Abstract
Background
Several recent studies have reported subacute combined degeneration (SCD) induced by nitrous oxide (N2O) abuse. However, the association between the evolution of dynamic neuroimaging and clinical manifestations has not been reported in patients with N2O-induced SCD.
Case presentation
We described the case of a 24-year-old man who developed SCD with inverted V-sign hyperintensities over the posterior aspect of the spinal cord caused by frequent, excessive N2O inhalation. One month after treatment, his weakness and paresthesia resolved and serum vitamin B12 levels exceeded the normal levels. However, the hyperintensities had extended horizontally and longitudinally on T2-weighted magnetic resonance imaging (MRI), compared to those on the initial scan. Two months after treatment, the patient experienced some residual numbness in the distal limbs, and his serum homocysteine levels were normal, but the abnormal signals seen on cervical T2-weighted MRI had decreased only slightly compared to those seen on the one-month follow-up MRI. The evolution of conventional MRI findings lagged compared to the clinical manifestation, which was suggestive of a clinical-radiological dissociation.
Conclusions
Clinical-radiological dissociation might have occurred in this case because T2-weighted imaging was not sensitive enough to reveal cytotoxic edema. Moreover, the serum vitamin B12 level is not a good indicator of cellular vitamin B12. Thus, clinicians should recognize this phenomenon, comprehensively assess the condition of patients with N2O-induced SCD, and avoid terminating treatment based on the resolution of clinical symptoms and serological results.
Similar content being viewed by others
Background
Subacute combined degeneration (SCD) is a neurological complication of vitamin B12 deficiency, which is typically observed in elderly individuals with malabsorption syndromes and inadequate intake or bioavailability of vitamin B12. [1]. Recently, several sporadic cases of otherwise healthy young adults with SCD induced by nitrous oxide (N2O) abuse have been described [2]. However, to the best of our knowledge, none of these studies have reported a relationship between the evolution of dynamic neuroimaging and clinical manifestations in a patient with N2O-induced SCD. Treatment is frequently discontinued by individuals who abuse N2O following improvement in the neurological symptoms [3]. Moreover, guidelines for treatment duration have not been established for patients with SCD. Therefore, we reported the case of a young man diagnosed with SCD caused by extensive N2O inhalation that led to clinical-radiological dissociation. Furthermore, we highlighted the fact that the condition of patients with N2O-induced SCD cannot be determined by imaging abnormalities, clinical manifestations, or serum vitamin B12 levels alone.
Case presentation
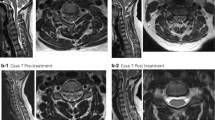
A 24-year-old man in a wheel-chair presented with numbness in all extremities and worsening lower-extremity weakness for approximately 20 days. He had inhaled N2O through an average of approximately 100–200 “whippit” cartridges per day for at least 3 months for recreational purposes. The patient had good dietary intake without alcohol use and did not have a history of smoking or illicit drug use. Neurological examination revealed clear consciousness with fluent speech. No abnormalities were detected in the cranial nerves. However, mild weakness in the upper limbs (grade 4), severe weakness in the lower limbs (grade 3), marked increase in the deep tendon reflexes, impaired joint position and vibration sensation, sensory ataxia, positive bilateral Babinski sign, and positive Romberg and Lhermitte’s signs were observed on neurological examination. Laboratory tests revealed decreased levels of serum red blood cell (RBC) (3.32 × 1012 /L, reference range: 4.30–5.80 × 1012 /L), hemoglobin (Hb) (118.4 g/L, reference range: 130–175 g/L), vitamin B12 (98.2 pmol/L, reference range: 145–637 pmol/L), and folic acid (8.38 nmol/L, reference range: 8.83–60.80 nmol/L). Serum homocysteine (Hcy) was considerably elevated (> 50 μmol/L, reference range: 5.46–16.20 μmol/L), which was indicative of functional vitamin B12 deficiency at the cellular level. Cerebrospinal fluid (CSF) assessment yielded normal results. The inflammatory, infectious, and immune biomarkers in CSF and serum were unremarkable. Sagittal spinal cord magnetic resonance imaging (MRI) revealed hyperintensities involving the posterior columns from C2 to C6 on T2-weighted images (Fig. 1a), with an inverted V-sign on axial MRI (Fig. 1b). Brain and thoracic MRI findings were normal.
