Abstract
Background
Little is known of the burden of Group B Streptococcus (GBS) colonization among pregnant women in Jordan. We conducted a pilot study to determine the prevalence of GBS among pregnant women in Amman, Jordan, where GBS testing is not routine. We also explored GBS serotypes and the performance of a rapid GBS antigen diagnostic test.
Methods
We collected vaginal-rectal swabs from women who presented for labor and delivery at Al-Bashir Hospital. Three methods were used to identify GBS: Strep B Rapid Test (Creative Diagnostics), blood agar media (Remel) with confirmed with BBL Streptocard acid latex test (Becton Dickinson), and CHROMagar StrepB (Remel). Results were read by a senior microbiologist. We defined our gold standard for GBS-positive as a positive blood agar culture confirmed by latex agglutination and positive CHROMagar. PCR testing determined serotype information. Demographic and clinical data were also collected.
Results
In April and May 2015, 200 women were enrolled with a median age of 27 years (IQR: 23–32); 89.0% were Jordanian nationals and 71.9% completed secondary school. Median gestational age was 38 weeks (IQR: 37–40); most women reported prenatal care (median 9 visits; IQR: 8–12). Median parity was 2 births (IQR: 1–3). Pre-pregnancy median BMI was 24.1 (IQR: 21.5–28.0) and 14.5% reported an underlying medical condition. Obstetric complications included gestational hypertension (9.5%), gestational diabetes (6.0%), and UTI (53.5%), of which 84.5% reported treatment. Overall, 39 (19.5%) of women were GBS-positive on blood agar media and CHROMagar, while 67 (33.5%) were positive by rapid test (36% sensitivity, 67% specificity). Serotype information was available for 25 (64%) isolates: III (48%), Ia (24%), II (20%), and V (8%). No demographic or clinical differences were noted between GBS+ and GBS-negative women.
Conclusions
Nearly one in five women presenting for labor in Jordan was colonized with GBS, with serotype group III as the most common. The rapid GBS antigen diagnostic had low sensitivity and specificity. These results support expanded research in the region, including defining GBS resistance patterns, serotyping information, and risk factors. It also emphasizes the need for routine GBS testing and improved rapid GBS diagnostics for developing world settings.
Similar content being viewed by others
Background
Group B Streptococcus (S. agalactiae; “GBS”) is a gram-positive bacterium with a special capacity to cause perinatal infections of the mother, fetus, and/or newborn. This pathogen causes chorioamnionitis [1], preterm birth [2], stillbirth [3], meningitis [4] and is a leading cause of both early-onset (< 7 days of life) and late-onset (7–89 days of life) neonatal sepsis [5]. Globally, the burden of GBS disease is estimated to be 0.49–0.53 per 1000 livebirths, with a case fatality rate of 8.4–9.6% [6, 7]. The incidence of early-onset GBS disease is estimated to be 0.43 per 1000 livebirths, with a case fatality rate of 12.1%, twice that of late-onset disease [6].
In the United States, recommendations for routine screening for GBS in women between 35 and 37 weeks pregnant, followed by antibiotic prophylaxis 4h prior to delivery for colonized patients, led to a dramatic decrease in the incidence of early-onset GBS disease in neonates [8], with no change in the incidence of late-onset GBS. This screen-and-treat paradigm, however, has not been widely adopted outside of the United States, and policy decisions have been challenged by the absence of solid estimates of the number of at-risk mothers and babies in many parts of the world [9]. One barrier to obtaining reliable epidemiological data from low- and middle-income countries has been the absence of accurate point-of-care tests to determine GBS colonization without the need for time-consuming and expensive, laboratory-based cultivation practices [10].
A 2016 meta-analysis including data for over 70,000 women in 37 countries estimated global prevalence of maternal GBS colonization at 17.9% (95%CI: 16.2–19.7%) [11]. However, the authors noted substantial heterogeneity across and within regions, and reported few studies from Middle East and North African (MENA) countries, highlighting a paucity of maternal GBS prevalence data from the region. From our own review, we found many reports from the MENA region estimating the frequency of GBS in pregnant mothers, with estimates ranging from 1.6 to 32% (Table 1), however nearly half of these studies were conducted in only two countries – Iran and Israel – and over one-third of the studies were published more than 10 years ago. In Jordan, universal screening is not routine. Only one manuscript, published in 1991, estimated the GBS prevalence among 500 pregnant women in Jordan, finding nearly one-third (30.4%) of women were colonized by vaginal, rectal, and/or urine specimens [35]. Prior to launching future studies to assess novel diagnostics and vaccines, it is essential to establish the current burden of GBS colonization in the region. Given the scarcity of information about GBS burden of colonization in Jordan, we conducted a pilot study to determine the prevalence of GBS recto-vaginal colonization among pregnant women presenting for labor at one high-volume government hospital in Amman, Jordan, where GBS testing is not routine. Secondary objectives were to assess capsular serotypes of GBS specimens and to compare the performance of a GBS rapid diagnostic to culture.
