcelona Clinic Liver Cancer staging (0.77), Okuda (0.62), Japan Integrated Staging (0.73), Cancer of the Liver Italian Program score (0.76), Chinese University Prognostic Index (0.68), and the Groupe d’ Etude et de Traitement du Carcinome Hepatocellulaire Prognostic classification (0.65) (p < 0.001 for all). The results were validated in the prospective validation cohort.
Conclusions
The prognostic nomogram resulted in more accurate individualized risk estimates for overall survival in HCC patients.
Similar content being viewed by others
Background
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the second highest mortality rate among cancers worldwide, accounting for more than 0.5 million deaths annually [1]. Furthermore, the incidence of HCC has been increasing in the last decades [2, 3]. Therefore, HCC has been a major health problem worldwide. In the past decades, several effective therapies have been developed, including surgical resection, liver transplantation, radiofrequency ablation (RFA), microwave ablation, percutaneous ethanol injection (PEI), and transcatheter arterial embolization or chemoembolization (TAE/TACE) [4]. Therefore, it is imperative to determine whether a patient would benefit from aggressive therapies, while avoiding overtreatment. Cancer staging is important for guiding therapeutic interventions and assessing prognosis that could be of significance for both the patients and clinicians in decision-making.
Currently, several staging systems are being used to predict survival in HCC patients, including the Tumor, Node, Metastasis (TNM) staging [5], Barcelona Clinic Liver Cancer (BCLC) staging [6], Okuda [7], Cancer of the Liver Italian Program (CLIP) score [8], Japan Integrated Staging Score (JIS) [9], Chinese University Prognostic Index (CUPI) [10], and the Groupe d’ Etude et de Traitement du Carcinome Hepatocellulaire Prognostic classification (GETCH) [11], all of which have their advantages and disadvantages. The Okuda, CUPI, and GETCH classifications properly stratified the prognosis of patients with advanced or terminal stage [12]. The TNM staging only accounts for tumor-related indicators reflecting the tumor morphology and pathology, without taking the liver functional features into consideration [13]. Meanwhile, these staging systems only serve to stratify patients into various groups with variable outcomes, but could not estimate the individual survival outcomes of HCC.
Nomograms are graphic calculating scales of predictive statistical models to optimize predictive accuracy of individuals [14, 15], and they have been developed for several carcinomas [16–19]. Because nomograms has been demonstrated to provide more precise prediction over the traditional staging systems in many types of cancers, it has been proposed as an alternative method or even as a new standard to guide the administration of appropriate treatment to cancer patients [16, 19, 20]. However, nomograms that predict overall survival (OS) in HCC patients are rare. Although Li shu et al. proposed a prognostic nomogram specifically developed for patients with unresectable HCCs after TACE, it did not cover the entire clinical spectrum of HCCs [21]. Patients who were suitable candidates for surgical resection or had advanced/end-stage cancers were excluded. In this study, the specific aim of this analysis was to develop a simple and clinically useful nomogram for patients with HCC and compare the performance of this model with the currently available staging systems.
Methods
Patients and design
We retrospectively analyzed 661 patients between October 2008 and July 2012 and prospectively studied 220 patients between August 2012 and March 2013, who were newly diagnosed with HCC at the Beijing Ditan Hospital (Beijing, China), Capital Medical University. The diagnosis of HCC was based on the European Association for the Study of the Liver (EASL) criteria [22]: a histopathologic confirmation, a positive lesion detected by at least 2 different imaging techniques, or a positive lesion detected by 1 imaging technique combined with α-fetoprotein (AFP) >400 ng/ml. The imaging techniques included transabdominal ultrasonography, angiogram, computed tomography (CT) and magnetic resonance imaging (MRI). Patient records and information was anonymized prior to analysis. This project was approved by the ethics committee of the Beijing Ditan Hospital (Beijing, China).
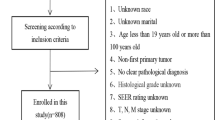
The inclusion criteria were age 18–75 years; newly diagnosed with HCC; and no history of previous anticancer therapy. The exclusion criteria were the diagnosis or history of other malignancies; tumors of uncertain origin or probable metastatic liver tumors; patients with missing key data concerning clinical information and laboratory data; or patients with no follow-up data.
