Abstract
Background
Troponin T (TnT) is a well-known risk factor for negative outcome in hemodialysis (HD) patients, but little is known about variation over time, and the impact of clinical and dialysis specific factors. This study investigated the effect of angiotensin II receptor blockade (ARB), short and long-term variation in TnT and associations with clinical parameters.
Methods
In this analysis based on the SAFIR-cohort (Clinical Trials ID: NCT00791830) 81 HD patients were randomized double-blind for placebo (n = 40) or angiotensin II receptor blocker (ARB) treatment (n = 41) with irbesartan (150–300 mg) and followed for 12 months with six serial measurements of TnT using a high-sensitivity assay.
Results
Fifty-four patients (67%) completed follow-up. Baseline TnT-medians (min-max) were (placebo/ARB): 45(14–295)/46(10–343) ng/L. ARB-treatment did not significantly affect mean TnT-levels over the 12-month study period. Median week-to-week and one-year TnT-variation (5th–95th-percentile range) using all samples regardless of intervention were: 0(− 14–10) ng/L (week-to-week) and 3(− 40–71) ng/L (12 months). Median TnT-amplitude, capturing the change from the lowest to the highest TnT-value observed during the one-year study period was 38% or 20.5 ng/L. Median ratios with 95% limits of agreement were: 1.00(0.73–1.37); P = 0.92 (1 week/baseline; n = 77) and 1.07(0.52–2.25); P = 0.19 (12 months/baseline; n = 54). Baseline TnT was positively correlated with diabetes, ultrafiltration volume, arterial stiffness, change in intradialytic total peripheral resistance and N-terminal pro b-type natriuretic peptide (NT-proBNP) and negatively correlated with hematocrit, residual renal function and change in intradialytic cardiac output. High baseline TnT was associated with a higher risk of admission and cardiovascular (CV) events during follow-up. Increase in TnT over time (ΔTnT = 12-months-baseline) was significantly associated with increase in left ventricular (LV) mass and NT-proBNP and decrease in LV ejection fraction and late intradialytic stroke volume. ΔTnT was not significantly associated with admissions, CV or intradialytic hypotensive events during follow-up. Admissions were significantly more likely with a high (TnT-amplitude> 20.5 ng/L) than a low TnT-amplitude. Peaks in TnT were less frequent in aspirin-treated patients.
Conclusion
ARB-treatment had no significant effect on TnT-levels. Week-to-week variation was generally low, yet over 12 months individual patients had considerable TnT fluctuations. Rise in TnT over time was significantly correlated with markers of cardiac deterioration.
Trial registration
ClinicalTrials.gov Identifier: NCT00791830. Date of registration: November 17, 2008. EudraCT no: 2008–001267-11.
Similar content being viewed by others
Background
Hemodialysis (HD) patients have a high prevalence of cardiovascular (CV) disease and increased risk of myocardial infarction (MI) [1]. Troponin T (TnT) is a small protein (37 kDa), which acts as the tropomyosin-binding and thin filament-anchoring subunit of the troponin complex, that regulates contraction in cardiac and skeletal muscles [2]. The development of high-sensitive assays permits detection of very low levels of cardiac TnT and a clinically relevant increase in TnT is stated as one that exceeds the 99th-percentile of a normal reference population [3]. HD patients, however, often have chronically elevated TnT as documented by multiple studies [4,5,6,7,8]. The underlying pathophysiology may reflect coronary artery disease [9, 10], subclinical myocardial injury [10], myocardial stunning [11], left ventricular (LV) hypertrophy [12], reduced renal clearance [13] and circulatory congestion [10]. The diagnosis of MI in these patients often relies on a higher cut-off value or assessment of the dynamic change in TnT, typically stated as a > 20% increase 6–9 h after presentation or by comparison with previous values [14]. In otherwise stable and asymptomatic HD patients, interpretation of elevated TnT remains a challenge for the clinician. Especially given the fact that even without suspected acute coronary syndrome higher TnT-levels are associated with a worse prognosis with a 2- to 4-fold increased 3-year mortality rate [15]. Yet, although, higher levels in cross-sectional studies are associated with worse outcome, relatively little is known about short- and long-term changes in individual HD patients. Thus, from a clinical viewpoint it is important to know the expected range, variation over time, and the impact of clinical and dialysis specific factors. In addition, few interventional studies exist. Angiotensin converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) consistently induce strong regression of LV-hypertrophy and fibrosis [16,17,18]. Improvement of cardiac performance via LV-regression due to blockade of the renin-angiotensin-aldosterone system (RAAS) should thus potentially lower TnT-levels. The aim of the present study was therefore to investigate the effect of the ARB irbesartan on TnT-levels, short and long-term variation in TnT and associations with various clinical and dialysis related parameters in a cohort of newly started HD patients participating in the SAFIR study [19,20,21,22].
Methods
Study design
Primary results regarding residual renal function and intermediate CV endpoints have been published previously together with the study protocol [19,20,21,22]. Briefly, the SAFIR-study (acronym for” SAving residual renal Function in hemodialysis patients receiving IRbesartan) was designed as a randomized double-blind placebo-controlled multicenter trial. Inclusion criteria were dialysis vintage < 1 year, urinary output > 300 mL/day and left ventricular ejection fraction > 30% (LV EF) assessed by echocardiography. Patients, who experienced myocardial infarction or unstable angina pectoris within three months prior to admission, were excluded. Patients were recruited from six hospitals in Denmark and followed for one year. Inclusion began in May 2009 and the last patient’s last visit was in December 2012.