Methods
Study design and population
This was a 2-month cross-sectional study to determine the prevalence of GBS colonization among women admitted for labor and delivery at Al-Bashir Hospital in Amman, Jordan. Al-Bashir Hospital is one of three major government-run referral medical centers in Amman, which is Jordan’s largest city and capital. There are an estimated 1300 deliveries per month. Intrapartum antibiotic prophylaxis (IAP) for suspected GBS colonization was provided per clinician discretion based on maternal risk factors and other indications for suspected infection. Other intrapartum antibiotic provision was at clinician discretion.
This study was approved by the institutional review boards of Vanderbilt University Medical Center, University of Jordan, and the Jordan Ministry of Health. All participants provided written informed consent prior to enrollment. To maintain participant confidentiality, unique study identification numbers were used in lieu of personal identifiers.
Data and specimen collection
After consent, trained local research staff interviewed the pregnant women using a standardized questionnaire to record maternal and paternal demographic characteristics, history of antenatal care, and medical and obstetric history. Subjects were queried in Arabic, and bilingual research staff transcribed the information onto an English-language case report form at the time of the interview. After subjects were discharged, charts were abstracted for maternal outcomes, antibiotic use, and length of stay.
GBS specimen collection and processing were conducted according to the 2010 US Centers for Disease Control and Prevention (CDC) recommendations [52]. A single combined vaginal-rectal swab was collected from each participant prior to delivery, placed into LIMBroth tubes, transported to a laboratory at the University of Jordan, placed in an incubator at 35–37 degrees Celsius, and then further sub-cultured after a minimum of 18h. Three methods were used to identify GBS. Point-of-care GBS testing was performed with Strep B Rapid Test (Creative Diagnostics), which was selected based on availability, cost, and ease of use. Two laboratory methods to identify GBS included blood agar media (Becton Dickinson) confirmed with BBL Streptocard acid latex test (Remel) and CHROMagar StrepB (Remel). Results were read by a senior microbiologist at the University of Jordan. We defined our gold standard for GBS-positive as a positive blood agar culture confirmed by both latex agglutination and positive CHROMagar.
Samples determined as GBS-positive were shipped from Jordan to the United States for additional confirmatory test on chromogenic agar and PCR of the conserved sip gene, as described [53]. About one-third (14/39, 35.9%) of the samples did not survive transport. For the 25 viable strains, GBS serotypes were determined using a nested PCR-based strategy [54] and confirmed with latex agglutination (IMMULEX™ STREP-B kit, Statens Serum Institut Diagnostica, Hillerød, Denmark).
Study data were collected and managed using REDCap electronic data capture tools hosted at Vanderbilt University [55], and analyzed using SAS, version 9.4 (SAS Institute, Inc., Cary, NC).
Analysis
We present counts and proportions for categorical variables and medians and interquartile ranges (IQR) for continuous variables. Patient characteristics were compared by GBS outcome using Wilcoxon rank sums and Chi-square statistics to assess differences in medians and distributions, respectively. In the event of small cell sizes (n < 5), Fisher’s exact test was substituted for Chi-square.