Resection and liver transplantation should be the first option for patients who have the optimal profile. Locoregional approaches including ablation and TAE were used for patients who were not suitable candidates for curative therapies. RFA, PEI, or microwave ablation was performed in HCC patients with 2–3 nodules ≤3 cm. TACE/Lp-TAE were performed in patients with 4 nodules >3 cm, or Child-Pugh A or B. Sorafenib and FOLFOX regimens were considered first-line treatment in patients with distant metastases who can no longer be treated with potentially more effective therapies. End stage includes those patients with severe impairment of liver function (Child-Pugh C) merely received the best supportive care [23, 24].
Data collection
A standardized data collection form was designed to retrieve all the relevant information on demographic data (age, sex, history of smoking, history of alcohol consumption, family history of HCC, and household registry); laboratory data (alanine aminotransferase [ALT], aspartate aminotransferase [AST], total bilirubin [TBil], serum albumin [ALB], alkaline phosphatase [ALP], ɣ-glutamyl transpeptidase [GGT], prothrombin activity [PTA], international normalized ratio [INR], AFP, white blood cell [WBC] count, absolute neutrophil count [NC], absolute lymphocyte count [LC], absolute platelet count [PLT], neutrophil-to-lymphocyte ratio [NLR]); and tumor-related indicators (tumor size and number, lymph node metastasis, distant metastasis, portal vein involvement). The relevant data were collected from the patient medical records or the hospital database at the time of HCC diagnosis and during the follow-up period. In addition, seven scoring systems associated with clinical prognosis were used at baseline, which were the TNM, BCLC, Okuda, CLIP, JIS, CUPI, and GETCH staging scores, as previously described [5–11].
Follow-up
All patients were followed-up at least once every 3 months during the first 2 years after treatment, and every 4–6 months annually thereafter. At each of these follow-up visits, a detailed history was taken and a complete physical examination was carried out. Abdominal CT or MRI was also done annually or earlier when tumour recurrence/metastasis was suspected. OS was defined as the interval between diagnosis and death from any cause or until the last known follow-up, obtained from the patient medical records, or through direct contact with the patients or their families.
Statistical analysis
All the statistical analyses were conducted with SPSS 20.0 statistical package (IBM, Armonk, NY, USA). Continuous variables were presented as mean ± standard deviation or medians with interquartile ranges, while categorical variables as the frequencies or percentages of events. The Student’s t-test or Mann–Whitney U test was used for continuous data. The Pearson chi-square or Fisher’s exact tests were used to compare differences in proportion between the groups, as appropriate. Cox univariate and multivariate regression analyses were performed to identify independent risk factors for predicting mortality.
Nomograms were formulated based on the results of the multivariate Cox regression analyses performed using the RMS packages [25] in R version 3.0.2 (http://www.r-project.org/). Final selection of the nomogram model was based on a backward step-down process with the Akaike information criterion [26]. The performance of the nomograms and other seven staging systems for predicting survival were evaluated by the concordance index (C-index), an equivalent variable of the area under curve (AUC) of the receiver operating characteristic (ROC) curve for censored data. The maximum C-index value is 1.0, which indicates a perfect prediction model whereas 0.5 indicates a random chance to correctly predict outcome by the model. Bootstraps with 1,000 resamples were used for validation to correct the C-index and explain the variance due to over-optimism. Comparisons between nomogram models and the other seven staging systems were performed with the rcorrp.cens function in the Hmisc package [27] in R. Calibration curves of the nomogram for 1-, 2-, and 3-year OS were applied to assess the agreement between the predicted survival and the observed survival. Clinical survival outcomes were assessed by Kaplan–Meier analysis and prognostic groups were compared by log-rank test. When externally validating the nomogram, the total points for each patient were computed according to the established nomogram, which were used as factors in the Cox regression model, and the C-index and calibration curves were derived based on the regression analysis. All statistical tests were two-sided with a statistical significance level set at p values < 0.05.
Results
Patient characteristics and outcomes
In total, 1221 patients newly diagnosed with HCC during the study period were enrolled in the study. Following the exclusion of those who did not meet the inclusion criteria, 661 patients were finally included in the primary cohort, and 220 in the prospective validation cohort. The baseline characteristics of the primary and validation cohorts are listed in Table 1. 356 (40.4%) of the patients had survived, whereas 525 (59.6%) of the patients had died by the end of the 3-year follow-up. The median OS periods were 25.0 months and 21.0 months for the primary and validation cohorts, respectively. The 1-, 2-, and 3-year OS rates were 66.1, 50.8, and 41.6% in the primary cohort, and 63.6, 42.3, and 36.4% in the prospective validation cohort, respectively.