Study medication and blood pressure
Patients were randomized to the ARB irbesartan 150 mg, or matching placebo. Initial dose was 150 mg/day with dosage increment after two weeks to 300 mg/day. As Irbesartan has a long plasma half-life of 15 h and is not removed by dialysis, timing of drug administration was not specified [23, 24]. Counting residual tablets monthly was used to check compliance. Patients receiving RAAS-blocking agents such as ACEI or ARB at inclusion stopped this treatment one week before baseline. A predialytic systolic blood pressure (BP) target of 140 mmHg was aimed for in all patients by adjusting dryweight and by use of all classes of antihypertensive drugs other than RAAS-blocking agents without any restrictions regarding timing and class of additional antihypertensive drugs. BP results have been published previously [19,20,21,22].
Laboratory procedures
After 30 min of rest in the supine position, venous blood samples were drawn before HD in lithium-heparin-coated tubes. Samples were centrifuged, and the separated plasma (5 mL) was stored at − 80 °C. Plasma levels of TnT were measured with a previously validated automated Roche high sensitivity TnT immunoassay (Troponin T hs STAT Roche Diagnostics, Mannheim, Germany) on a Cobas e601 analyser according to the instructions of the manufacturer. The assay uses two cardiac TnT-specific mouse monoclonal antibodies in a sandwich format. The antibodies recognize epitopes located in the central part of the TnT molecule (amino acid positions 125–131 and 135–147, respectively). The assay does not exhibit significant cross-reaction with other troponins (skeletal muscle troponin T, cardiac/skeletal troponin I or human troponin C). Detection limit is 5 ng/L with a total imprecision of less than 10% at a level of 13 ng/L, and in 616 healthy volunteers, the upper 99th percentile was 13.5 ng/L [25]. Analytical within assay coefficient of variation in HD patients is approximately 1.7–6% according to previous studies [26,27,28]. N-terminal pro b-type natriuretic peptide (NT-proBNP) methodology has previously been described in detail [21].
Arterial stiffness
Pulse wave velocity (PWV) was measured with the SphygmoCor system (version 7.0 and 8.2, Atcor Medical, Sydney, Australia) by sequential 10–20 s pulse wave recordings at the carotid artery and femoral artery using the intersecting tangent algorithm as previously described [19, 21].
Intradialytic parameters
Intradialytic measurements of cardiac output (CO) was done within the first and the last 30 min of the dialysis session by injecting a bolus of 30 mL 37 °C isotonic saline into the venous blood line using a validated method (Hemodialysis Monitor HD02/HD03, Flow-QC tubing sets, and clipon flow/dilution sensors Transonic Systems Inc., Ithaca, NY, USA) previously described [22]. The mean arterial blood pressure (MAP), total peripheral resistance (TPR), and stroke volume (SV) were derived by:
Intradialytic hypotension (IDH) was defined as symptomatic hypotension requiring administration of intravenous fluid or preterm ending of the dialysis session and was recorded at all dialysis sessions as previously described [22].
Echocardiography
Echocardiography with quantification of cardiac chamber size, LV mass and function was performed as previously described [19, 21] in accordance with current guidelines [29].
Statistics
Data were analyzed with Stata/IC 12.1 (StataCorp LP, College Station, TX 77845 USA). The assumption of normality was checked with QQ-plots, and analyses were performed using naturally log-transformed TnT due to skewness. Baseline data (qualitative variables) and various patient distributions were analyzed with χ2-test and continuous variables were analyzed with t-test or Wilcoxon signed-rank test. Students t-test and a multivariate repeated measurements model (xtmixed) with time and drug (placebo or ARB) and the interaction between them as factors, which allows for missing values and dropout were used for comparison of placebo vs. ARB as previously described [21, 30]. Variation over time was assessed with Bland-Altman plots and paired t-tests. Sets of duplicate log (TnT)-values (e.g. baseline vs. 1 week) were used to calculate average within- and between subject coefficients of variation (CV1 and CVG) using variance component estimates obtained by xtmixed and the following equations:
Bootstrapping was used to obtain a 95% confidence interval (95% CI) for both CVI and CVG. Univariate and multivariate linear regression analysis was performed with baseline log (TnT) (3 multivariate models with five fixed parameters) or change (Δ = 12 months-baseline) in log (TnT) as outcome (2 multivariate models with 3 fixed parameters). Different predictors were added and tested in these models as the sixth/fourth variable. Admissions, CV-events, IDH-episodes and TnT-peaks were dichotomized to 0 or ≥ 1 events and used as outcome in univariate logistic regression analysis based on various baseline parameters and/or changes (Δ = 12 months-baseline) in TnT over time. Pearson’s r was used to describe linear relationships. Intention-to-treat analyses were performed and P < 0.05 was considered statistically significant. Values are presented as means with 95% CI unless otherwise stated. Additional details are given in the Supplement.
Results
Patient characteristics
Eighty-two patients were included in the study with forty-one in each group. One patient in the placebo group did not consent to storage of plasma samples for TnT-analysis and was therefore excluded (Fig. 1). Overall, the groups were similar at baseline (Table 1). Twenty-six patients did not complete the study, eleven in the placebo and fifteen in the ARB group. Reasons for dropout were not significantly different [21].
Consort Flow Chart. Inclusion and exclusion criteria have been published previously [19]. Briefly, the main inclusion criteria were urine output > 300 ml/day, dialysis vintage < 1 year and left ventricular ejection fraction > 30%
Impact of ARB-treatment
Individual and mean changes over time in placebo and ARB-treated groups are shown in Fig. 2. Median values with ranges can be found in Table 2 in order to facilitate interpretation. There were no significant differences between the groups during the study period and ARB-treatment did not significantly affect mean TnT-levels over the 12-month study period (P ≥ 0.19 in all tests for parallel curves, equal levels, and constant levels) as shown in Fig. 2. Changes between baseline and 12 months are given as median ratios (12 months/baseline) with 95% confidence intervals (95% CI) due to back-transformation from natural log-transformed mean values in the placebo and ARB group. Overall, median ratios were not significantly different, neither when using all available data (estimates from the multivariate repeated measurement model 1), nor when excluding patients with incomplete data (estimates based on Student’s t-test) as shown in Fig. 2. CV-events, admissions and IDH episodes were not significantly different after 12 months and total number of events in the two groups (placebo/ARB) were: 90/61 (Admissions); 18/14 (CV-events) and 50/63 (IDH-events). CV-events consisted of (placebo/ARB): MI: 0/2; angina: 8/5; percutaneous coronary intervention (PCI): 2/1; coronary artery bypass grafting (CABG): 0/1; arrythmia: 4/3 and valvular diseases: 4/2.