Results
Patient characteristics
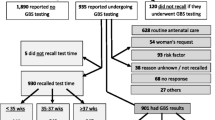
Overall, 226 women who presented in labor during April and May 2015 at Al-Bashir Hospital were approached; 26 women refused enrollment, for total cohort of 200 women. Demographic and clinical characteristics of the 200 participants are displayed in Table 2. The median participant age was 27 years (IQR: 23–32). Most participants were Jordanian nationals (89.0%), had completed secondary school (71.9%), and had not been employed in the past year (86.2%). Median gestational age at delivery was 38 weeks (IQR: 37–40). All but one woman (99.5%) reported attending antenatal care, and most made frequent visits (median 9 visits; IQR: 8–12). Median maternal pre-pregnancy BMI was 24.1 (IQR: 21.5–28.0); median gravidity was 3 pregnancies (IQR: 2–5), and parity was 2 births (IQR: 1–3). Cesarean-sections were common: overall, 96/200 (48.0%) deliveries were by cesarean; of these, 79/98 (80.6%) were repeat C-sections. Few women reported smoking cigarettes (8.5%) and narghile (tobacco hookah, 4.5%) during this pregnancy. However, most women reported living in a household with at least one cigarette smoker (63.0%) and some reported household narghile smoking (16.5%). No demographic or clinical differences were noted between GBS-positive and GBS-negative participants (Table 2).
Few underlying medical conditions were reported, most frequently high blood pressure (7.0%) and diabetes (3.0%). Obstetric complications during the current pregnancy reported included gestational hypertension (9.5%) and gestational diabetes (6.0%). Over half the women (53.5%) reported a urinary tract infection during the current pregnancy, of which 84.5% reported treatment (prior to the intrapartum period). Penicillin allergy was reported in 5.0% of all participants; no other drug allergies were reported.
GBS colonization
In our cohort, 39/200 (19.5%) women were positive for GBS on both blood agar media with positive latex test and CHROMagar, our gold standard. The two tests used in our gold standard were 100% concordant. On the rapid antigen test, 67/200 (33.5%) were positive, of which, only 14/67 (21%) were confirmed GBS-positive by culture. There were 25 false-negative results and 53 false-positive results using the rapid test; sensitivity and specificity for the rapid test were 36 and 67%, respectively, compared to the gold standard of blood agar media with positive latex test and CHROMagar. The positive predictive value of the rapid test was 20.9%, the negative predictive value was 81.2%, the positive likelihood ratio was 1.09, and the negative likelihood ratio was 0.96.
Antibiotic use
Data for intrapartum antibiotic prophylaxis (IAP) and antibiotic use were known for 190 subjects, and 43 (22.6%) of these subjects received an intrapartum antibiotic. Of the 39 women who were GBS-positive by culture, only nine (23.1%) received IAP; 5/9 (55.6%) of whom also had a positive rapid result. One-quarter of women who received an intrapartum antibiotic were GBS-positive by rapid test but not culture (11/43, 25.6%). Reasons for intrapartum antibiotic use are shown in Table 3.
Serotyping analysis
Serotyping data are displayed in Fig. 1. The most prevalent GBS serotype identified was Type III (12/25, 48%), followed by Type Ia (6/25, 24%) and Type II (5/25, 20%). Only 8% (2/25) of cases were Type V. There were no Type Ib or Type IV GBS isolates identified.
Discussion
Our surveillance study revealed nearly one in five women presenting for labor at Al-Bashir Hospital in Jordan were colonized with GBS. This represents a substantial burden of colonization within a context of no routine screening for GBS among pregnant women. Our findings are consistent with a worldwide-adjusted estimate for maternal GBS colonization of 18%, but a higher prevalence compared to Southern Asia (10%) and Eastern Asia (9.1%) [9]. However, our prevalence estimate is lower than the only other local data from Jordan, which reported 30.4% GBS colonization [35]. That study enrolled 500 women and included a positive GBS urine culture, rectal swab, and/or vaginal swab as part of their GBS colonization definition, but these data are nearly 30 years old [35]. The frequency of GBS colonization we found is average when compared to other countries in the MENA region (Table 1), where the average was 16.9% among 18,805 total subjects, with minimum of 1.6% and peak of 32% of subjects with GBS colonization. Thus, our study highlights that GBS colonization is common and potentially an important cause of neonatal disease. While our study did not include neonatal data, a 2017 study exploring neonatal sepsis at a tertiary hospital in Jordan found that GBS was isolated from 10% of neonatal sepsis cases; however, the authors did not explore overall prevalence of GBS among pregnant women [56]. Therefore, our study emphasizes that routine testing for GBS in mothers would be valuable in Jordan so IAP could be introduced to prevent early-onset GBS neonatal disease.