Univariate and multivariate analyses in the primary cohort
For OS, the significant inferior prognostic factors included the male sex, history of alcohol consumption, ALT, AST, TBil, ALB, ALP, GGT, WBC, NC, LC, NLR, Cr, PTA, AFP ≥ 400 ng/mL, tumor number ≥ 3, tumor diameter ≥ 5 cm, lymph node metastasis, and portal vein involvement (p < 0.05). The above variables were entered into multivariate Cox proportional hazard regression analyses. The results indicated AST, GGT, WBC, NLR, PTA, AFP ≥ 400 ng/mL, tumor number ≥ 3, tumor diameter ≥ 5 cm, lymph node metastasis, and portal vein involvement to be independent prognostic variables. The detailed results of the multivariate analysis are shown in Table 2.
Prognostic nomogram for survival
The coefficients obtained from the Cox regression model were used to construct the nomograms for OS (Fig. 1). Each subtype within the variables was assigned a score. By adding up the total score from all the variables and locating it to the total point scale, we could determine the probabilities of the outcomes by drawing a vertical line to the total score. The nomograms included three liver function indices (AST, GGT, PTA), two inflammatory indices (WBC, NLR), and five tumor-related indicators (AFP, tumor number, tumor size, lymph node metastasis, and portal vein involvement), of which PTA, NLR, and portal vein involvement were the most important contributing factors for OS prediction. Details concerning the point assignment from the nomograms and the prognostic score are shown in Table 3.
A hepatocellular carcinoma survival nomogram is depicted. To use the nomogram, the value of an individual patient is located on each variable axis, and a line is drawn upward to determine the number of points received for the value of each variable. The sum of these numbers is located on the total point axis, and a line is drawn downward to the survival axes to determine the likelihood of 1-, 2-, and 3-year survivals. AST, aspartate aminotransferase; GGT, γ-glutamyl transpeptidase; WBC, white blood cell; NLR, neutrophil-to-lymphocyte ratio; PTA, prothrombin activity; AFP, alpha fetoprotein
Validation of the prognostic nomogram
The C-index for the established nomogram for predicting the OS was 0.81 (95% confidence interval (CI), 0.79–0.82) in the primary cohort. When the validation cohort was subjected to the nomogram, the C-index was 0.78 (95% CI, 0.74–0.82), which was greater than 0.7, suggesting the suitability of the new model for patients with HCC. The calibration plots showed fair agreements between the nomogram predictions and actual observations for the 1-, 2-, and 3-year OS in the primary cohort (Fig. 2a-c) and the prospective validation cohort (Fig. 2d-f).
The calibration curve of overall survival at 1, 2, and 3 years for the primary cohort (a-c) and the prospective validation cohort (d-f). Nomogram-predicted probability of survival is plotted on the x-axis, and the actual survival is plotted on the y-axis. Dashed lines along the 45° line through the point of origin represent the perfect calibration models where the predicted probabilities are identical to the actual probabilities
According to the total scores, patients were divided into four quartiles (quartile 1: 0–7; quartile 2: 8–10; quartile 3: 10–15; and quartile 4: > 15). After dividing the survival rates into quartiles, we further identified the prognostic discrimination of the nomograms by Kaplan–Meier analysis. The nomogram could accurately stratify patients into the 4 risk groups with significant differences in the 1-, 2-, and 3-year OS rates in the primary (3-year OS rate: 76.2% in quartile 1, 65.6% in quartile 2, 25.7% in quartile 3, and 3.2% in quartile 4; p < 0.001) and the validation cohort (3-year OS rate: 64.6% in quartile 1, 55.1% in quartile 2, 28.6% in quartile 3, and 0% in quartile 4; p < 0.001) (Fig. 3h,p).
