Abstract
Background
Carbon dioxide (CO2) accumulation is a challenging issue in critically ill patients. CO2 can be eliminated by renal replacement therapy but studies are scarce and clinical relevance is unknown. We prospectively studied CO2 and O2 behavior at different sample points of continuous veno-venous hemofiltration (CVVH) and build a model to calculate CO2 removal bedside.
Methods
In 10 patients receiving standard CVVH under citrate anticoagulation, blood gas analysis was performed at different sample points within the CVVH circuit. Citrate was then replaced by NaCl 0.9% and sampling was repeated. Total CO2 (tCO2), CO2 flow (V̇CO2) and O2 flow (V̇O2) were compared between different sample points. The effect of citrate on transmembrane tCO2 was evaluated. Wilcoxon matched-pairs signed rank test was performed to evaluate significance of difference between 2 data sets. Friedman test was used when more data sets were compared.
Results
V̇CO2 in the effluent (26.0 ml/min) correlated significantly with transmembrane V̇CO2 (24.2 ml/min). This represents 14% of the average expired V̇CO2 in ventilated patients. Only 1.3 ml/min CO2 was removed in the de-aeration chamber, suggesting that CO2 was almost entirely cleared across the membrane filter. tCO2 values in effluent, before, and after the filter were not statistically different. Transmembrane tCO2 under citrate or NaCl 0.9% predilution also did not differ significantly. No changes in V̇O2 were observed throughout the CVVH circuit. Based on recorded data, formulas were constructed that allow bedside evaluation of CVVH-attributable CO2 removal.
Conclusion
A relevant amount of CO2 is removed by CVVH and can be quantified by one simple blood gas analysis within the circuit. Future studies should assess the clinical impact of this observation.
Trial registration
The trial was registered at https://clinicaltrials.gov with trial registration number NCT03314363 on October 192,017.
Similar content being viewed by others
Background
Red blood cells and plasma harbour carbon dioxide (CO2) in the form of dissolved CO2, bicarbonate, and carbamino compounds which are in equilibrium with each other [1]. The sum of all components is expressed as total CO2 (tCO2).
CO2 accumulation causes hypercarbia which may be a challenge in intensive care unit (ICU) patients. It has propagated a more extensive use of extracorporeal techniques to enable ultra-protective ventilation in acute respiratory distress syndrome or to avoid intubation in patients with severe exacerbation of chronic obstructive lung disease [2]. Although renal replacement therapy (RRT) is advocated to generate small amounts of CO2 due to the red blood cell passing through the filter [3], the net effect is a removal of CO2 in an intermittent hemodialysis (IHD) model with acetate [3, 4] This CO2 extraction of 41 ml/min seemed to correlate with a deficit of 46 ml/min in expired CO2 [5]. Different methods have been explored to increase the removal of CO2 in effluent by increasing pH with THAM or NaOH but it seemed too complex and too dangerous to be used in humans. [6] Extraction reached up to 120 ml/min in an in vitro model of IHD [7]. “CO2 loss” induced by RRT may become clinically relevant as mean expired CO2 in ICU patients is 180 ml/min [8]. Continuous RRT (CRRT) is progressively supplanting intermittent dialysis in the ICU. CRRT is hemodynamically well-tolerated, may provide easier control of metabolic alterations and fluid overload, and is associated with less chronic kidney disease in the post-ICU phase [9,10,11]. The impact of CRRT on CO2 metabolism is remarkably poorly documented. In addition, trisodium citrate - the preferred anticoagulant for CRRT- acts as a weak acid [12]. This will alter the Henderson-Hasselbalch equation, disrupt the balance between the different CO2 forms, and thus potentially influence CO2 extraction during CRRT.
We designed a study to better understand CO2 and O2 extraction during CRRT. Based on obtained data, formulas were constructed to assess CRRT-related CO2 clearance at the bedside.
Methods
A prospective study was performed in critically ill patients undergoing continuous veno-venous hemofiltration (CVVH). The study was approved by the Ethical Committee of the University Hospital Brussels (reference BUN 143201731636) and registered at https://clinicaltrials.gov (reference NCT03314363). Informed consent was obtained from the patient or a legal representative.
