Abstract
Background
The exact relationship between 25-hydroxyvitamin D [25(OH) D] levels and small vessel disease (SVD) are not clear in China. The aim of this study was to determine such the association between 25(OH) D and SVD in China.
Methods
We retrospectively enrolled 106 patients with SVD and 115 controls between Jan 2017 and Dec 2017. All the subjects were categorized into three subgroups according to the level of 25 (OH) D: vitamin D deficiency (< 12 ng/ml), insufficiency (12–20 ng/ml) and sufficiency (> 20 ng/ml).
Results
Among 106 SVD patients, 80 (75.5%) were men and the mean age was 61.6 ± 13.2 years. The deficiency of 25(OH) D was observed in 76 (71.7%) of SVD patients and 47 (40.9%) of controls (P = 0.001). Compared with controls, patients with SVD were more likely to be male, a stroke history, smokers, with hyperlipidemia, higher systolic and diastolic blood pressure and low-density lipoprotein, and lower of 25(OH)D level (P < 0.05). Logistic regression analysis revealed the level of 25 (OH) D as an independent predictor of SVD (OR 0.772, 95% CI 0.691–0.862, P = 0.001). Compared with the sufficient 25 (OH) D group, the ORs of SVD in deficient and insufficient 25(OH)D group were 5.609 (95% CI 2.006–15.683) and 1.077 (95% CI: 0.338–3.428) after adjusting for potential confounders, respectively. In hypertensives with vitamin D deficient and insufficient group compared with sufficient group, the ORs of SVD increased to 9.738 (95% CI 2.398–39.540) and 1.108 (95% CI 0.232–5.280), respectively (Pinteraction = 0.001).
Conclusion
We found significant associations between SVD and 25(OH)D deficiency. The combined presence of hypertension and vitamin D deficiency increased the probability of developing SVD. Our findings will warrant further prospective studies in the future.
Similar content being viewed by others
Background
Stroke is now recognized to be the second leading cause of death worldwide [1, 2] and the first one in China [3]. Small vessel disease (SVD) is considered to cause 20 to 25% of strokes. According to the STandards for ReportIng Vascular changes on nEuroimaging (STRIVE), there are several neuroimaging features of SVD, including acute small subcortical infarcts, white matter hyperintensities (WMHs), lacunes, microbleeds (CMBs), perivascular spaces (PVS) and brain atrophy [4]. To date, SVD has been recognized to be the second commonest cause of dementia, gait disturbances, affective disorders and late-onset depression [5]. SVD is becoming a major public health issue in Asian countries especially in China [6].
However, SVD is not silent, permanent or untreatable. Identification of controllable and treatable risk factors is essential for effective prevention of SVD. Importantly, accumulating evidence opened new insights and offered new therapeutic targets in recent years. As a fat-soluble vitamin, an increasing body of evidence supported a vital role for vitamin D in brain function and development. Vitamin D may also prevent vascular injury by inhibiting the renin-angiotensin-aldosterone system and atherogenesis [7] and lowering blood pressure. The prevalence of 25-hydroxyvitamin D [25(OH) D] deficiency is high in patients with acute stroke, and it may be associated with greater clinical severity and poor functional prognosis. However, there are only quite a few reports about the exact relationship between 25(OH) D and the occurrence of SVD. It was reported 25(OH)D was inversely associated with lacunes, WMHs and deep CMBs in Korea, which was linked to chronic brain injury associated with SVD [8]. The findings from India also indicated the combined presence of hypertension and vitamin D deficiency increased the probability of developing vascular dementia (VaD) due to SVD, and the intervention of vitamin D status and hypertension could be helpful to reduce the risk of VaD [9]. However, whether these findings could be reproduced in other ethnicities should be further confirmed.
To date, as far as we know, only one study from China reported an association between vitamin D deficiency and total MRI burden of SVD in the south of China [10]. Whether the effect of 25(OH)D on SVD could be modified by the history of hypertension needs to be further elucidated in China. We hypothesized there might be some relationships between 25(OH)D deficiency and the occurrence of SVD in the north of China. Thus, in our present study, we aimed to investigate the exact relationship between 25(OH) D levels and the predictors of SVD in the north of China.