Kaplan–Meier curves of risk group stratification for OS in the primary cohort (a-h) and the prospective validation cohort (i-p) categorized according to different staging systems. a,i Tumor, Node, Metastasis (TNM); b,j Barcelona Clinic Liver Cancer (BCLC); c,k Okuda; d,l Japan Integrated Staging Score (JIS); e,m Cancer of the Liver Italian Program (CLIP); f,n Chinese University Prognostic Index (CUPI); g,o Groupe d’ Etude et de Traitement du Carcinome Hepatocellulaire Prognostic classification (GETCH); and h,p the prognostic nomogram
The performance of the nomogram compared to other staging systems
As shown in Fig. 3a-p, the Kaplan–Meier curves were generated for all the staging systems in the primary and validation cohorts. Although all the staging systems showed good prognostic stratification (p < 0.001 for all cases) in the primary cohort, some overlapping of the survival curves for TNM, BCLC, JIS, and CLIP was observed. In the validation cohort, all the staging systems also showed clear prognostic stratification (p < 0.001); however, some overlapping of the survival curves was observed for the TNM, BCLC, Okuda, JIS, and CLIP. In both cohorts, the TNM staging was not good at stratifying patients with stage III–IV, and the BCLC was not good at stratifying the patients between stages C and D. Moreover, the Okuda, CUPI, and the GETCH classifications could stratify patients with stage I–III to a certain extent, whereas they were unsatisfactory in stratifying patients in the early stages of HCC in both cohorts.
Our nomogram displayed better accuracy in predicting survival of patients with HCC in both cohorts. As shown in Table 4, the nomogram for OS had a bootstrap-corrected C-index of 0.81, which was significantly better than that of the TNM (0.71), BCLC (0.77), Okuda (0.62), JIS (0.62), CLIP (0.76), CUPI (0.68), and GETCH (0.65) staging systems in the primary cohort (p < 0.001 for all). In the prospective validation cohort, the nomograms had the highest areas under the curve (0.78), but without statistical significance only in comparison to BCLC.
Discussion
In this study, we established a novel, easy-to-use, and effective nomogram capable of estimating individual survival outcomes for HCC. Moreover, a robust HCC nomogram including the inflammatory indices (WBC, NLR) was developed to improve the predictive power of the current prognostic scores.
Distinct from other solid cancers, the prognosis for HCC patients relies not only on tumor progression but also on the extent of liver dysfunction; approximately 70 to 90% of HCCs occur in the context of chronic liver inflammation and cirrhosis [28, 29]. Consequently, staging systems such as TNM that depend solely on pathological characteristics retain limited prognostic impact on HCC [13]. A number of alternative systems have been proposed for HCC, including the BCLC, CLIP, CUPI, and JIS. However, there is no universally accepted consensus about the best staging system for predicting the outcome of HCC patients.
Numerous clinical and experimental data demonstrated that host inflammatory response to cancer cells is associated with tumor progression [30, 31]. The link between inflammation and cancer is well established. Various markers of systemic inflammation response, including WBC count [32, 33], cytokines [34, 35], and absolute count of blood neutrophils or lymphocytes as well as the neutrophil-to-lymphocyte (NLR) ratio [36–38] have been explored for their prognostic impact in various cancer populations including HCC. In this study, we also found that the WBC count and NLR have moderate contributions to the nomogram prediction of OS. Elevated neutrophils are regarded as a reservoir of the circulating vascular endothelial growth factor, which plays a key role in the promotion of angiogenesis [39], and neutrophils could contribute to metastasis by promoting the motility of tumor cells and the adhesion of metastatic tumor cells to liver sinusoids [40, 41]. Conversely, reduced lymphocyte infiltration, reflecting the suppression of the host immune surveillance, has been shown to attenuate lymphocyte-mediated antitumor immune response [42]. The presence of high intratumoral activated CD8 cytotoxic cells is associated with improved survival in HCC patients [43]. Consequently, when taken together, NLR could reflect the balance between host inflammation and immunity, which has been reported to be a predictor of survival in HCC patients who underwent hepatic resection, RFA, TACE, and liver transplantation [36, 44–46]. In the future, manipulating the inflammatory status and the immune function of HCC patients might be a promising strategy for further improving the clinical outcomes.
