Abstract
Background
CIC-mutant oligodendroglial tumours linked to better prognosis. We aim to investigate associations between CIC gene mutation status, MR characteristics and clinical features.
Methods
Imaging and genomic data from the Cancer Genome Atlas and the Cancer Imaging Archive (TCGA/TCIA) for 59 patients with oligodendroglial tumours were used. Differences between CIC mutation and CIC wild-type were tested using Chi-square test and binary logistic regression analysis.
Results
In univariate analysis, the clinical variables and MR features, which consisted 3 selected features (subventricular zone[SVZ] involvement, volume and seizure history) were associated with CIC mutation status (all p < 0.05). A multivariate logistic regression analysis identified that seizure history (no vs. yes odd ratio [OR]: 28.960, 95 confidence interval [CI]:2.625–319.49, p = 0.006) and SVZ involvement (SVZ- vs. SVZ+ OR: 77.092, p = 0.003; 95% CI: 4.578–1298.334) were associated with a higher incidence of CIC mutation status. The nomogram showed good discrimination, with a C-index of 0.906 (95% CI: 0.812–1.000) and was well calibrated. SVZ- group has increased (SVZ- vs. SVZ+, hazard ratio [HR]: 4.500, p = 0.04; 95% CI: 1.069–18.945) overall survival.
Conclusions
Absence of seizure history and SVZ involvement (−) was associated with a higher incidence of CIC mutation.
Similar content being viewed by others
Background
Low-grade gliomas (LGGs) exhibiting oligodendroglial features include oligodendrogliomas and oligoastrocytomas [1]. The updated 2016 edition of the World Health Organization (WHO) Classification of tumors of the Central Nervous System (CNS) uses molecular parameters and the histology to define the main tumor categories for the first time. This represents a shift from the traditional principle of using neuropathological diagnoses, which are primarily based on the microscopic features, to using molecularly-oriented diagnoses [2, 3]. Therefore, neurosurgeons increasingly depend on molecular genetic features to guide their clinical judgement and decision-making processes [4, 5]. LGG samples with an isocitrate dehydrogenase (IDH) mutation and the codeletion of 1p and 19q had the most favourable outcomes for treatment [6, 7].
The Capicua transcriptional repressor (CIC) gene is often mutated in oligodendroglial tumours with the codeletion of 1p and 19q. CIC-mutant oligodendroglial tumours are also associated with better prognoses [8, 9] and to better treatment outcomes and overall survival [8,9,10]. In the field of MR radiogenomics research, many imaging-based characteristics (tumour localization, mass effect, tumour contrast enhancement, etc.) are correlated with molecular genetic biomarkers (EGFR, IDH/1p19q subtype, TP53 mutation status, etc.) that are used to identify their phenotypes upon imaging [8, 11,12,13,14,15]. Recent studies have also found that the heterogeneity in patient prognoses might be linked to neuronal stem cells (NSCs), located in the subventricular zone (SVZ) [8, 16, 17].
To date, although several studies have evaluated MRI characteristics as they relate to IDH/1p19q status [18, 19], no study has investigated associations between CIC mutation status and MR imaging features in oligodendroglial tumours. Radiological detection of CIC mutation status may facilitate the preoperative prediction of a patient’s prognosis. Therefore, this paper reports preliminary research that can be used to determine the associations between CIC gene mutation status, MR characteristics and clinical features.
Methods
Patient population
All patient data was acquired from the published The Cancer Genome Atlas LowGrade Glioma (TCGA-LGG) project and within this publication it is stated “Specimens were obtained from patients, with appropriate consent from institutional review boards”. (http://cancergenome.nih.gov/).
The clinical files of oligodendroglial tumours were downloaded from the TCGA data portal (https://tcgadata.nci.nih.gov/tcga/dataAccessMatrix.htm;updated: 2018-08-23). MR datawere provided by TCIA (updated: 2014-09-04).TCGA and TCIA are publicly available databases that contain no linkage to patient identifiers. All patients must meet the following criteria to enter our study: available pathologic diagnosis of oligodendroglial tumours (oligodendroglioma or oligoastrocytoma) from TCGA; available CIC mutation status (CIC mutation or CIC wild-type) from TCGA [20] (extracted from a previous study [N Engl J Med. 2015; 372 (26):2481–2498]); and available MR images (T1WI, T2WI, Flair and post-contrast) from TCIA. Finally, 59 patients with oligodendroglial tumours were used in this institutional review board approval–exempt study.