Based on clinical trials and observational studies, early-onset GBS disease can be prevented by the administration of IAP during labor to GBS colonized women, with potential efficacy of 80% [57, 58]. Therefore, two screening methods have been implemented to identify GBS antenatally: risk-based or universal culture-based screening between 35 and 37 weeks. In 2002, CDC recommended switching to culture-based universal screening. Subsequently, an uptake of prenatal screening and IAP was rapid and widespread [8]. In addition, the incidence of invasive early-onset GBS disease decreased by more than 80% from 1.8 cases/1000 live births in the early 1990s to 0.26 cases/1000 live births in 2010, estimating that over 70,000 cases of early-onset GBS invasive disease were prevented in the United States [8]. However, globally, the majority of countries that routinely screen for GBS are risk-based, and unfortunately very few of these countries are from the MENA area, including Jordan which does not routinely test for GBS colonization. In our study, only 9/39 (23.1%) women with confirmed GBS received an intrapartum antibiotic; five of these were also diagnosed by rapid test.
There are no established international standards for sampling GBS colonization; however, CDC recommends rectovaginal swabs at 35–37 weeks with selective enrichment broth culture, but this approach is not always feasible for low and middle-income settings [9]. Additional barriers to routine testing in these low resource settings may include the lack of microbiology capacity to perform routine testing for GBS, lack of timely knowledge of the results being reported back to the clinicians because of separate settings where prenatal care is performed and where lab testing would be performed, and lack of electronic medical records for the obstetricians to obtain antenatal results. One way to address these issues is the introduction of point-of-care testing when women present in labor. Unfortunately, the rapid diagnostic used in this pilot study was substantially less sensitive and specific than culture. Furthermore, over one-quarter of women who received an intrapartum antibiotic due to a positive rapid GBS antigen test (11/43, 25.6%) were found later to be GBS culture-negative, leading to unnecessary use of antibiotics. Our GBS antigen test results were poorer than other studies using other antigen-based tests that yielded 57.3% sensitivity and 99.5% specificity in Canada [59] and 100% sensitivity and 92.9% specificity in India [60]. Other point-of-care testing methods such as PCR testing have been reported to have high sensitivity (92.9–100%) and specificity (81.1–97.5%) [19, 61, 62] and may be a better solution. However, these higher-performing tests typically are cost-prohibitive for lower-resource settings [12, 63], and further development of affordable point-of-care GBS tests are urgently needed, particularly those that also simultaneously provide information about antibiotic sensitivities.
GBS has 10 known serotypes; however, five (Ia, Ib, II, III, and V) colonize the rectovaginal tracts of women in all regions, accounting for 98% of serotypes globally [9]. Moreover, these five serotypes represent 97% of invasive isolates in all regions with serotype data [7], with serotype III the most prevalent serotype across the United Nations sub-regions. Our study also identified serotype III as the most prevalent, followed by Ia and II serotypes. This is similar to other studies that report serotype III as the first- or second-most common isolate colonizing women [64]. Knowing which serotypes are most widespread will be important when GBS conjugate vaccines become available [65]. Therefore, further studies are needed to know which serotypes are responsible for both colonization and invasive neonatal disease in the MENA region, including Jordan.
Our results should be viewed in light of a number of limitations. This study was conducted at one facility – a government hospital – over 2 months and may not be generalizable to other settings in Jordan or the MENA region. However, the frequency of GBS colonization is similar to the average reported for all MENA regions. Furthermore, we are unable to report neonatal outcome information and our use of one senior microbiologist may have affected our results. In addition, we did not test for antibiotic susceptibility, which is important considering reports of increasing erythromycin and clindamycin resistance in GBS [66]. Still, given the dearth of information about GBS in Jordan and the wider MENA region, our results help to shed light on the high prevalence of GBS colonization among pregnant women.
Conclusions
We found high prevalence of GBS and both under- and over-treatment of GBS among pregnant women in Jordan. These results highlight the unmet need for routine GBS testing during pregnancy and support expanded research in the region, including defining the GBS resistance patterns, serotyping information, risk factors, and neonatal outcomes. They also emphasize the need for improved rapid GBS diagnostics for developing world settings.
References
Benitz WE, Gould JB, Druzin ML. Risk factors for early-onset group B streptococcal sepsis: estimation of odds ratios by critical literature review. Pediatrics. 1999;103(6):e77.
Tudela CM, Stewart RD, Roberts SW, Wendel GD, Stafford IA, McIntire DD, et al. Intrapartum evidence of early-onset group B streptococcus. Obstet Gynecol. 2012;119(3):626–9.
Nan C, Dangor Z, Cutland CL, Edwards MS, Madhi SA, Cunnington MC. Maternal group B Streptococcus-related stillbirth: a systematic review. BJOG. 2015;122(11):1437–45.
Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, et al. Bacterial meningitis in the United States, 1998-2007. N Engl J Med. 2011;364(21):2016–25.
Melin P, Efstratiou A. Group B streptococcal epidemiology and vaccine needs in developed countries. Vaccine. 2013;31(Suppl 4):D31–42.
Edmond KM, Kortsalioudaki C, Scott S, Schrag SJ, Zaidi AKM, Cousens S, et al. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet. 2012;379(9815):547–56.
Madrid L, Seale AC, Kohli-Lynch M, Edmond KM, Lawn JE, Heath PT, et al. Infant group B streptococcal disease incidence and serotypes worldwide: systematic review and meta-analyses. Clin Infect Dis. 2017;65(suppl_2):S160–72.
Schrag SJ, Verani JR. Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine. 2013;31(Suppl 4):D20–6.
Russell NJ, Seale AC, O’Driscoll M, O’Sullivan C, Bianchi-Jassir F, Gonzalez-Guarin J, et al. Maternal colonization with group B Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin Infect Dis. 2017;65(suppl_2):S100–11.
Di RGC, Melin P, Berardi A, Blennow M, Carbonell-Estrany X, Donzelli GP, et al. GBS screening and antibiotic prophylaxis: a European consensus conference. J Matern Fetal Neonatal Med. 2015;28(7):766–82.
Kwatra G, Cunnington MC, Merrall E, Adrian PV, Ip M, Klugman KP, et al. Prevalence of maternal colonisation with Group B streptococcus: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16(9):1076–84.
Wassef M, Ghaith D, Abdella RMA, Kamel M. Rapid screening for Group B Streptococcus in near-term pregnant women by Granada™ biphasic broth. J Matern Fetal Neonatal Med. 2017;30(13):1540–3.
Shabayek S, Abdalla S, Abouzeid AM. Serotype and surface protein gene distribution of colonizing Group B streptococcus in women in Egypt. Epidemiol Infect. 2014;142(1):208–10.
Elbaradie SMY, Mahmoud M, Farid M. Maternal and neonatal screening for Group B streptococci by SCP B gene based PCR: a preliminary study. Indian J Med Microbiol. 2009;27(1):17–21.
Shabayek SAAE-K, Abdalla SM, Abouzeid AMH. Vaginal carriage and antibiotic susceptibility profile of Group B Streptococcus during late pregnancy in Ismailia, Egypt. J Infect Public Health. 2009;2(2):86–90.
Darabi R, Tadi S, Mohit M, Sadeghi E, Hatamizadeh G, Kardeh B, et al. The prevalence and risk factors of Group B streptococcus colonization in Iranian pregnant women. Electron Physician. 2017;9(5):4399–404.
Mousavi SM, Hosseini SM, Mashouf RY, Arabestani MR. Identification of Group B streptococci using 16S rRNA, cfb, scpB, and atr genes in pregnant women by PCR. Acta Med Iran. 2016;54(12):765–70.
Sadeh M, Firouzi R, Derakhshandeh A, Bagher Khalili M, Kong F, Kudinha T. Molecular characterization of Streptococcus agalactiae isolates from pregnant and non-pregnant women at Yazd University hospital, Iran. Jundishapur J Microbiol. 2016;9(2):e30412.
Bidgani S, Navidifar T, Najafian M, Amin M. Comparison of Group B streptococci colonization in vaginal and rectal specimens by culture method and polymerase chain reaction technique. J Chin Med Assoc. 2016;79(3):141–5.
Goudarzi G, Ghafarzadeh M, Shakib P, Anbari K. Culture and real-time PCR based maternal screening and antibiotic susceptibility for Group B Streptococcus: an Iranian experience. Glob J Heal Sci. 2015;7(6):45075.
Hadavand S, Ghafoorimehr F, Rajabi L, Davati A, Zafarghandi N. Frequency of Group B streptococcal colonization in pregnant women aged 35- 37 weeks in clinical centers of Shahed University, Tehran, Iran. Iran J Pathol. 2015;10(2):120–6.
Shirazi M, Abbariki E, Hafizi A, Shahbazi F, Bandari M, Dastgerdy E. The prevalence of Group B streptococcus colonization in Iranian pregnant women and its subsequent outcome. Int J Fertil Steril. 2014;7(4):267–70.