The proposed nomogram included three liver function indices (AST, GGT, PTA), five tumor-related indicators (AFP, tumor number and size, lymph node metastasis, and portal vein involvement), and two inflammatory indices (WBC, NLR), which performed well in predicting the survival outcome of HCC patients, and the prediction was supported by the C-index (0.82 and 0.78 for the primary and validation cohorts, respectively) and the calibration curves. In the current study, the nomogram showed the highest predictive accuracy for OS in patients with HCC, compared to the other seven staging systems. Although there was no statistical significance in comparison to the BCLC in the validation cohort, it is worth noting that the nomogram could more effectively stratify patients with advanced stage cancers compared to the TNM, BCLC, Okuda, and CLIP, and more effectively stratify patients in the early stages of HCC than the Okuda, CUPI, and GETCH in both cohorts.
Our nomogram has some limitations. First, the nomogram was established based on a single-center cohort study. Second, the nomograms only included basic clinical and laboratory data. However, the present study aimed to build reliable prediction models. Objective variables are therefore the ideal factors to be included in the models, while subjective variables might negatively affect the models due to inevitable bias. Third, the study was conducted retrospectively and selection bias might exist. However, we have included a relatively large training cohort to build the nomograms and validated them by a prospective dataset. The results consistently showed the satisfactory performance of the established models.
Conclusions
In conclusion, we developed and validated nomograms predicting individual prognosis in patients with HCC. The proposed nomogram in this study provided better predictive accuracy and discrimination than the TNM, BCLC, Okuda, JIS, CLIP, CUPI, and GETCH staging systems, and it offers a useful tool for providing patient counseling and timing surveillance, as well as clinical assessments. In order to standardize the use of this nomogram, validation with data from other institutions and other patient groups is required.
Abbreviations
- AFP:
-
Alpha fetoprotein
- ALB:
-
Albumin
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BCLC:
-
Barcelona Clinic Liver Cancer
- CIs:
-
Confidence intervals
- CLIP:
-
Cancer of the Liver Italian Program
- CUPI:
-
Chinese University Prognostic Index
- EASL:
-
The European Association for the Study of the Liver
- GETCH:
-
Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire
- GGT:
-
ɣ-Glutamyltranspeptidase
- HCC:
-
Hepatocellular carcinoma
- HRs:
-
Hazard ratios
- INR:
-
International normalized ratio
- JIS:
-
Japan integrated staging
- LC:
-
Absolute lymphocyte count
- NC:
-
Absolute neutrophil count
- NLR:
-
Neutrophil-to-lymphocyte ratio
- OS:
-
Overall survival
- PLT:
-
Absolute platelet count
- PTA:
-
Prothrombin activity
- RFA:
-
Radiofrequency ablation
- TACE:
-
Transcatheter arterial chemoembolization
- TBil:
-
Total bilirubin
- TNM:
-
The Tumor, node, metastasis
- WBC:
-
White blood cell
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
McGuire S. World cancer report 2014. Geneva, Switzerland: World Health Organization, International Agency for research on cancer, WHO press, 2015. Adv Nutr. 2016;7:418–9.
El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–27.
Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–5S16.
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. p. 191–200.
Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–38.
Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–28.
Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan integrated staging score (JIS score). J Gastroenterol. 2003;38:207–15.
The Cancer of the Liver Italian Program (CLIP) inverstigators. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. Hepatology. 1998;28:751–5.
Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–9.
Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hepatocellulaire. J Hepatol. 1999;31:133–41.
Huitzil-Melendez FD, Capanu M, O’Reilly EM, Duffy A, Gansukh B, Saltz LL, et al. Advanced hepatocellular carcinoma: which staging systems best predict prognosis. J Clin Oncol. 2010;28:2889–95.
Zhou L, Rui JA, Wang SB, Chen SG, Qu Q. LCSGJ-T classification, 6th or 5th edition TNM staging did not independently predict the long-term prognosis of HBV-related hepatocellular carcinoma after radical hepatectomy. J Surg Res. 2010;159:538–44.
Touijer K, Scardino PT. Nomograms for staging, prognosis, and predicting treatment outcomes. Cancer. 2009;115:3107–11.
Kattan MW, Scardino PT. Evidence for the usefulness of nomograms. Nat Clin Pract Urol. 2007;4:638–9.
Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188–95.
Han DS, Suh YS, Kong SH, Lee HJ, Choi Y, Aikou S, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol. 2012;30:3834–40.
Karakiewicz PI, Briganti A, Chun FK, Trinh QD, Perrotte P, Ficarra V, et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol. 2007;25:1316–22.
Sternberg CN. Are nomograms better than currently available stage groupings for bladder cancer. J Clin Oncol. 2006;24:3819–20.