Image feature analysis
The MR images were presented to two radiologists for interpretation and measurement and in cases of disagreement a consensus was reached after discussion. Both radiologists were blind to CIC mutation status and clinical information. The following 7 qualitative MR imaging features [11, 12, 21, 22] were evaluated: (1) volume (< 60 cm3 vs. ≥60 cm3); (2) multifocal (no vs. yes); (3) intratumoural haemorrhaging (no vs. yes); (4) enhancing margin (well defined vs. poorly defined); (5) necrosis (no vs. yes); (6) proportion of contrast-enhanced tumour (< 5% vs. ≥5%); and (7) SVZ Involvement (no vs. yes). The tumours that were located in close contact with the SVZ were classified as SVZ+, while the tumours that were located distantly from the SVZ were classified as SVZ-17.
Statistical analysis
Differences between CIC mutation and CIC wild-type were tested using the Chi-square test and binary logistic regression analysis (version 22.0; SPSS Company, Chicago, IL). Odd ratios (OR) and 95% confidence intervals (CI) are reported. The area under the receiver operator characteristic curve (AUC) was estimated for prediction of CIC gene mutation status. The sensitivity, specificity, positive likelihood ratio (PLR) and negative likelihood ratio (NLR) of the model in the prediction of CIC mutations were obtained. Survival analysis (SVZ- vs. SVZ+) was estimated using the Cox proportional hazards models. Hazard ratios (HR) and 95% CI are reported. The statistical significance threshold was set at a P-value of 0.05 (two-sided) to indicate statistical significance.
Results
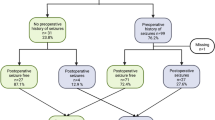
Our series of 59 patients (45.4 ± 14.0 years, range: 18–74 years) included 29 men (49.2%) and 30 (50.8%) women. CIC mutations were found in 10 (16.9%) of 59 patients. There were 35 patients (59.3%) with tumours that involved the SVZ (Fig. 1). Specifically, 8 tumours involved the frontal horn, 5 the body, 1 the occipital horn, 17 the temporal horn and 4 others. The cohort mean OS was 37.5 months (range: 0.03–156.11 and median = 21.4), and 44 patients were still alive (74.6%) at the end of the study while 15 were deceased (25.4%). Clinical data are summarized in Table 1 and Additional file 1: Table S1.
In univariate analysis, the clinical variables and MR features, which consisted of three selected features (SVZ involvement, volume and seizure history) were significantly associated with CIC mutation status (all P < 0.05). We demonstrated that a smaller tumour volume (OR: 9.100, P = 0.004), SVZ-(OR: 20.400 P = 0.006) and a history absent of seizures (OR: 6.462, P = 0.014) were associated with a significantly higher incidence of CIC mutations (Table 1 Fig. 2).
In multivariate logistic regression analysis, only two risk factors were significant independent predictors (Table 2). We demonstrated that seizure history (no vs. yes OR: 28.960, 95CI:2.625–319.49, P = 0.006) and subventricular zone involvement (SVZ- vs. SVZ+ OR: 77.092, P = 0.003; 95% CI: 4.578–1298.334) were associated with a higher incidence of CIC mutation status. The nomogram displayed high discrimination, with a C-index of 0.906 (95% CI: 0.812–1.000) and was well calibrated (Fig. 3). The sensitivity, specificity, positive likelihood ratio (PLR) and negative likelihood ratio (NLR) of this model in the prediction of CIC mutations were 0.90, 0.71, 3.09 and 0.14, respectively. Subventricular zone involvement (−) of oligodendroglial tumours in combination with absence of seizure history may therefore be used to better prognosticate CIC mutation status than the use of each variable alone (Fig. 4).
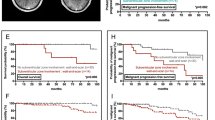
Patients (follow up: 0.03–156.11 months) with SVZ- had a longer median overall survival (133.6 vs.65.7) months (SVZ- vs. SVZ+, hazard ratio (HR): 4.500, P = 0.04; 95% CI: 1.069–18.945) than patients with SVZ+(Fig. 5). SVZ+ tumors were significantly larger than SVZ- tumors (181 ± 92.1 cm3 vs.117 ± 116 cm3, P < 0.05).