Materials and methods
Subjects
Our study was a case-control study, and we retrospectively recruited 106 patients with SVD within 7 days of onset with local neurological deficits lasting more than 24 h and caused by cerebrovascular etiology in the Department of Neurology, Beijing Chaoyang Hospital and Jinan City people’s hospital between Jan 2017 and Dec 2017. The 115 healthy volunteers who were matched with the patients were also recruited. All the subjects were enrolled by randomization and blindness in order to avoid bias.
As for the sample size, vitamin D deficiency (78%) was very common in ischemic stroke in China [11], and the incidence of vitamin D deficiency in the control group was about 60%. We used the following values to calculate the sample size in our study: α = 0.05, β = 0.20, p1=78% (the proportion of vitamin D deficiency in SVD), p0=60% (the proportion of vitamin D deficiency in controls), q0 = 1-p0 = 40%, q1 = 1-p1 = 22%, 1:1 design, two sides, the sample size N was about 100. The following formulae were used, N = 2(Zα/2 + Zβ )2(p0q0 + p1q1) /(p0 − p1)2.
Detailed neurological examinations were performed in all subjects. The NIH Stroke Scale (NIHSS) and the Rankin scale were to evaluate the stroke severity and disability after a stroke. Magnetic resonance imaging (MRI) was performed in all the patients to determine the presence of SVD, including lacunar infarction, WMHs, CMBs, and PVS according to STRIVE [4]. The exclusion criteria were: (1) age < 18 years old; (2) unable to perform MRI; (3) cardioembolic source of stroke and more than 50% stenosis of the affected large artery; (4) pre-stroke diagnosis of active or chronic inflammatory diseases, depression or other psychiatric disorders, malignant tumor, intracerebral hemorrhage, thyroid diseases, autoimmune diseases, and a history of any central nervous system disease. Our work was approved by the Ethics Committee of Beijing Chaoyang Hospital and Jinan City people’s hospital. Written informed consent was obtained from all participants.
Clinical variables
We obtained the following clinical data: age, gender; vascular risk factors such as hypertension, diabetes mellitus, hyperlipidemia, stroke, coronary heart disease, hyperlipidemia, atrial fibrillation, peripheral arterial disease and smoking. The laboratory blood tests were obtained including the counts of red blood cell, white blood cell, platelet, fibrinogen, fasting blood glucose, hemoglobin A1c, glycated albumin, uric acid, homocysteine, total cholesterol, low-density lipoprotein, high-density lipoprotein, triglyceride, C-reactive protein, albumin, 25-hydroxy vitamin D. As for the vitamin D level, serum levels of 25(OH)D were measured using enzyme immunoassay kits. The subjects were stratified into three subgroups: vitamin D deficiency (< 12 ng/ml), insufficiency (12–20 ng/ml) and sufficiency (> 20 ng/ml) [9, 12], according to the guidelines of the National Osteoporosis Society [12].
Statistical analysis
The data were described using the mean and standard deviation values for continuous variables, the median and interquartile range values for categorical variables, and absolute numbers and percentages for nominal and categorical variables, and we compared the groups using the nonparametric Mann-Whitney U test. We performed a chi-square test to determine the correlation between categorical variables and a t-test between continuous variables. The association between 25(OH)D and SVD was tested using logistic regression analyses, and the odds ratios (ORs) and 95% confidence interval (CI) were estimated. We used the Statistical Package for Social Sciences (SPSS) version 16.0 (SPSS Inc., Chicago, IL, USA) for data analysis. A P value less than 0.05 was considered statistically significant.
Results
A total of 221 subjects were enrolled, and 106 were patients with SVD and 115 for controls. Among 106 SVD patients, 80 (75.5%) were men and the mean age was 61.6 ± 13.2 years. The scores of NIHSS and Rankin scale in SVD group were 3 (1–5) and 1 (0–2), respectively. The demographics, vascular risk factors, and the laboratory findings were presented in Table 1. Compared with controls, patients with SVD were more likely to be male, smokers, with hyperlipidemia, higher systolic and diastolic blood pressure, higher low-density lipoprotein, and a stroke history (P < 0.05).