Javanmanesh F, Eshraghi N. Prevalence of positive recto-vaginal culture for Group B streptococcus in pregnant women at 35-37 weeks of gestation. Med J Islam Repub Iran. 2013;27(1):7–11.
Tajbakhsh S, Norouzi Esfahani M, Emaneini M, Motamed N, Rahmani E, Gharibi S. Identification of Streptococcus agalactiae by fluorescent in situ hybridization compared to culturing and the determination of prevalence of Streptococcus agalactiae colonization among pregnant women in Bushehr, Iran. BMC Infect Dis. 2013;13:420.
Hamedi A, Akhlaghi F, Seyedi SJ, Kharazmi A. Evaluation of group B streptococci colonization rate in pregnant women and their newborn. Acta Med Iran. 2012;50(12):805–8.
Hassanzadeh P, Motamedifar M, Gharaghani MN. Carriage rate of group B streptococci in pregnant women in three teaching hospitals in shiraz, Iran. Med Princ Pract. 2011;20(3):277–82.
Namavar Jahromi B, Poorarian S, Poorbarfehee S. The prevalence and adverse effects of group B streptococcal colonization during pregnancy. Arch Iran Med. 2008;11(6):654–7.
Hakim M, Jabour A, Anton M, Hakim M, Kheirallah S. Screening Arab Israeli pregnant women for Group B Streptococcus by the ampliVue GBS assay: are the rates higher than the national average? Isr Med Assoc J. 2018;5(20):291–4.
Sefty H, Klivitsky A, Bromberg M, Dichtiar R, Ben AM, Shohat T, et al. Factors associated with choice of approach for Group B streptococcus screening. Isr J Heal Policy Res. 2016;5(1):42.
Kabiri D, Hants Y, Yarkoni TR, Shaulof E, Friedman SE, Paltiel O, et al. Antepartum membrane stripping in GBS carriers, is it safe? (the STRIP-G study). Tse H, editor PLoS One. 2015;10(12):e0145905.
Ganor-Paz Y, Kailer D, Shechter-Maor G, Regev R, Fejgin MD, Biron-Shental T. Obstetric and neonatal outcomes after preterm premature rupture of membranes among women carrying Group B streptococcus. Int J Gynaecol Obstet. 2015;129(1):13–6.
Eisenberg VH, Raveh D, Meislish Y, Rudensky B, Ezra Y, Samueloff A, et al. Prevention of early-onset neonatal Group B streptococcal infection: is universal screening by culture universally applicable? Isr Med Assoc J. 2006;8(10):698–702.
Marchaim D, Hallak M, Gortzak-Uzan L, Peled N, Riesenberg K, Schlaeffer F. Cell wall proteins of Group B Streptococcus and low incidence of neonatal disease in southern Israel. J Reprod Med. 2003;48(9):697–702.
Eidelman AI, Rudensky B, Turgeman D, Nubani N, Schimmel MS, Isacsohn M. Epidemiology of Group B streptococci colonization and disease in mothers and infants: update of ongoing 10-year Jerusalem study. Isr J Med Sci. 1990;26(2):71–3.
Sunna E, El-Daher N, Bustami K, Na’was T. A study of Group B streptococcal carrier state during late pregnancy. Trop Geogr Med. 1991;43(1–2):161–4.
Ghaddar N, Alfouzan W, Anastasiadis E, Al Jiser T, Itani SE, Dernaika R, et al. Evaluation of chromogenic medium and direct latex agglutination test for detection of Group B streptococcus in vaginal specimens from pregnant women in Lebanon and Kuwait. J Med Microbiol. 2014;63(Pt 10):1395–9.
Al-Sweih N, Hammoud M, Al-Shimmiri M, Jamal M, Neil L, Rotimi V. Serotype distribution and mother-to-baby transmission rate of Streptococcus agalactiae among expectant mothers in Kuwait. Arch Gynecol Obstet. 2005;272(2):131–5.
Seoud M, Nassar AH, Zalloua P, Boghossian N, Ezeddine J, Fakhoury H, et al. Prenatal and neonatal Group B Streptococcus screening and serotyping in Lebanon: incidence and implications. Acta Obstet Gynecol Scand. 2010;89(3):399–403.
Moraleda C, Benmessaoud R, Esteban J, López Y, Alami H, Barkat A, et al. Prevalence, antimicrobial resistance and serotype distribution of Group B streptococcus isolated among pregnant women and newborns in Rabat. Morocco J Med Microbiol. 2018;67(5):652–61.