Individual and mean log-transformed TnT at various time points. Mean changes (baseline-12 months) and mean differences between groups are given as median ratios with 95% CI due to back-transformation using estimates from both xtmixed (all available patient data regardless of time in the study) and Student’s t-test thereby excluding patients with incomplete data (only patients with complete 12-months follow up). Corresponding median TnT-values and ranges (min-max) are shown in Table 2
Variation in TnT over time
Since ARB-treatment had no significant impact on TnT-levels, all samples were pooled into one group regardless of treatment status for analysis of variation over time. Figure 3 shows Bland-Altman plots, median ratios and corresponding within-subject (CVI) and between-subject (CVG) coefficients of variation regardless of treatment status. Median ratios with 95% limits of agreement (reference range for difference) were: 1.00(0.73–1.37); P = 0.92 (Baseline vs. 1 week) and 1.07(0.52–2.25); P = 0.19 (Baseline vs. 12 months), respectively. Corresponding CVI and CVG (95% CI) were: CVI: 11.8 (5.8–14.5)%; CVG 111.6 (91.3–130.3)% (Baseline vs. 1 week) and CVI: 31.1 (15.2–34.0)%; CVG: 109.9 (90.1–129.7)% (Baseline vs. 12 months). Median change (min; max) after 12 months was: 3(− 101; 368) ng/L (all samples; n = 54). Median (5th–95th-percentile range) week-to-week and one-year individual TnT-variation were: 0(− 14–10) ng/L (baseline-1 week: n = 77) and 3(− 40–71) ng/L (baseline-12 months: n = 54) as shown in Fig. 4a-b. Using a 20% change in TnT as cut-off (typically used for MI-diagnosis), 7.8% (6/77 patients) had > 20% increase in TnT and 2.6% (2/77 patients) had > 20% decrease in TnT one week after baseline. At 12 months 20.4% (11/54 patients) had > 20% increase in TnT and 22.2% (12/54 patients had > 20% decrease in TnT (Fig. 4c-d). Median (5-95th-percentile range) TnT amplitude in patients with 12-months of follow-up (n = 54) was 20.5(4–395) ng/L (Fig. 4e). Using data from all patients regardless of time in the study (n = 81), individual median amplitude (max-min) over the entire 12-month period was 14(0–1611) ng/L corresponding to range/median 30(0–4131)%. After removal of 3 outliers (TnT > 400 ng/L) individual median amplitude (min-max) over the entire 12-month period was 14(0–114) ng/L (Fig. 4f) corresponding to range/median 29(0–208)%. Using only samples from patients with complete 12-months follow-up (n = 54), individual median amplitude (max-min) over the entire 12-month period was 20.5(3–1611) ng/L corresponding to range/median 38(15–4131)%. After removal of 3 outliers (TnT > 400 ng/L) individual median amplitude (min-max) over the entire 12-month period was 18(3–114) ng/L corresponding to range/median 36(15–165)%. Most patients exhibited minor TnT-fluctuations close to the median. However, a low median TnT-level did not exclude subsequent rise in TnT (Fig. 4g-h).
Bland-Altman plots (a-e) showing variation in TnT over time (baseline vs. subsequent measurements). Median ratios due to back-transformation with 95% limits of agreement (reference range for difference) and corresponding within-subject (CVI) & between-subject (CVG) coefficients of variation with 95% confidence intervals (95% CI) are also given. Note logarithmic scale on Y-axis in all Bland-Altman plots
a Shows short-term TnT variation baseline vs. 1 week with patients sorted according to magnitude of change in TnT (lowest to highest). b Long-term TnT variation baseline vs. 12 months with individual patients sorted according to magnitude of change (lowest to highest). c Similar to A but with patients sorted according to magnitude of TnT ratio (1 week/baseline). d Similar to B but with patients sorted according to magnitude of TnT ratio (12 months/baseline). e TnT amplitude (max-min) from patients with complete 12 months follow-up with patients sorted according to magnitude of change (lowest to highest). f TnT amplitude (max-min) from all patients, except outliers, regardless of time in the study with patients sorted according to magnitude of change (lowest to highest). g Range (min & max) and median TnT from all patients sorted according to intervention (Placebo/ARB) regardless of time in the study. h Range (min & max) and median TnT from all patients, except outliers, sorted according to median TnT regardless of time in the study
Baseline TnT correlations (univariate analysis)
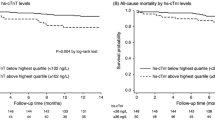
Baseline log-transformed TnT was positively correlated with age, diabetes, Charlson comorbidity index, Ultrafiltration (UF) volume, arterial stiffness (PWV and PWV-tertiles)), change in intradialytic total peripheral resistance (ΔTPR = TPRend-TPRstart) and NT-proBNP. Baseline TnT was negatively correlated with change in intradialytic cardiac output (ΔCO=COend-COstart), hematocrit and residual renal function (urine volume and GFR) as shown in Table 3. Echocardiographic parameters such as LV mass index and LV EF were not significantly associated with baseline TnT in univariate analysis. The impact of arterial stiffness on baseline TnT was examined further by splitting baseline PWV into tertiles as shown in Fig. 5. Known heart disease at baseline was only borderline significant (P = 0.06). Multivariate regression analysis was also performed with baseline log (TnT) as outcome and results are shown in the Supplement (Table S1).