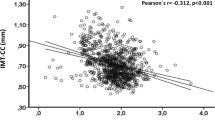
The levels of 25 (OH) D were classified as sufficient in 11 patients (10.4%) vs 25 controls (21.7%), insufficient in 19 patients (17.9%%) vs 43 controls (37.4%), and deficient in 76 patients (71.7%) vs 47 controls (40.9%). The mean serum 25(OH)D level was much lower in SVD patients (10.2 ± 5.6 ng/ml) compared with controls (14.6 ± 7.5 ng/ml) (P = 0.001).
Logistic regression analysis revealed the level of 25(OH)D as an independent predictor of SVD (OR 0.772, 95% CI 0.691–0.862, P = 0.001) after adjusting for the other covariates, shown in Table 2. Compared with the sufficient group of 25(OH)D, we also found the ORs of SVD in the deficient and insufficient group were 5.609 (95% CI 2.006–15.683, P = 0.001) and 1.077 (95% CI 0.338–3.428, P = 0.9) after adjusting for potential confounders, respectively (Table 3).
We also found there was a significant interaction between vitamin D status and hypertension on the presence of SVD (Pinteraction = 0.001). Compared with sufficient group, in hypertensives with vitamin D deficient and insufficient group, the ORs increased to 9.738 (95% CI 2.398–39.540, P = 0.001) and 1.108 (95% CI 0.232–5.280, P = 0.898), respectively (Table 4). However, we did not find such interaction between vitamin D status and hypertension in non-hypertensive patients.
Discussion
In our present study, we found significant associations between SVD and 25(OH)D deficiency. We also found there was a significant interaction between vitamin D status and hypertension in patients with SVD. Random clinical trials should also focus on supplementation of vitamin D in subjects with vitamin D deficiency and particularly those with a history of hypertension.
As a neurosteroid, vitamin D plays a vital role in preventing vascular injury through the mechanism of inhibiting the renin-angiotensin-aldosterone system and atherogenesis [7], lowering blood pressure, reduction in endothelial dysfunction [13], and neuroprotective actions in stroke [14]. Accumulating data have supported the hypothesis lower 25(OH)D level was associated with ischemic stroke, higher mortality and poor outcome [15,16,17,18,19,20,21,22,23,24,25,26], and a higher vitamin D was linked to a decreased risk of cerebrovascular disease [27]. These findings were also substantiated by the evidence of meta-analysis [28]. However, there are very limited studies linking SVD and vitamin D [29, 30]. Recently, some cross-sectional studies proved that 25(OH)D was inversely associated with lacunes, WMHs and deep CMBs [8]. The findings from India also revealed that deficient vitamin D was associated with a 2.2-fold increase in odds of small-vessel related dementia [9]. As for the total burden of SVD, lower 25(OH)D was associated with greater total SVD burden in ischemic stroke [10]. However, the above studies had a limited representation of individuals from China. In our study, we further confirmed patients with SVD were correlated with the deficiency of 25(OH)D. Our study was in line with the former studies from different races including in Nepal, India, Iran and Italy [15, 31,32,33,34,35,36,37].
SVD is associated with vascular risk factors, among which hypertension is considered as an important, preventable risk factor for cardiovascular disease and stroke. Several physiological mechanisms link vitamin D to hypertension because calcitriol acts as an endogenous inhibitor of the Renin-Angiotensin System. There is a growing body of evidence about the association between vitamin D and hypertension in different ethnicities. In Indian population, an inverse association was found between 25(OH)D and risk of ischemic stroke, which indicated the management of hypertension and severe vitamin D deficiency, particularly in hypertensive subjects, could be helpful to prevent stroke effectively [38]. Another observational study also supported a close relationship between 25(OH)D and higher blood pressure [39]. Our results are consistent with the prior studies [9, 38], with the evidence that hypertension may amply aggravate the risk of SVD with lower vitamin D levels [40]. Our findings were also in accordance with the evidence of cardiovascular disease from our research team (Li et al) [41]. As known, both hypertension and vitamin D deficiency are controllable and treatable parameters, thus, monitoring and management of vitamin D and hypertension may reduce the risk of SVD.