Magnetic resonance imaging of the cervical spinal cord. a. Sagittal T2-weighted imaging showed increased intramedullary signal intensity along the posterior column of the spinal cord extending from C2 to C6 on August 3, 2018. b. A V-shaped hyperintensity on axial T2-weighted imaging at the C4 level was seen within the dorsal cervical spinal cord on August 3, 2018. c. Sagittal T2-weighted imaging showed abnormal, longitudinally and horizontally extensive hyperintensities involving the lateral and posterior columns of the spinal cord extending from C1 through T2 on September 3, 2018. d. Axial T2-weighted imaging at the C4 level showed a ball-shaped hyperintensity on September 3, 2018. e. Sagittal T2-weighted imaging showed hyperintensity along the posterior column of the spinal cord extending from C1 to T1 on October 4, 2018. f. Axial T2-weighted imaging at the C4 level showed that the V-shaped hyperintensity had decreased compared to that in September (d)
The patient was diagnosed with SCD of the spinal cord induced by N2O abuse. Treatment with a high dose of supplementary intramuscular vitamin B12 injections (1.5 mg per day), oral folic acid (15 mg per day), and abstinence from N2O led to a gradual improvement in the patient’s symptoms. One month later, the symptoms of weakness and paresthesia were resolved; the patient could walk unsupported with some residual gait impairment. The serum RBC, Hb, and folic acid levels improved to normal, and the serum vitamin B12 level increased to more than 1476 pmol/L (the maximum measurable value). However, the patient’s serum Hcy remained elevated (19.02 μmol/L). At this time, we observed an interesting phenomenon. The hyperintensities on T2-weighted images had extended, both horizontally and longitudinally, from C1 to T2 (Fig. 1c), resembling a “ball” on the axial images, despite the improvement in the patient’s clinical symptoms and laboratory values (Fig. 1. d). Importantly, the patient had had no exposure to hormones or N2O. He was discharged with a prescription for vitamin B12 supplements. The patient’s gait had improved, and he experienced only mild paresthesia of the distal limbs during the two-month follow-up. Serum Hcy had improved to normal levels. Moreover, the abnormal signals seen on T2-weighted images had decreased compared to those on the one-month follow-up MRI, but were still more extensive than those seen on the initial MRI (Fig. 1 e, f). It seemed that a clinical-radiological dissociation had occurred, and conventional MRI findings had consistently lagged behind the clinical and laboratory manifestations.
Discussion and conclusions
Although several cases of SCD associated with vitamin B12 deficiency induced by N2O abuse have been described, the relationship between the evolution of dynamic neuroimaging and clinical manifestations has never been reported. To the best of our knowledge, this is the first report of clinical-neuroimaging dissociation in a patient with N2O-induced SCD.
N2O induces SCD by irreversibly oxidizing the cobalt ion of vitamin B12 (cobalamin). The highly nucleophilic cobalamin (1+) ion, which is a product of the methylation of Hcy (to form methionine), commonly reacts with methyltetrahydrofolate to regenerate methylcobalamin [4]. Once the cobalt ion is oxidized by N2O, methylcobalamin, as a cofactor of methionine synthase in the transfer of Hcy to methionine, subsequently inhibits S-adenosylmethionine, which is essential for the methylation of myelin sheath phospholipids [5]. Thus, inactivation of vitamin B12 metabolism results in the demyelination of the spinal cord [6].
Few patients with cobalamin deficiency have normal serum vitamin B12 levels. According to the metabolic pathway described above, a normal serum level of vitamin B12 is not indicative of the precise or timely cellular availability of vitamin B12. Instead, elevated serum levels of Hcy or methylmalonic acid are better biomarkers for the diagnosis of cellular vitamin B12 deficiency [7]. Although the serum levels of vitamin B12 and folic acid returned to normal in this patient, elevated Hcy levels showed greater value as an indicator of cellular vitamin B12 deficiency. Thus, demyelination of the cervical spinal cord may still exist in this patient even if the serum vitamin B12 and folic acid levels are normal.
Moreover, the lag in the conventional MRI findings compared to clinical manifestations was similar to that seen in central pontine myelinolysis (CPM). In 1996, SCD was classified as a pure myelinolytic disease with no apparent loss of myelin or areas of partial neuropathological remyelination [8]. Hence, we surmise that the clinical-radiological dissociation observed in our case may be related to the neuropathological basis of intramedullary and interstitial edema, similar to that observed in CPM. Hyperintensity on spinal cord diffusion-weighted imaging (DWI) and a corresponding hypointensity on the apparent diffusion coefficient maps have been previously reported in patients with SCD [9, 10]. These acute demyelinating lesions manifest as restricted diffusion, indicating an energy failure, which results in cytotoxic edema.