Prediction of clinical events based on baseline TnT
High baseline TnT increased the risk of admission and CV-events (0 vs. ≥1 admissions/CV-events) during follow-up with natural log-transformed TnT odds-ratios (ORs): 2.62(1.22–5.64); P = 0.01 and 2.25(1.04–4.86); P = 0.04, respectively (Table 5). Baseline TnT was also borderline significant in terms of predicting IDH-events (0 vs. ≥1 events) during follow-up with natural log-transformed TnT OR: 1.87(0.98–3.59); P = 0.06.
Parameters associated with change in TnT
Increase in TnT over time (Δ = 12-months-baseline) was significantly associated with increase in LV mass and NT-proBNP and decrease in LV EF and late intradialytic stroke volume 30 min before end of HD (ΔSV2) in univariate analysis (Table 4). Results from multivariate analysis are shown in the Supplement (Table S2).
Changes in TnT over time and correlations with clinical outcome
Three different approaches were used to investigate the relationship between change in TnT over time and clinical outcome in terms of hospital admissions, CV-events and IDH episodes (Table 5).
Regardless of whether change in TnT (Δ = 12 months-baseline) was assessed as Δlog (TnT) or as a dichotomized outcome increase (ΔTnT> 0) vs. decrease (ΔTnT≤0) it was not significantly associated with admissions and CV-events during follow-up. In logistic regression analysis, TnT-increase after 12-months was associated with a lower risk of IDH-events with (TnT-increase vs. TnT-decrease) OR: 0.31(0.10–0.96); P = 0.04.
TnT-amplitude
TnT-amplitude (max-min) was used to capture the change from the lowest to the highest TnT-value during the entire study period. If analysis was restricted to patients with complete 12-months follow-up (n = 54), the median TnT-amplitude was 38% or 20.5 ng/L as previously mentioned. By dichotomizing the amplitude into low (TnT ≤ 20.5 ng/L) or high (TnT > 20.5 ng/L), high TnT-amplitude was significantly associated with increased number of admissions, borderline significant in terms of IDH episodes and non-significant in terms of CV-events (Table 5). Using univariate logistic regression analysis, ORs (0 vs. ≥ 1 event) for comparison of high vs. low TnT-amplitude were: 4.60(1.24–16.97); P = 0.02 (admissions) and 3.08(0.97; 9.67); P = 0.05 (IDH-episodes).
TnT-peak frequency
The number of TnT-peaks (defined as 20% increase above the individual patient TnT-median calculated from all available samples regardless of time in the study) were assessed. TnT-peak distribution in our cohort was (number of patients): No peak: 46(57%); 1 peak: 25(31%); 2 peaks: 9(11%); and 3 peaks: 1(1%). Baseline TnT-level, ARB-treatment, known heart disease, diabetes and arterial stiffness (baseline PWV-tertiles) had no significant impact on peak frequency. In univariate logistic regression analysis, the risk of admission tended to increase with the number of TnT-peaks with OR (0 vs. ≥ 1 admission) 2.30(1.00–5.30); P = 0.05. The frequency of CV-events or IDH-episodes was not significantly different when comparing patients without peaks to those with peaks (Table 5).
Aspirin vs. non-aspirin treatment
In univariate logistic regression analysis, aspirin treatment decreased the risk of TnT-peaks (0 vs. ≥ 1 TnT peak) with OR: 0.28(0.11–0.72); P = 0.008. Admissions, CV and IDH events were not significantly different in aspirin vs. non aspirin treated. Additional details are given in the Supplement.
Discussion
This study found no significant impact of long-term treatment with the ARB irbesartan on predialytic TnT-levels in HD patients. Overall, TnT was quite stable with an individual median ratio over the entire 12-month period with 95% limits of agreement of 1.07(0.51–2.25). Yet, during 12 months of observation some patients exhibited a significant rise in TnT and a low median TnT-level did not exclude subsequent rise in TnT. Our study investigated various clinical and dialysis related parameters associated with TnT. Diabetes, UF volume, arterial stiffness (PWV), change in intradialytic total peripheral resistance and NT-proBNP were positively correlated with baseline TnT whereas hematocrit, residual renal function (GFR or urine volume) and change in intradialytic cardiac output were negatively correlated with baseline TnT. Patients with preserved renal function are less prone to volume overload possibly explaining why TnT was lower in these patients in line with the positive correlations found between TnT and UF volume and NT-proBNP, respectively. Moreover, a better clearance of TnT fragments in patients with preserved renal function could also be a relevant factor to consider, as demonstrated by a previous study [13].
The strengths of this study include serial measurements which allowed us to describe both short (one week) and long-term (12 months) TnT-changes in our cohort. Within-subject and between-subject coefficients of variation were similar to previous studies in HD patients using high-sensitivity TnT-assays when comparing short-term estimates [27, 28]. Unlike most previous studies our study included intervention with an ARB, and patients were well characterized regarding cardiac status (e.g. LV mass, LV EF and NT-proBNP), arterial stiffness, intradialytic hemodynamics, medications and clinical events. To the best of our knowledge, our study is the first to examine the impact of long-term ARB-treatment on TnT-levels in HD patients in a randomized double-blind placebo-controlled design.