However, the mechanism of deficiency of vitamin D and the developing of SVD is not fully understood. It has been reported vitamin D may play an important role in neuroprotection, perhaps through detoxification pathways, stimulation of neurotrophic factors, inhibition of inducible nitric oxide synthase, antioxidation/anti-inflammatory, neuronal calcium regulation, and enhanced nerve conduction [42]. Serum vitamin D was inversely associated with the levels of interleukin-6 and C reactive protein, suggesting a potential anti-inflammatory role for vitamin D underlying in stroke [43, 44]. It is also plausible that vitamin D supplementation could be a beneficial intervention for stroke prevention. Both hypertension and SVD may also have a close relationship with arterial stiffness [45], which may be also due to the deficiency of vitamin D [46], however, data from randomized clinical trials are needed to clarify these speculations [47].
Our study also had some limitations. First, this study was designed with limited samples especially after categorized into three subgroups, and we could not prove a causal relationship between 25(OH)D and SVD. Studies with larger numbers from multiple centers in China are needed urgently to further confirm our findings. Second, the multiple other factors such as nutrition status, physical activity, serum calcium and phosphate, health education, social status, inflammatory markers, seasonal categories were not available in our study [9, 38, 48]. In addition, serum parathyroid hormone and alkaline phosphatase must be taken into account when considering vitamin D metabolism [49, 50]. Furthermore, vitamin D receptor gene polymorphism may protect against neurological deficits and neuronal death, however, we did not test vitamin D receptor activation in our study [51]. Third, we did not classify SVD into different types according to STRIVE such as lacunes, WMHs, CMBs or PVS [8, 52], and we did not assess the total SVD burden (0 to 4) [10]. Further studies are required to confirm this association and explore the association among different subtypes of SVD [8, 17]. Fourth, there were some different categories according to levels of vitamin D (stratified into 2 or 3 or 5) in different studies [38, 53], such as deficiency (< 25 nmol/L or < 20 ng/mL), insufficiency (25–50 nmol/L or 20–30 ng/mL), sufficiency (≥50 nmol/L or ≥ 30 ng/mL) [44, 54, 55]. In spite of these limitations, to the best of our knowledge, this is the first study to report a link between vitamin D status and hypertension associated with SVD in the north of China. However, our findings also need to be validated in a larger study in other regions in China.
Conclusion
We found significant associations between SVD and 25(OH)D deficiency. The combined presence of hypertension and vitamin D deficiency increased the probability of SVD. Our findings will warrant further prospective studies or interventional studies with large samples in the future.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon request.
Abbreviations
- 25(OH) D:
-
25-hydroxyvitamin D
- CI:
-
confidence interval
- CMBs:
-
microbleeds
- OR:
-
odds ratio
- PVS:
-
perivascular spaces
- STRIVE:
-
the STandards for ReportIng Vascular changes on nEuroimaging
- SVD:
-
small vessel disease
- VaD:
-
vascular dementia
- WMHs:
-
white matter hyperintensities
References
Donkor ES. Stroke in the 21(st) century: a snapshot of the burden, epidemiology, and quality of life. Stroke Res Treat. 2018;2018:3238165.
Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38(2):208–11.
Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, et al. Prevalence, incidence, and mortality of stroke in China: results from a Nationwide population-based survey of 480 687 adults. Circulation. 2017;135(8):759–71.
Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–38.
Wardlaw JM, William M. Feinberg award for excellence in clinical stroke: small vessel disease; a big problem. But Fixable Stroke. 2018;49(7):1770–5.
Hilal S, Mok V, Youn YC, Wong A, Ikram MK, Chen CL. Prevalence, risk factors and consequences of cerebral small vessel diseases: data from three Asian countries. J Neurol Neurosurg Psychiatry. 2017;88(8):669–74.