Discussion
Molecular genetic studies demonstrated distinct glioma entities with specific epigenetic and genetic profiles [23]. Some oligoastrocytomas and most oligodendrogliomas are characterized by a typical and unique unbalanced translocation, der (1, 19), resulting in a 1p/19q co-deletion (codeletion of 1p and 19q). Candidate tumour suppressor genes (TSGs) targeted by these losses, including FUBP1 on 1p31.1 and CIC on 19q13.2, were only recently discovered [10]. CIC-mutant oligodendroglial tumours are also linked to better prognoses [8, 9].
There are a number of studies regarding the relationship between imaging features and gene mutations. Rios Velazquez E et al. [24] confirmed that quantitative features related to intratumour heterogeneity that were able to successfully discriminate (AUC = 0.69) between EGFR- and EGFR+ lesions. Brendle C et al. [25] showed that the differentiation of high-grade gliomas and low-grade gliomas (sensitivity: 100% specificity: 80%) is made possible by the dynamic contrast-enhanced MR perfusion parameter Ve (P = 0.024), while arterial spin labelling perfusion shows the potential for the discrimination of the ATRX and IDH mutation statuses (sensitivity: 75% specificity: 88%, P = 0.014). Dagher J [26] implied that wild-type von Hippel-Lindau (VHL) renal cell carcinomas were associated with lymph nodal metastases. Rizzo S [27] found that a pleural effusion related to ALK mutations while nodules located in non-tumour lobes or round lesion shapes were related to a KRAS mutation in subgroups of non-small cell lung cancer patients. So far, no study has investigated associations between CIC mutation status and MR imaging features in oligodendroglial tumours. This study suggests that SVZ involvement and seizure history can be conveniently used to facilitate the prediction of CIC mutation status.
The subventricular zone has been associated with the origination and development, as well as the biological behaviour of LGGs [22]. Recently, limited studies have reported associations between SVZ involvement and patient prognosis. Nakagawa Y et al. [28] implied that the loss of 19q and lack of SVZ+ might be prognostic for longer survival. Liu S et al. [22] reported that multivariate analysis showed that a shorter distance between the tumor centroid and the SVZ (p = 0.039) was significantly associated with poor overall survival in SVZ-involved patients (low-grade astrocytoma). Liu S et al. [22] also reported that a longer distance between the SVZ and the tumour centroid was significantly related to better overall survival in SVZ-involved LGG patients. Adeberg S et al. [8] confirmed that the tumour location with regard to the subventricular zone is related to a patient’s prognosis (p < 0.05).
Nomograms are user-friendly tools that give relative contexts and probabilities of cancer prognoses [11]. In the present study, we constructed three models for the preoperative prediction of CIC mutation statuses in oligodendroglial tumours. ROC curve analysis showed that use of seizure history combined with SVZ involvement (AUC = 0.906) was superior to simply the use of seizure history (AUC = 0.725) or SVZ involvement alone (AUC = 0.804). The discriminatory power of the nomogram which combined SVZ involvement and seizure history was also very strong and well calibrated.
The limitations of our study included the retrospective nature of our data collection, the relatively limited number of cases (n = 59) and the automatic imaging feature extraction not being implemented. In addition, we do not know whether re-classification by an expert neuropathologist has been performed, however, similar to other papers (2017–2019 more than 250 articles) published using this dataset, we therefore believe our conclusions are not largely influence by such factors. Further study should be conducted by using a larger pool of oligodendroglial tumours patients with the use quantitative image analysis tools being required [29]. The newly emerged field of radiogenomics allows specific MR imaging phenotypes to be linked with gene expression profiles. Further work is needed to better define the relationships identified in our study and to explore additional relationships (SVZ involvement and CD133, SVZ involvement and CD44, SVZ involvement and MMPs).
Conclusions
In conclusion, this study presents that SVZ involvement (−) and absence of seizure history may therefore be used to facilitate the prediction of CIC mutation status. Patients with SVZ- had a longer median overall survival months than patients with SVZ+.This work represents a practical application of imaging findings for personalized medicine. External validation of this model in other cohorts of patients is needed.
Availability of data and materials
All data generated or analyzed in this study are included in this published article and its Additional files.