DWI provides quantitative and qualitative functional information on the microdiffusion of water molecules at the cellular level and has been widely used for the evaluation of a variety of brain disorders, such as acute cerebral infarction [11]. Similarly, DWI is superior to T2-weighted imaging for the diagnosis of cytotoxic edema in the early stages. Hence, we hypothesize that T2-weighted imaging is not sensitive enough to reflect the early intramedullary and interstitial cytotoxic edema caused by SCD, which may be another possible reason for the clinical-imaging dissociation in the present case.
In conclusion, we recommend that N2O abuse should be considered in patients presenting with SCD, especially if the patient is young and otherwise healthy. The inability of serum vitamin B12 to reflect cellular vitamin B12 levels and that of T2-weighted imaging in revealing cytotoxic edema in the early stages may have contributed to the clinical-imaging dissociation. Thus, clinicians should comprehensively assess the condition of patients with N2O-induced SCD, avoid terminating treatment due to the resolution of clinical symptoms and serological findings, and carefully evaluate worsening imaging results for possible clinical-imaging dissociation.
Availability of data and materials
Data has not been made accessible in the interest of protecting the patient’s privacy.
Abbreviations
- CPM:
-
central pontine myelinolysis
- CSF:
-
cerebrospinal fluid
- DWI:
-
diffusion-weighted imaging
- Hb:
-
hemoglobin
- Hcy:
-
homocysteine
- MRI:
-
magnetic resonance imaging
- N2O:
-
nitrous oxide
- RBC:
-
red blood cells
- SCD:
-
subacute combined degeneration
References
Green R, Allen LH, Bjørke-Monsen AL, Brito A, Guéant JL, Miller JW, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3:17040.
Patel KK, Mejia Munne JC, Gunness VRN, Hersey D, Alshafai N, Sciubba D, et al. Subacute combined degeneration of the spinal cord following nitrous oxide anesthesia: a systematic review of cases. Clin Neurol Neurosurg. 2018;173:163–8.
Cao J, Su ZY, Xu SB, Liu CC. Subacute combined degeneration: a retrospective study of 68 cases with short-term follow-up. Eur Neurol. 2018;79:247–55.
Jordan JT, Weiser J, Van Ness PC. Unrecognized cobalamin deficiency, nitrous oxide, and reversible subacute combined degeneration. Neurol Clin Pract. 2014;4:358–61.
Hathout L, El-Saden S. Nitrous oxide-induced B12 deficiency myelopathy: perspectives on the clinical biochemistry of vitamin B12. J Neurol Sci. 2011;301:1–8.
Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ. 2014;349:g5226.
Briani C, Dalla Torre C, Citton V, Manara R, Pompanin S, Binotto G, et al. Cobalamin deficiency: clinical picture and radiological findings. Nutrients. 2013;5:4521–39.
Powers J. Pathology of myelin. Mol Chem Neuropathol. 1996;27:31–8.
Tian C. Hyperintense signal on spinal cord diffusion-weighted imaging in a patient with subacute combined degeneration. Neurol India. 2011;59:429–31.
Kim EY, Lee SY, Cha SH, Yi KS, Cho BS, Kang MH. Subacute combined degeneration revealed by diffusion-weighted imaging: a case study. Clin Neuroradiol. 2013;23:157–9.
Simonsen CZ, Madsen MH, Schmitz ML, Mikkelsen IK, Fisher M, Andersen G. Sensitivity of diffusion- and perfusion-weighted imaging for diagnosing acute ischemic stroke is 97.5%. Stroke. 2015;46:98–101.
Acknowledgments
The authors would like to thank the patient and his family for their participation and help.
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This study was supported by the National Natural Science Foundation of China (81871104). The funding body supplied this manuscript with funding for data collection, analysis, and interpretation, as well as writing the manuscript.
Author information
Authors and Affiliations
Contributions
JJ: conceptualization and design of the study, interpretation of data, drafting and revising the manuscript. XS: critical revision of the manuscript for important intellectual content, study supervision. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent for publication was obtained from the patient. A copy of the written consent is available for review by the editor upon request.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, J., Shang, X. Clinical-radiological dissociation in a patient with nitrous oxide-induced subacute combined degeneration: a case report. BMC Neurol 20, 99 (2020). https://doi.org/10.1186/s12883-020-01685-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-020-01685-5