Michos ED, Melamed ML. Vitamin D and cardiovascular disease risk. Curr Opin Clin Nutr Metab Care. 2008;11(1):7–12.
Chung PW, Park KY, Kim JM, Shin DW, Park MS, Chung YJ, et al. 25-hydroxyvitamin D status is associated with chronic cerebral small vessel disease. Stroke. 2015;46(1):248–51.
Prabhakar P, Chandra SR, Supriya M, Issac TG, Prasad C, Christopher R. Vitamin D status and vascular dementia due to cerebral small vessel disease in the elderly Asian Indian population. J Neurol Sci. 2015;359(1–2):108–11.
Feng C, Tang N, Huang H, Zhang G, Qi X, Shi F. 25-Hydroxy vitamin D level is associated with total MRI burden of cerebral small vessel disease in ischemic stroke patients. Int J Neurosci. 2018:1–6.
Tu WJ, Zhao SJ, Xu DJ, Chen H. Serum 25-hydroxyvitamin D predicts the short-term outcomes of Chinese patients with acute ischaemic stroke. Clin Sci (Lond). 2014;126(5):339–46.
Aspray TJ, Bowring C, Fraser W, Gittoes N, Javaid MK, Macdonald H, et al. National Osteoporosis Society vitamin D guideline summary. Age Ageing. 2014;43(5):592–5.
Al-Daghri NM, Bukhari I, Yakout SM, Sabico S, Khattak MNK, Aziz I, et al. Associations of serum nitric oxide with vitamin D and other metabolic factors in apparently healthy adolescents. Biomed Res Int. 2018;2018:1489132.
Mozos I, Marginean O. Links between vitamin D deficiency and cardiovascular diseases. Biomed Res Int. 2015;2015:109275.
Sun Q, Pan A, Hu FB, Manson JE, Rexrode KM. 25-Hydroxyvitamin D levels and the risk of stroke: a prospective study and meta-analysis. Stroke. 2012;43(6):1470–7.
Chowdhury R, Stevens S, Ward H, Chowdhury S, Sajjad A, Franco OH. Circulating vitamin D, calcium and risk of cerebrovascular disease: a systematic review and meta-analysis. Eur J Epidemiol. 2012;27(8):581–91.
Zhou R, Wang M, Huang H, Li W, Hu Y, Wu T. Lower vitamin D status is associated with an increased risk of ischemic stroke: a systematic review and meta-analysis. Nutrients. 2018;10(3):E277.
Kojima G, Bell C, Abbott RD, Launer L, Chen R, Motonaga H, et al. Low dietary vitamin D predicts 34-year incident stroke: the Honolulu heart program. Stroke. 2012;43(8):2163–7.
Pilz S, Tomaschitz A, Drechsler C, Zittermann A, Dekker JM, Marz W. Vitamin D supplementation: a promising approach for the prevention and treatment of strokes. Curr Drug Targets. 2011;12(1):88–96.
Witham MD, Dove FJ, Sugden JA, Doney AS, Struthers AD. The effect of vitamin D replacement on markers of vascular health in stroke patients - a randomised controlled trial. Nutr Metab Cardiovasc Dis. 2012;22(10):864–70.
Park KY, Chung PW, Kim YB, Moon HS, Suh BC, Won YS, et al. Serum vitamin D status as a predictor of prognosis in patients with acute ischemic stroke. Cerebrovasc Dis. 2015;40(1–2):73–80.
Daumas A, Daubail B, Legris N, Jacquin-Piques A, Sensenbrenner B, Denimal D, et al. Association between admission serum 25-Hydroxyvitamin D levels and functional outcome of Thrombolyzed stroke patients. J Stroke Cerebrovasc Dis. 2016;25(4):907–13.
Thapa L, Shrestha A, Pradhan M, Bhandari TR, Shrestha S, Poudel RS, et al. Status of vitamin D and its association with stroke risk factors in patients with acute ischemic stroke in a tertiary care hospital. JNMA J Nepal Med Assoc. 2014;52(195):935–9.