LV hypertrophy (LVH) is frequent in end-stage renal disease (ESRD) [31,32,33] and with manifest LVH, myocardial capillary growth is expected to lack behind cardiomyocyte hypertrophy causing cardiomyocyte/capillary mismatch leading to increased oxygen diffusion distance, reduced ischemic tolerance of the heart, which in turns leads to subclinical ischemia of the myocardium and thereby amplified leakage of cardiac troponins including TnT [10]. RAAS-blocking agents such as ACEI or ARB are generally considered to be beneficial in terms of regression of LVH [18] and improvement in LV EF [34]. As previously reported [21], we found no significant effect of ARB-treatment on BP, LV mass and LV EF and our patients did not exhibit pronounced LVH or heart failure, which may explain why there was no significant impact of ARB-treatment on TnT in our study. Not many studies have examined long-term changes in TnT with serial measurements beyond six months like our study. Conway et al. examined 75 HD patients out of which 46 completed 4 serial pre- and post HD TnT measurements after 15 months [35]. TnT was frequently elevated and baseline TnT-levels were associated with an increased risk of mortality and acute coronary syndrome. Bloch et al. followed 238 HD patients out of which 164 completed 24 months of follow-up using pre-HD TnT-measurements at baseline, 18 and 24 months, respectively [4]. TnT increased by 50% in < 1/3 of patients and doubled in only 10% of patients during 18- and 22-months follow-up. Baseline TnT was a significant predictor of all cause and CV death. Mongeon et al. followed 100 HD patients out of which 78 completed 12 months of follow-up using both pre- and post-HD measurements at baseline, 6 months and 12 months, respectively [36]. TnT was found to be stable over a 12-month period although levels tended to increase more between 6 and 12 months. Pre- and post-HD levels were similar, but higher TnT-levels were found in patients with coronary artery disease. Finally, Roberts et al. studied the impact of carvedilol vs. placebo on TnT in a mixed cohort of 72 patients including both HD and peritoneal dialysis (PD) patients [37]. TnT was measured at baseline, 6 months and 12 months, respectively. Forty-nine patients completed run-in and 31 completed 12-month follow-up. TnT-levels at baseline and during follow-up were similar to our study and there was no significant change in mean TnT-levels +/− Carvedilol treatment. Individual variation and correlation with clinical outcome were not reported. Our study thus adds significantly to our understanding of especially the temporal variation and on the impact of ARB-treatment on TNT in HD-patients. High baseline TnT was in our study associated with a higher risk of admission and CV-events during follow-up. Elevated TnT is a well-known risk factor for negative outcome even when MI is not suspected in large cohorts of HD patients [15]. Our findings are in line with this although strictly speaking our study was not powered for hard endpoints. Moreover, our study was able to demonstrate that rise in TnT over 12 months was significantly correlated with deterioration of cardiac status (increase in LV mass and NT-proBNP and decrease in LV ejection fraction and late intradialytic stroke volume). Despite this, rise in TnT after 12 months was not significantly associated with admissions, CV or IDH events during follow-up. We suspect this could be due to the relatively low number of patients in our study. We did demonstrate that a high TnT-amplitude (> 20.5 ng/L) was significantly associated with increased number of admissions and borderline significant in terms of IDH-episodes suggesting a link between IDH and myocardial damage in accordance with a previous study [38]. Similarly, we found that admissions tended to be more likely in patients with ≥1 peak in TnT (defined as a 20% increase above the individual patient TnT-median) compared to non-peakers.
The relatively low TnT-levels in our study suggest occurrence of predominately minor myocyte injury. Previous studies investigating the impact of dialysis on TnT-levels showed that TnT may increase after HD due to hemoconcentration [35] but generally reported little change [5, 36] or even a slight reduction in TnT after HD [39]. Nevertheless, the hemodynamic stress associated with dialysis including fluctuations in electrolytes and large UF volumes could be associated with a transient increase in TnT and repeated HD sessions could lead to a progressive increase in TnT over time in some patients as previously reported [4, 35, 36].
The relationship between increased arterial stiffness, a hallmark of ESRD, and TnT-levels was a novel finding which may reflect compromised myocardial perfusion due to early return of the arterial pulse wave during systole rather than diastole [40]. Increased arterial stiffness and low hematocrit combined with LVH and a high prevalence of coronary artery disease substantially augments the risk of ischemia and TnT-release. Impairment of myocardial function induced by dialysis treatment, known as cardiac stunning, may also contribute [11]. Jefferies et al. demonstrated that the prevalence of myocardial stunning can be reduced with increasing intensity (frequency and duration) of HD and that there was a strong positive relationship between UF rate and severity of stunning as well as a tendency towards lower levels of TnT with frequent dialysis [41]. Our study found that TnT-levels tended to increase with increased UF volume and a drop in intradialytic cardiac output. Since our study did not include home dialysis patients, we could not explore trends regarding HD frequency.
Interestingly, we found that use of aspirin vs. non-use was associated with fewer TnT-peaks during follow-up. There is a paucity of definitive data concerning the efficacy of aspirin in dialysis patients and most observational studies suffer from confounding by indication explaining why some studies found aspirin use to be associated with increased CV mortality or adverse CV-events [42,43,44]. Our study, although not designed to study the effects of aspirin, suggest that aspirin may be beneficial in terms of preventing asymptomatic ischemia in HD patients consistent with the Kidney Disease Outcomes Quality Initiative (KDOQI) clinical practice guideline [45].
Clinical implications
Despite the fact the TnT is a strong marker for poor outcome, the usefulness of TnT sampling in dialysis patients could be questioned. So far, there are no intervention studies that compare treatment strategies stratified by TnT levels in dialysis patients without MI. Our study showed that a rise in TnT reflected deterioration of cardiac function, indicating that frequent TnT monitoring could be clinically relevant. From a pragmatic point of view, measuring TnT e.g. twice yearly gives each patient’s baseline TnT-values for comparison when acute MI is suspected and may improve the clinician’s ability to diagnose deterioration of cardiac function in otherwise asymptomatic patients and facilitate pre-emptive cardiac evaluation with additional screening (stress testing, echocardiography, coronary angiography) or intensified prevention strategies (platelet inhibitors, anemia correction, correction of volume overload). This approach could be tested in future studies and may potentially alleviate CV disease burden in dialysis patients alongside clarifying the added value of frequent TnT sampling.