Bassir A, Dhibou H, Farah M, Mohamed L, Amal A, Nabila S, et al. Portage vaginal du streptocoque du groupe B chez la femme enceinte au niveau de la région de Marrakech. Pan Afr Med J. 2016;23:107.
Khan MA, Faiz A, Ashshi AM. Maternal colonization of Group B streptococcus: prevalence, associated factors and antimicrobial resistance. Ann Saudi Med. 2015;35(6):423–7.
Zamzami TY, Marzouki AM, Nasrat HA. Prevalence rate of Group B streptococcal colonization among women in labor at king Abdul-Aziz University hospital. Arch Gynecol Obstet. 2011;284(3):677–9.
El-Kersh TA, Al-Nuaim LA, Kharfy TA, Al-Shammary FJ, Al-Saleh SS, Al-Zamel FA. Detection of genital colonization of Group B streptococci during late pregnancy. Saudi Med J. 2002;23(1):56–61.
Jerbi M, Hidar S, Hannachi N, El Moueddeb S, Djebbari H, Boukadida J, et al. Risk factors for Group B streptococcal colonization in pregnant women at term: prospective study of 294 cases. Gynecol Obs Fertil. 2007;35(4):312–6.
Ferjani A, Ben Abdallah H, Ben Saida N, Gozzi C, Boukadida J. Vaginal colonization of the Streptococcus agalactiae in pregnant woman in Tunisia: risk factors and susceptibility of isolates to antibiotics. Bull Soc Pathol Exot. 2006;99(2):99–102.
Alp F, Findik D, Dagi HT, Arslan U, Pekin AT, Yilmaz SA. Screening and genotyping of group B streptococcus in pregnant and non-pregnant women in Turkey. J Infect Dev Ctries. 2016;10(3):222–6.
Eren A, Küçükercan M, Oğuzoğlu N, Unal N, Karateke A. The carriage of group B streptococci in Turkish pregnant women and its transmission rate in newborns and serotype distribution. Turk J Pediatr. 2005;47(1):28–33.
Kadanali A, Altoparlak U, Kadanali S. Maternal carriage and neonatal colonisation of group B streptococcus in eastern Turkey: prevalence, risk factors and antimicrobial resistance. Int J Clin Pract. 2005;59(4):437–40.
Barbaros I, Murat C, Mehmet V, Ismet TA, Can K, Sukufe D, et al. The colonization incidence of group B streptococcus in pregnant women and their newborns in Istanbul. Pediatr Int. 2005;47(1):64–6.
Amin A, Abdulrazzaq YM, Uduman S. Group B streptococcal serotype distribution of isolates from colonized pregnant women at the time of delivery in United Arab Emirates. J Inf Secur. 2002;45(1):42–6.
Sidky I, Thomas M. Prevalence of Group B streptococcal infection colonisation in pregnant women and their offspring in the Middle East. J Obstet Gynaecol. 2002;22(2):179–80.
Verani JR, McGee L, Schrag SJ. Prevention of perinatal Group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59(RR-10):1–36.
Khatami A, Randis TM, Chamby A, Hooven TA, Gegick M, Suzman E, et al. Improving the sensitivity of real-time PCR detection of Group B Streptococcus using consensus sequence-derived oligonucleotides. Open Forum Infect Dis. 2018;5(7):ofy164.
Khatami A, Randis TM, Tavares L, Gegick M, Suzman E, Ratner AJ. Vaginal co-colonization with multiple Group B Streptococcus serotypes. Vaccine. 2019;37(3):409–11.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–81.
Yusef D, Shalakhti T, Awad S, Khasawneh W. Clinical characteristics and epidemiology of sepsis in the neonatal intensive care unit in the era of multi-drug resistant organisms: a retrospective review. Pediatr Neonatol. 2018;59:35–41.
Boyer KM, Gotoff SP. Prevention of early-onset neonatal Group B streptococcal disease with selective intrapartum chemoprophylaxis. N Engl J Med. 1986;314(26):1665–9.
Tuppurainen N, Hallman M. Prevention of neonatal Group B streptococcal disease: intrapartum detection and chemoprophylaxis of heavily colonized parturients. Obstet Gynecol. 1989;73(4):583–7.