Qiu H, Wang M, Mi D, Zhao J, Tu W, Liu Q. Vitamin D status and the risk of recurrent stroke and mortality in ischemic stroke patients: data from a 24-month follow-up study in China. J Nutr Health Aging. 2017;21(7):766–71.
Leung RY, Han Y, Sing CW, Cheung BM, Wong IC, Tan KC, et al. Serum 25-hydroxyvitamin D and the risk of stroke in Hong Kong Chinese. Thromb Haemost. 2017;117(1):158–63.
Schneider MA. The importance of educating patients with stroke about vitamin D. J Neurosci Nurs. 2017;49(6):387–9.
Kilkkinen A, Knekt P, Aro A, Rissanen H, Marniemi J, Heliovaara M, et al. Vitamin D status and the risk of cardiovascular disease death. Am J Epidemiol. 2009;170(8):1032–9.
Brondum-Jacobsen P, Nordestgaard BG, Schnohr P, Benn M. 25-hydroxyvitamin D and symptomatic ischemic stroke: an original study and meta-analysis. Ann Neurol. 2013;73(1):38–47.
Carluccio MA, Di Donato I, Pescini F, Battaglini M, Bianchi S, Valenti R, et al. Vitamin D levels in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Neurol Sci. 2017;38(7):1333–6.
Levi-Vardi R, Yagil Y. Vitamin D, Hypertension, and ischemic stroke: an unresolved relationship. Hypertension. 2017;70:496–498.
Pokharel BR, Kharel G, Thapa LJ, Rana PV. Vitamin D and other risk factors among stroke patients. Kathmandu Univ Med J (KUMJ). 2015;13(49):71–3.
Narasimhan S, Balasubramanian P. Role of vitamin D in the outcome of ischemic stroke- a randomized controlled trial. J Clin Diagn Res. 2017;11(2):CC06–10.
Kim K, Cho KH, Im SH, Choi J, Yu J, Kim M. Decrement of serum vitamin D level after stroke. Ann Rehabil Med. 2017;41(6):944–50.
Pilz S, Dobnig H, Fischer JE, Wellnitz B, Seelhorst U, Boehm BO, et al. Low vitamin d levels predict stroke in patients referred to coronary angiography. Stroke. 2008;39(9):2611–3.
Afshari L, Amani R, Soltani F, Haghighizadeh MH, Afsharmanesh MR. The relation between serum vitamin D levels and body antioxidant status in ischemic stroke patients: a case-control study. Adv Biomed Res. 2015;4:213.
Moretti R, Caruso P, Dal Ben M, Conti C, Gazzin S, Tiribelli C. Vitamin D, Homocysteine, and folate in subcortical vascular dementia and Alzheimer dementia. Front Aging Neurosci 2017; 9: 169.
Manouchehri N, Vakil-Asadollahi M, Zandifar A, Rasmani F, Saadatnia M. Vitamin D status in small vessel and large vessel ischemic stroke patients: a case-control study. Adv Biomed Res. 2017;6:146.
Majumdar V, Prabhakar P, Kulkarni GB, Christopher R. Vitamin D status, hypertension and ischemic stroke: a clinical perspective. J Hum Hypertens. 2015;29(11):669–74.
Afzal S, Nordestgaard BG. Vitamin D, Hypertension, and ischemic stroke in 116 655 individuals from the general population: a genetic study. Hypertension. 2017; 499–507.
Vimaleswaran KS, Cavadino A, Berry DJ, Jorde R, Dieffenbach AK, Lu C, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719–29.
Li K, Zhao W, Wang L, Yang X, Yang X. Effect modification of hypertension on the association of vitamin D deficiency with severity of coronary stenosis. Blood Press. 2018;27(3):134–40.
Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing "D"ecline? Mol Asp Med. 2008;29(6):415–22.
Wang Q, Zhu Z, Liu Y, Tu X, He J. Relationship between serum vitamin D levels and inflammatory markers in acute stroke patients. Brain Behav. 2018;8(2):e00885.