Limitations
First of all, our findings are limited to HD patients without recent episodes of angina or MI, no heart failure, with some preserved renal function and a relatively short time on dialysis. CV disease and instability during HD may be more prevalent among more morbid and fragile patients. We did not collect blood samples after dialysis and our results therefore reflect the predialytic state. In addition, samples were not collected based on suspicion of myocardial ischemia and may therefore underestimate the true variance. Due to preserved urine output in the majority of our patients, relatively small UF volumes were prescribed during HD compared to other studies [46, 47]. In HD patients with more pronounced CV disease or larger fluid fluctuations, the fluctuations in TnT may differ as well as the response to ARB-treatment. In our cohort, ARB-treatment did not significantly reduce BP and results could have been different in the presence of a BP difference. Patients treated with PD may respond differently in terms of TnT-fluctuations and response to ARB [48].
Conclusions
The ARB irbesartan had no significant impact on predialytic TnT-levels. Week-to-week TnT-variation was low, yet over 12 months individual patients had considerable TnT fluctuations. The median TnT-amplitude, capturing the change from the lowest to the highest TnT-value observed during the 12 months study period was 38% or 20.5 ng/L. High TnT at baseline was associated with a higher risk of admission and CV-events during follow-up. Rise in TnT over time was significantly correlated with markers of cardiac deterioration and admissions during follow-up were significantly more likely with a high (TnT-amplitude> 20.5 ng/L) than a low TnT-amplitude. Aspirin use was associated with fewer peaks in TnT and may prove beneficial in terms of preventing cardiac damage in HD patients. Regular monitoring of TnT may improve the ability to diagnose deterioration of cardiac status in otherwise asymptomatic HD patients but requires further studies prior to implementation into clinical praxis.
Availability of data and materials
The datasets that support the findings of the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACEI:
-
Angiotensin converting enzyme inhibitor
- ARB:
-
Angiotensin II receptor blocker
- BP:
-
Blood pressure
- CABG:
-
Coronary artery bypass grafting
- CO:
-
Cardiac output
- ΔCO:
-
Change in intradialytic cardiac output (ΔCO = COend-COstart)
- CV:
-
Cardiovascular
- CVG :
-
Between-subject coefficient of variation
- CVI :
-
Within-subject coefficient of variation
- DDD:
-
Defined daily doses
- EF:
-
Ejection fraction
- EVF:
-
Erythrocyte volume fraction
- ESRD:
-
End-stage renal disease
- GFR:
-
Glomerular filtration rate
- HD:
-
Hemodialysis
- IDH:
-
Intradialytic hypotensive episodes
- LV:
-
Left ventricular
- LVH:
-
LV hypertrophy
- MAP:
-
Mean arterial blood pressure
- MI:
-
Myocardial infarction
- NT-proBNP:
-
N-terminal pro b-type natriuretic peptide
- OR:
-
Odds ratio
- PCI:
-
Percutaneous coronary intervention
- PD:
-
Peritoneal dialysis
- preHD:
-
Pre-hemodialysis
- PWV:
-
Carotid-femoral pulse wave velocity
- RAAS:
-
Renin-angiotensin-aldosterone system
- SAFIR:
-
Acronym for SAving residual renal Function in hemodialysis patients receiving Irbesartan
- SV:
-
Stroke volume
- SV1:
-
Early intradialytic stroke volume within 30 min after start of HD
- SV2:
-
Late intradialytic stroke volume 30 min before end of HD
- TnT:
-
Troponin T
- TPR:
-
Total peripheral resistance
- ΔTPR:
-
Change in intradialytic total peripheral resistance ((ΔTPR = TPRend-TPRstart)
- UF:
-
Ultrafiltration
References
Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, et al. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO study. Kidney Int. 2004;65(6):2380–9.
Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, et al. National Academy of Clinical Biochemistry Laboratory medicine practice guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13):e356–75.
Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, et al. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–53.
Honneger Bloch S, Honneger S, Semple D, Sidhu K, Stewart R, Pilmore H. Prognostic value and long-term variation of high sensitivity troponin T in clinically stable haemodialysis patients. N Z Med J. 2014;127(1402):97–109.
Pianta TJ, Horvath AR, Ellis VM, Leonetti R, Moffat C, Josland EA, et al. Cardiac high-sensitivity troponin T measurement: a layer of complexity in managing haemodialysis patients. Nephrology (Carlton). 2012;17(7):636–41.
Iliou MC, Fumeron C, Benoit MO, Tuppin P, Courvoisier CL, Calonge VM, et al. Factors associated with increased serum levels of cardiac troponins T and I in chronic haemodialysis patients: chronic Haemodialysis and new cardiac markers evaluation (CHANCE) study. Nephrol Dial Transplant. 2001;16(7):1452–8.
Wolley M, Stewart R, Curry E, Davidson J, White H, Pilmore H. Variation in and prognostic importance of troponin T measured using a high-sensitivity assay in clinically stable haemodialysis patients. Clin Kidney J. 2013;6(4):402–9.
Apple FS, Murakami MM, Pearce LA, Herzog CA. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation. 2002;106(23):2941–5.
deFilippi C, Wasserman S, Rosanio S, Tiblier E, Sperger H, Tocchi M, et al. Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA. 2003;290(3):353–9.
Wang AY, Lai KN. Use of cardiac biomarkers in end-stage renal disease. J Am Soc Nephrol. 2008;19(9):1643–52.
Breidthardt T, Burton JO, Odudu A, Eldehni MT, Jefferies HJ, McIntyre CW. Troponin T for the detection of dialysis-induced myocardial stunning in hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(8):1285–92.