Rallu F, Barriga P, Scrivo C, Martel-Laferrière V, Laferrière C. Sensitivities of antigen detection and PCR assays greatly increased compared to that of the standard culture method for screening for Group B streptococcus carriage in pregnant women. J Clin Microbiol. 2006;44(3):725–8.
Konikkara KP, Baliga S, Shenoy S, Bharati B. Evaluation of culture, antigen detection and polymerase chain reaction for detection of vaginal colonization of Group B Streptococcus (GBS) in pregnant women. J Clin Diagn Res. 2014;8(2):47–9.
Helmig RB, Gertsen JB. Diagnostic accuracy of polymerase chain reaction for intrapartum detection of Group B streptococcus colonization. Acta Obs Gynecol Scand. 2017;96(9):1070–4.
El Helali N, Nguyen J, Ly A, Giovangrandi Y, Trinquart L. Diagnostic accuracy of a rapid real-time polymerase chain reaction assay for universal intrapartum Group B Streptococcus screening. Clin Infect Dis. 2009;49(3):417–23.
Gray JW, Milner PJ, Edwards EH, Daniels JP, Khan KS. Feasibility of using microbiology diagnostic tests of moderate or high complexity at the point - of - care in a delivery suite. J Obs Gynaecol. 2012;32(5):458–60.
Madzivhandila M, Adrian PV, Cutland CL, Kuwanda L, Schrag SJ, Madhi SA. Serotype distribution and invasive potential of Group B Streptococcus isolates causing disease in infants and colonizing maternal-newborn dyads. PLoS One. 2011;6(3):e17861.
Kobayashi M, Vekemans J, Baker CJ, Ratner AJ, Le Doare K, Schrag SJ. Group B Streptococcus vaccine development: present status and future considerations, with emphasis on perspectives for low and middle income countries. F1000Res. 2016;5:2355.
Borchardt SM, DeBusscher JH, Tallman PA, Manning SD, Marrs CF, Kurzynski TA, et al. Frequency of antimicrobial resistance among invasive and colonizing Group B streptococcal isolates. BMC Infect Dis. 2006;6:57.
Acknowledgements
We gratefully acknowledge the study participants, Hanan Amin, Hanaa Khalaf, and staff at Al-Bashir Hospital, without whom this study would not be possible.
Funding
Support for this study was received from the Anne Potter Wilson Foundation through the Vanderbilt Institute for Global Health. K.C. was supported by award number K01 MH107256 from the National Institute of Mental Health (NIMH). A.J.R. was supported by award R56 AI136499 from the National Institute of Allergy and Infectious Diseases (NIAID). D.M.A. was supported by NIH grants R01 HD090061, R01 AI134036 and a March of Dimes Research Grant. REDCap software and support was provided by grant support to the Vanderbilt Institute for Clinical and Translational Research (UL1 TR000445 from NCATS/NIH). The funding agencies played no role in the design of the study, collection, analysis, interpretation of data, or in writing the manuscript.
Availability of data and materials
Informed consent was not obtained for publication of participant data. The datasets used and/or analyzed during the current study are available to fellow researchers from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
NH, KC, KBF, DMA, NK-B, AS, AMS and SF conceived the study. NH directed all study activities. KC led the statistical analysis and wrote the first draft of the paper. AS led the microbiology testing and result interpretation. ABC, TMR and AJR led the serotyping analysis. KC, AS, AMS, SF, NK-B, AAJ, JC, KBF, ABC, TMR, AJR, DMA and NH contributed to the drafting and revising of the final manuscript, have approved the submitted version, and have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study conducted in accordance with the Declaration of Helsinki and was approved by the Human Research Protections Program at Vanderbilt University Medical Center, University of Jordan Institutional Review Board, and the Jordan Ministry of Health. All participants provided written informed consent prior to enrollment. To maintain participant confidentiality, unique study identification numbers were used in lieu of personal identifiers.
Consent for publication
Not applicable (no individual data).
Competing interests
N.H. has received past vaccine and lab support from Sanofi, and previously served as a consultant for Moderna. A.J.R. has been a consultant for Pfizer. None of these past interests were related to the present study. We declare no other conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Clouse, K., Shehabi, A., Suleimat, A.M. et al. High prevalence of Group B Streptococcus colonization among pregnant women in Amman, Jordan. BMC Pregnancy Childbirth 19, 177 (2019). https://doi.org/10.1186/s12884-019-2317-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-019-2317-4