Alfieri DF, Lehmann MF, Oliveira SR, Flauzino T, Delongui F, de Araujo MC, et al. Vitamin D deficiency is associated with acute ischemic stroke, C-reactive protein, and short-term outcome. Metab Brain Dis. 2017;32(2):493–502.
Del Brutto OH, Mera RM, Penaherrera R, Penaherrera E, Zambrano M, Costa AF. Arterial stiffness and total cerebral small vessel disease score in community-dwelling older adults: results from the Atahualpa project. Vasc Med. 2019;24(1):6–11.
Mozos I, Stoian D, Luca CT. Crosstalk between vitamins a, B12, D, K, C, and E status and arterial stiffness. Dis Markers. 2017;2017:8784971.
Makariou SE, Michel P, Tzoufi MS, Challa A, Milionis HJ. Vitamin D and stroke: promise for prevention and better outcome. Curr Vasc Pharmacol. 2014;12(1):117–24.
Supriya M, Chandra SR, Prabhakar P, Prasad C, Christopher R. Vitamin D receptor (VDR) gene polymorphism and vascular dementia due to cerebral small vessel disease in an Asian Indian cohort. J Neurol Sci. 2018;391:84–9.
Xiangyu P, Zhao J, Wu Y. Serum alkaline phosphatase level is correlated with the incidence of cerebral small vessel disease. Clin Invest Med. 2019;42(1):E47–52.
Zhang X, Tu W, Manson JE, Tinker L, Liu S, Cauley JA, et al. Racial/ethnic differences in 25-Hydroxy vitamin D and parathyroid hormone levels and cardiovascular disease risk among postmenopausal women. J Am Heart Assoc. 2019;8(4):e011021.
Yuan J, Guo X, Liu Z, Zhao X, Feng Y, Song S, et al. Vitamin D receptor activation influences the ERK pathway and protects against neurological deficits and neuronal death. Int J Mol Med. 2018;41(1):364–72.
Chaudhuri JR, Mridula KR, Alladi S, Anamika A, Umamahesh M, Balaraju B, et al. Serum 25-hydroxyvitamin d deficiency in ischemic stroke and subtypes in Indian patients. J Stroke. 2014;16(1):44–50.
Zhang B, Wang Y, Zhong Y, Liao S, Lu Z. Serum 25-hydroxyvitamin D deficiency predicts poor outcome among acute ischemic stroke patients without hypertension. Neurochem Int. 2018;118:91–5.
Cranney A, Horsley T, O'Donnell S, Weiler H, Puil L, Ooi D, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007;1–235.
Chen H, Liu Y, Huang G, Zhu J, Feng W, He J. Association between vitamin D status and cognitive impairment in acute ischemic stroke patients: a prospective cohort study. Clin Interv Aging. 2018;13:2503–9.
Acknowledgments
We express our gratitude to all the patients and the staff who participated in our study. We thank Lili He, Yicheng Xu, Weixue Wang, Shuang Wang, Zihan Yan, Yu Zhang for their great help for the data collection and analysis.
Funding
This work was supported by the National Natural Science Foundation of China (81301016) and the Beijing Municipal Administration of Hospitals Incubating Program (PX2019009). The funders did not play any role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; nor in the preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
JLY and PYS conceived and designed the experiments. JZS analyzed the data and drafted the manuscript. JZS and KBL collected data. All authors have read and approved the final manuscript to be published.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Our work was approved by the Ethics Committee of Beijing Chaoyang Hospital and Jinan City people’s hospital. This study was performed in accordance with the Declaration of Helsinki and all authors agreed the publish statements of BMC Neurology.
Consent for publication
Written informed consent was obtained from all the subjects.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Si, J., Li, K., Shan, P. et al. The combined presence of hypertension and vitamin D deficiency increased the probability of the occurrence of small vessel disease in China. BMC Neurol 19, 164 (2019). https://doi.org/10.1186/s12883-019-1395-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-019-1395-2