Mallamaci F, Zoccali C, Parlongo S, Tripepi G, Benedetto FA, Cutrupi S, et al. Troponin is related to left ventricular mass and predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2002;40(1):68–75.
Diris JH, Hackeng CM, Kooman JP, Pinto YM, Hermens WT, van Dieijen-Visser MP. Impaired renal clearance explains elevated troponin T fragments in hemodialysis patients. Circulation. 2004;109(1):23–5.
Wu AH, Jaffe AS, Apple FS, Jesse RL, Francis GL, Morrow DA, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem. 2007;53(12):2086–96.
Michos ED, Wilson LM, Yeh HC, Berger Z, Suarez-Cuervo C, Stacy SR, et al. Prognostic value of cardiac troponin in patients with chronic kidney disease without suspected acute coronary syndrome: a systematic review and meta-analysis. Ann Intern Med. 2014;161(7):491–501.
Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G. de FU, et al. cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995–1003.
Malmqvist K, Kahan T, Edner M, Held C, Hägg A, Lind L, et al. Regression of left ventricular hypertrophy in human hypertension with irbesartan. J Hypertens. 2001;19(6):1167–76.
Yang LY, Ge X, Wang YL, Ma KL, Liu H, Zhang XL, et al. Angiotensin receptor blockers reduce left ventricular hypertrophy in dialysis patients: a meta-analysis. Am J Med Sci. 2013;345(1):1–9.
Peters CD, Kjærgaard KD, Jespersen B, Christensen KL, Jensen JD. Renal and cardiovascular effects of irbesartan in dialysis patients - a randomized controlled trial protocol (SAFIR study). Dan Med J. 2013;60(4):A4602.
Kjaergaard KD, Peters CD, Jespersen B, Tietze IN, Madsen JK, Pedersen BB, et al. Angiotensin blockade and progressive loss of kidney function in hemodialysis patients: a randomized controlled trial. Am J Kidney Dis. 2014;64(6):892–901.
Peters CD, Kjaergaard KD, Jensen JD, Christensen KL, Strandhave C, Tietze IN, et al. No significant effect of angiotensin II receptor blockade on intermediate cardiovascular end points in hemodialysis patients. Kidney Int. 2014;86(3):625–37.
Peters CD, Kjaergaard KD, Jensen JD, Christensen KL, Strandhave C, Tietze IN, et al. Short and long-term effects of the angiotensin II receptor blocker Irbesartan on Intradialytic central hemodynamics: a randomized double-blind placebo-controlled one-year intervention trial (the SAFIR study). PLoS One. 2015;10(6):e0126882.
Sica DA, Marino MR, Hammett JL, Ferreira I, Gehr TW, Ford NF. The pharmacokinetics of irbesartan in renal failure and maintenance hemodialysis. ClinPharmacolTher. 1997;62(6):610–8.
Marino MR, Langenbacher K, Ford NF, Uderman HD. Pharmacokinetics and pharmacodynamics of irbesartan in healthy subjects. J Clin Pharmacol. 1998;38(3):246–55.
Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56(2):254–61.
Aakre KM, Roraas T, Petersen PH, Svarstad E, Sellevoll H, Skadberg O, et al. Weekly and 90-minute biological variations in cardiac troponin T and cardiac troponin I in hemodialysis patients and healthy controls. Clin Chem. 2014;60(6):838–47.
Corte Z, Garcia C, Venta R. Biological variation of cardiac troponin T in patients with end-stage renal disease and in healthy individuals. Ann Clin Biochem. 2015;52(Pt 1):53–60.
Mbagaya W, Luvai A, Lopez B. Biological variation of cardiac troponin in stable haemodialysis patients. Ann Clin Biochem. 2015;52(Pt 5):562–8.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–63.
Peters CD, Kjaergaard KD, Nielsen CH, Christensen KL, Bibby BM, Jensen JD, et al. Long-term effects of angiotensin II blockade with irbesartan on inflammatory markers in hemodialysis patients: a randomized double blind placebo controlled trial (SAFIR study). Hemodial Int. 2017;21(1):47–62.
Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47(1):186–92.
Takeda A, Toda T, Iwamoto H, Watanabe K, Matsui N. Long-term evolution and changing associations of left ventricular hypertrophy after starting hemodialysis. Nephron ClinPract. 2008;110(2):c126–c32.
Silberberg JS, Barre PE, Prichard SS, Sniderman AD. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989;36(2):286–90.
Cice G, Di Benedetto A, D'Isa S, D'Andrea A, Marcelli D, Gatti E, et al. Effects of Telmisartan added to angiotensin-converting enzyme inhibitors on mortality and morbidity in hemodialysis patients with chronic heart failure a double-blind, placebo-controlled trial. J Am Coll Cardiol. 2010;56(21):1701–8.
Conway B, McLaughlin M, Sharpe P, Harty J. Use of cardiac troponin T in diagnosis and prognosis of cardiac events in patients on chronic haemodialysis. Nephrol Dial Transplant. 2005;20(12):2759–64.
Mongeon FP, Dorais M, Lorier JL, Froment D, Letendre E, Rinfret S. Effect of hemodialysis, coronary artery disease and diabetes on cardiac troponin T: a prospective survey over one year. Open Cardiovasc Med J. 2009;3:69–77.
Roberts MA, Darssan D, Badve SV, Carroll RP, Fahim MA, Haluska BA, et al. Carvedilol and cardiac biomarkers in Dialysis patients: secondary analysis of a randomized controlled trial. Kidney Blood Press Res. 2017;42(6):1033–44.
Hung SY, Hung YM, Fang HC, Yeh JH, Hung GC, Wu CJ, et al. Cardiac troponin I and creatine kinase isoenzyme MB in patients with intradialytic hypotension. Blood Purif. 2004;22(4):338–43.