Abbreviations
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- CIC:
-
Capicua transcriptional repressor
- HR:
-
Hazard ratio
- IDH:
-
Isocitrate dehydrogenase
- LGG:
-
Low-grade gliomas
- NLR:
-
Negative likelihood ratio
- NSCs:
-
Neuronal stem cells
- OR:
-
Odds ratio
- PLR:
-
Positive likelihood ratio
- SVZ:
-
Subventricular zone
- TCIA:
-
The Cancer Imaging Archive
- TSGs:
-
Tumor suppressor genes
References
Michaud K, de Tayrac M, D'Astous M, Duval C, Paquet C, Samassekou O, et al. Contribution of 1p, 19q, 9p and 10q automated analysis by FISH to the diagnosis and prognosis of Oligodendroglial tumors according to WHO 2016 guidelines. PLoS One. 2016;11(12):e0168728.
Komori T. The 2016 WHO classification of Tumours of the central nervous system: the major points of revision. Neurol Med Chir (Tokyo). 2017;57(7):301–11.
Carter JH, McNulty SN, Cimino PJ, Cottrell CE, Heusel JW, Vigh-Conrad KA, et al. Targeted next-generation sequencing in molecular subtyping of lower-grade diffuse gliomas: application of the World Health Organization’s 2016 revised criteria for central nervous system tumors. J Mol Diagn. 2017;19(2):328–37.
Sturm D, Pfister SM, Jones DTW. Pediatric gliomas: current concepts on diagnosis, biology, and clinical management. J Clin Oncol. 2017;35(21):2370–7.
Michotte A, Neyns B, Chaskis C, Sadones J. Neuropathological and molecular aspects of low-grade and high-grade gliomas. Acta Neurol Belg. 2004;104(4):148–53.
Liu Z, Zhang T, Jiang H, Xu W, Zhang J. Conventional MR-based preoperative nomograms for prediction of IDH/1p19q subtype in low-grade glioma. Acad Radiol. 2018.
Wu CC, Jain R, Radmanesh A, Poisson LM, Guo WY, Zagzag D, et al. Predicting genotype and survival in glioma using standard clinical MR imaging apparent diffusion coefficient images: a pilot study from the Cancer genome atlas. AJNR Am J Neuroradiol. 2018;39(10):1814–20.
Adeberg S, Bostel T, Konig L, Welzel T, Debus J, Combs SE. A comparison of long-term survivors and short-term survivors with glioblastoma, subventricular zone involvement: a predictive factor for survival? Radiat Oncol. 2014;9:95.
Gleize V, Alentorn A, Connen de Kerillis L, Labussiere M, Nadaradjane AA, Mundwiller E, et al. CIC inactivating mutations identify aggressive subset of 1p19q codeleted gliomas. Ann Neurol. Sep 2015;78(3):355–74.
Eisenreich S, Abou-El-Ardat K, Szafranski K, Campos Valenzuela JA, Rump A, Nigro JM, et al. Novel CIC point mutations and an exon-spanning, homozygous deletion identified in oligodendroglial tumors by a comprehensive genomic approach including transcriptome sequencing. PLoS One. 2013;8(9):e76623.
Wang Y, Liu S, Fan X, Li S, Wang R, Wang L, et al. Age-associated brain regions in gliomas: a volumetric analysis. J Neuro-Oncol. 2015;123(2):299–306.
Pope WB, Chen JH, Dong J, Carlson MR, Perlina A, Cloughesy TF, et al. Relationship between gene expression and enhancement in glioblastoma multiforme: exploratory DNA microarray analysis. Radiology. 2008;249(1):268–77.
Carlson MR, Pope WB, Horvath S, Braunstein JG, Nghiemphu P, Tso CL, et al. Relationship between survival and edema in malignant gliomas: role of vascular endothelial growth factor and neuronal pentraxin 2. Clin Cancer Res May. 2007;13(9):2592–8.
Jamshidi N, Diehn M, Bredel M, Kuo MD. Illuminating radiogenomic characteristics of glioblastoma multiforme through integration of MR imaging, messenger RNA expression, and DNA copy number variation. Radiology. 2014;270(1):1–2.
Diehn M, Nardini C, Wang DS, McGovern S, Jayaraman M, Liang Y, et al. Identification of noninvasive imaging surrogates for brain tumor gene-expression modules. Proc Natl Acad Sci U S A Apr. 2008;105(13):5213–8.