Mariani L, Miceli R, Kattan MW, Brennan MF, Colecchia M, Fiore M, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer. 2005;103:402–8.
Xu L, Peng ZW, Chen MS, Shi M, Zhang YJ, Guo RP, et al. Prognostic nomogram for patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. J Hepatol. 2015;63:122–30.
Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30.
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2.
Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74.
Frank E, Harrell J. Rms: Regression Modeling Strategies. R Package version 3.4-0. https://CRAN.R-project.org/package=rms. Accessed 17 Jan 2012.
Harrell Jr FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87.
Frank E, Harrell Jr. Harrell Miscellaneous. R Package version 3.9-2. https://CRAN.R-project.org/package=Hmisc. Accessed 10 Feb 2012.
Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009;29:502–10.
Schutte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma--epidemiological trends and risk factors. Dig Dis. 2009;27:80–92.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99.
Erlinger TP, Muntner P, Helzlsouer KJ. WBC count and the risk of cancer mortality in a national sample of U.S. adults: results from the second National health and nutrition examination survey mortality study. Cancer Epidemiol Biomarkers Prev. 2004;13:1052–6.
Grimm Jr RH, Neaton JD, Ludwig W. Prognostic importance of the white blood cell count for coronary, cancer, and all-cause mortality. JAMA. 1985;254:1932–7.
Liao R, Sun J, Wu H, Yi Y, Wang JX, He HW, et al. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res. 2013;32:3.
Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184–90.
Mano Y, Shirabe K, Yamashita Y, Harimoto N, Tsujita E, Takeishi K, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013;258:301–5.
Sharaiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI, et al. Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol. 2011;18:3362–9.
Gao F, Li X, Geng M, Ye X, Liu H, Liu Y, et al. Pretreatment neutrophil-lymphocyte ratio: an independent predictor of survival in patients with hepatocellular carcinoma. Medicine (Baltimore). 2015;94:e639.
Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6:283–7.
McDonald B, Spicer J, Giannais B, Fallavollita L, Brodt P, Ferri LE. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J Cancer. 2009;125:1298–305.
Wu Y, Zhao Q, Peng C, Sun L, Li XF, Kuang DM. Neutrophils promote motility of cancer cells via a hyaluronan-mediated TLR4/PI3K activation loop. J Pathol. 2011;225:438–47.
Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70.
Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–93.
Chen TM, Lin CC, Huang PT, Wen CF. Neutrophil-to-lymphocyte ratio associated with mortality in early hepatocellular carcinoma patients after radiofrequency ablation. J Gastroenterol Hepatol. 2012;27:553–61.
McNally ME, Martinez A, Khabiri H, Guy G, Michaels AJ, Hanje J, et al. Inflammatory markers are associated with outcome in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. Ann Surg Oncol. 2013;20:923–8.
Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58:58–64.
Acknowledgements
We gratefully recognize the patients who participated in this study. We thank Yan Sang for her help with the data.
Funding
Supported by grants from the National Natural Science Foundation of China (grant nos. 81273743 and 81473641), the 215 Program from Beijing Municipal Health Bureau (2013-2-11) and the Collaborative Innovation Center of Infectious diseases (PXM 2015-014226-000058).
Availability of data and materials
The datasets generated and analysed during the current study are available from the corresponding author at wangxb@ccmu.edu.cn on reasonable request.
Authors’ contributions
XBW and WG conceived and designed the study. WG, FYG, LJC, YXL, MFG, LS and YF were responsible for the acquisition and analysis of data. FYG, LJC and YF drafted the manuscript. All authors participated in interpretation of the findings. XBW revised the manuscript, and all authors have read and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests with regard to the publication of this research report.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study protocol was in accordance with the ethical guidelines of the Declaration of Helsinki and was approved by the ethics committee of the Beijing Ditan Hospital (Beijing, China). All patients in the prospective validation cohort provided written informed consent. The present study in the retrospective cohort was the medical chart review and observational study. The ethics committee of Beijing Ditan Hospital (Beijing, China) approved the waiver of informed consent.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wan, G., Gao, F., Chen, J. et al. Nomogram prediction of individual prognosis of patients with hepatocellular carcinoma. BMC Cancer 17, 91 (2017). https://doi.org/10.1186/s12885-017-3062-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-017-3062-6