Badiou S, Boudet A, Leray-Moragues H, Rodriguez A, Bargnoux AS, Dupuy AM, et al. Monthly reference change value of cardiac troponin in hemodialysis patients as a useful tool for long-term cardiovascular management. Clin Biochem. 2016;49(15):1195–8.
Nichols WW, Michael F. O'Rourke and Charalambos Vlachopoulos. McDonald's Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles 6th Edition. London: Hodder Arnold; 2011.
Jefferies HJ, Virk B, Schiller B, Moran J, McIntyre CW. Frequent hemodialysis schedules are associated with reduced levels of dialysis-induced cardiac injury (myocardial stunning). Clin J Am Soc Nephrol. 2011;6(6):1326–32.
Chan KE, Lazarus JM, Thadhani R, Hakim RM. Anticoagulant and antiplatelet usage associates with mortality among hemodialysis patients. J Am Soc Nephrol. 2009;20(4):872–81.
Ethier J, Bragg-Gresham JL, Piera L, Akizawa T, Asano Y, Mason N, et al. Aspirin prescription and outcomes in hemodialysis patients: the Dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis. 2007;50(4):602–11.
Palmer SC, Di Micco L, Razavian M, Craig JC, Perkovic V, Pellegrini F, et al. Antiplatelet agents for chronic kidney disease. Cochrane Database Syst Rev. 2013;2:CD008834.
KDOQI Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients http://www.kidney.org/professionals/KDOQI/guidelines_commentaries.cfm. Accesed 2 Jan 2020.
Tislér A, Akócsi K, Hárshegyi I, Varga G, Ferenczi S, Grosz M, et al. Comparison of dialysis and clinical characteristics of patients with frequent and occasional hemodialysis-associated hypotension. Kidney Blood Press Res. 2002;25(2):97–102.
Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66(3):1212–20.
Suzuki H, Kanno Y, Sugahara S, Okada H, Nakamoto H. Effects of an angiotensin II receptor blocker, valsartan, on residual renal function in patients on CAPD. AmJKidney Dis. 2004;43(6):1056–64.
Acknowledgements
We acknowledge the great efforts and contributions of the study nurses and study participants from the six hospitals participating in the SAFIR study. Lab technicians Ilse Rasmussen and Lotte Kristine Cornelius Lassen are thanked for their assistance with blood samples and assays. A subset of data was presented as posters at American Society of Nephrology Kidney Week 2016 in Chicago, IL, USA (Abstract FR-PO925 available from: https://www.asn-online.org/abstracts/) and at Artery 2018 meeting in Guimarães, Portugal (https://doi.org/10.1016/j.artres.2018.10.203).
Funding
The SAFIR study was primarily supported by The Danish Council for Independent Research Medical Sciences, but also by (in alphabetical order): Aase og Ejnar Danielsens Fond, Beckett-Fonden, Civilingeniør Frode Nygaard og Hustrus Fond, The Danish Society of Hypertension, The Danish Society of Nephrology, Direktør Kurt Bønnelycke & Hustru Grethe Bønnelyckes Fond, Fabrikant Karl G Andersens Fond, Fausbølls Helsefond, Fonden til udvikling og uddannelse ved Nyremedicinsk Afdeling ved Aarhus Universitetshospital, Fresenius Medical Care Denmark, Frimodt-Heineke Fonden, Helen & Ejnar Bjørnows Fond, The Institute of Clinical Medicine at Aarhus University, Kirsten Anthonius’ Fond, Leo Pharmas Hypertensionslegat, Nyreforeningens Forskningsfond, Overlæge Poul M Christiansen & Hustrus Fond, Region Midtjyllands Sundhedsvidenskabelige Forskningsfond, Snedkermester Sophus Jacobsen and Hustru Astrid Jacobsens Fond. Sanofi Denmark provided original study medication (placebo and active tablets) free of charge and financially supported some meeting activity. NorDiaTech Denmark provided one Transonic Flow-QC Hemodialysis Monitor for three years including yearly calibration. The funders including Sanofi Denmark and NorDiaTech Denmark had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
KK, CDP, BJ and JDJ conceptualized the study. CDP is the principle author and drafted the manuscript. KLC is a senior cardiology consultant who provided valuable input given his extensive experience with blood pressure research and greatly aided in the interpretation of the data. JDJ is a senior hemodialysis consultant who contributed to data analysis, preparation of figures and drafting of the manuscript. BJ is a senior consultant and professor in nephrology responsible for the SAFIR-study and oversaw the work. CDP and KK were both principal investigators in the SAFIR-study and contributed to data collection, database preparation, literature review and writing. BMB provided statistical support and contributed to writing the statistical part of the Methods section and Results section. All authors contributed to, reviewed and approved the manuscript including all its drafts. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with good clinical practice (GCP) and the ethical standards described in the Helsinki Declaration. All participating sites were monitored by a local independent GCP-Unit. Written informed consent was obtained from all participants. The Central Denmark Region Committees on Biomedical Research Ethics, the Danish Health and Medicines Authority, and the Danish Data Protection Agency approved the study. ClinicalTrials.gov Identifier: NCT00791830 EudraCT no: 2008–001267-11.
Consent for publication
Consent for publication is not applicable to this manuscript.
Competing interests
Dr. Bibby reports personal fees from Kidney International and Kidney International Reports although not related to this paper. Besides funding sources, the other authors have declared that no competing interests exist.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Methodology. Additional results, Table S1, Table S2
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Peters, C.D., Kjaergaard, K.D., Christensen, K.L. et al. High-sensitivity Troponin T in hemodialysis patients: a randomized placebo-controlled sub-study investigating angiotensin-II-blockade, variation over time and associations with clinical outcome. BMC Nephrol 21, 452 (2020). https://doi.org/10.1186/s12882-020-02103-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-020-02103-1