Liu S, Wang Y, Fan X, Ma J, Qiu X, Jiang T. Association of MRI-classified subventricular regions with survival outcomes in patients with anaplastic glioma. Clin Radiol. 2017;72(5):426 e421–6.
Gollapalli K, Ghantasala S, Kumar S, Srivastava R, Rapole S, Moiyadi A, et al. Subventricular zone involvement in Glioblastoma - A proteomic evaluation and clinicoradiological correlation. Sci Rep. 2017;7(1):1449.
Jaffe CC. Imaging and genomics: is there a synergy? Radiology. 2012;264(2):329–31.
Rutman AM, Kuo MD. Radiogenomics: creating a link between molecular diagnostics and diagnostic imaging. Eur J Radiol. 2009;70(2):232–41.
Cancer Genome Atlas Research N, Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 2015;372(26):2481–2498.
Gutman DA, Dunn WD Jr, Grossmann P, Cooper LA, Holder CA, Ligon KL, et al. Somatic mutations associated with MRI-derived volumetric features in glioblastoma. Neuroradiology. 2015;57(12):1227–37.
Liu S, Wang Y, Fan X, Ma J, Ma W, Wang R, et al. Anatomical involvement of the subventricular zone predicts poor survival outcome in low-grade Astrocytomas. PLoS One. 2016;11(4):e0154539.
Malzkorn B, Reifenberger G. Practical implications of integrated glioma classification according to the World Health Organization classification of tumors of the central nervous system 2016. Curr Opin Oncol. 2016;28(6):494–501.
Rios Velazquez E, Parmar C, Liu Y, Coroller TP, Cruz G, Stringfield O, et al. Somatic mutations drive distinct imaging phenotypes in lung Cancer. Cancer Res. 2017;77(14):3922–30.
Brendle C, Hempel JM, Schittenhelm J, Skardelly M, Tabatabai G, Bender B, et al. Glioma grading and determination of IDH mutation status and ATRX loss by DCE and ASL perfusion. Clin Neuroradiol. 2017;28(3):421–8.
Dagher J, Kammerer-Jacquet SF, Brunot A, Pladys A, Patard JJ, Bensalah K, et al. Wild-type VHL clear cell renal cell carcinomas are a distinct clinical and histologic entity: a 10-year follow-up. Eur Urol Focus. 2016;1(3):284–90.
Rizzo S, Petrella F, Buscarino V, De Maria F, Raimondi S, Barberis M, et al. CT Radiogenomic characterization of EGFR, K-RAS, and ALK mutations in non-small cell lung Cancer. Eur Radiol. 2016;26(1):32–42.
Nakagawa Y, Sasaki H, Ohara K, Ezaki T, Toda M, Ohira T, et al. Clinical and molecular prognostic factors for long-term survival of the patients with glioblastomas in a single institutional consecutive cohort. World Neurosurg. 2017;106:165–73.
Bai HX, Lee AM, Yang L, Zhang P, Davatzikos C, Maris JM, et al. Imaging genomics in cancer research: limitations and promises. Br J Radiol. 2016;89(1061):20151030.
Acknowledgements
None.
Funding
Supported by a grant from the Medical and Health Science and Technology Project of Guangzhou (No. 20161A011022) and partially supported by the fund from Guangzhou Institute of Pediatrics/Guangzhou Women and Children’s Medical Center/(NO:IP-2016-002). The funding body did not play any role in design, in the collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
ZYL and HSL performed the statistical analysis and drafted the manuscript, JZ, ZQL, HSL and ZYL participated in the design of the study and helped to draft the manuscript, JZ and ZYL collected the data, JZ and ZYL helped to perform the statistical analysis, JZ conceived of the study and participated in the design of the study. All authors read the approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All patient data was acquired from the published The Cancer Genome Atlas Low Grade Glioma (TCGA-LGG) project and within this publication it is stated “Specimens were obtained from patients, with appropriate consent from institutional review boards”. (http://cancergenome.nih.gov/).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Table S1. Clinical data of this study. (XLS 71 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, Z., Liu, H., Liu, Z. et al. Oligodendroglial tumours: subventricular zone involvement and seizure history are associated with CIC mutation status. BMC Neurol 19, 134 (2019). https://doi.org/10.1186/s12883-019-1362-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-019-1362-y