CVVH was performed with the Prismaflex®(Lund, Sweden) Baxter® device equipped with a Prismaflex® Baxter®, AN69 surface treated (ST) filter of 1.5 square meter (Meyzieu, France). Prismocitrate®18/0 (Sondalo, Italy) Baxter® was used as predilution and Prismocal® B22 (Sondalo, Italy) Baxter® or NaCl 0.9% as postdilution fluid. Dosing and postdilution fluid use were initiated and adapted according to an implemented standard CVVH protocol.
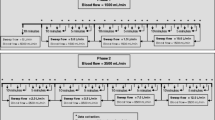
Inclusion and exclusion criteria are listed in Additional file 1. Blood samples were taken at 5 different sample points (SP) (Fig. 1). SP were located at the distal end of the arterial dialysis catheter lumen (SP1); between citrate predilution infusion port and filter (SP2); directly after the filter (SP3); at the effluent conduit (SP4); proximal to the venous dialysis catheter lumen (SP5). At every SP, pH, HCO3-, tCO2, pCO2, pO2, hemoglobin (Hb) and Hb saturation were measured using a blood gas analyzer [ABL90 Flex®, Radiometer(Bronshoj, Denmark)]. Subsequently, citrate predilution was stopped and replaced for at least 20 min by NaCl 0.9% at similar flow. Blood gas analysis was repeated according to the same protocol.
O2 content (tO2) was calculated as Hb x Hb saturation × 1.35 [1]. CO2 (V̇CO2) and O2 flow (V̇O2) at the specific SP were calculated by multiplying the set fluid flow (Q) on CVVH with respectively tCO2 and tO2. Results were adjusted from mmol to ml by using Boyle’s gas law: pV = nRT (p: pressure of the gas, V: volume of gas, n: amount of substance of gas, R: gas constant, T absolute temperature of the gas). The average air pressure recorded by the Belgian national weather institute was applied and ambient temperature was measured. “Transmembrane” (i.e before and after the filter) V̇CO2 was calculated by subtracting V̇CO2 at SP3 from V̇CO2 at SP2. “Transmembrane tCO2” was calculated in the same way.
tCO2 of bicarbonate fluid was 22 mmol/l, converted by gas law to ml/l depending on ambient conditions, and calculated in ml/min based on the flow set on CVVH. When bicarbonate was used, the “expected V̇CO2 at SP5” was calculated by adding the calculated V̇CO2 of the postdilution fluid to the V̇CO2 at SP3.
Relevant parameters such as CRRT settings were collected tobe used in a predictive equation.
Statistical analysis
Data were analyzed using Prism Graphpad® version 7(La Jolla, USA). As data sets contained 18 values at the most, normality was not assessed. Data are expressed as mean ± standard deviation. Wilcoxon matched-pairs signed rank test was performed to evaluate significance of difference between 2 data sets. Friedman test was used when more data sets were compared. Differences in measured data, V̇CO2 and V̇O2 between SP were evaluated.
V̇CO2 in the effluent (SP4) was compared with “transmembrane V̇CO2”. “Expected V̇CO2 at SP5” was compared with V̇CO2 at SP5. A subgroup analysis was performed to compare the influence of citrate on CO2 extraction by the filter by comparing “transmembrane tCO2” with and without citrate.
Results
Summary of patient characteristics are depicted in Table 1. CVVH settings of patients are provided in Additional file 2. Predilution citrate was not replaced by NaCl 0.9% in 2 patients because pre-existing hypercoagulability could compromise filter function.
Comparison of V̇CO2
V̇CO2 (SP1) was higher than V̇CO2 (SP5) and V̇CO2 (SP2) [111.3 ± 8.1 ml/min vs. respectively 87.4 ± 14.6 ml/min (p < 0.01) and 110.5 ± 9.6 ml/min (p = 0.03)]. V̇CO2 dropped significantly between SP2 and SP3 [from 110.5 ± 9.6 ml/min to 84.5 ± 6.5 ml/min (p < 0.01)]. V̇CO2 at SP4 (26.0 ± 5.8 ml/min) and transmembrane V̇CO2 at SP4 (24.2 ± 2.6 ml/min) were not statistically different (p = 0.39).
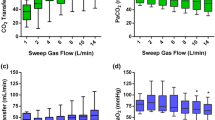
Results for NaCl 0.9% postdilution were plotted in Fig. 2. Patients receiving postdilution NaCl 0.9% exhibited higher V̇CO2 (SP1) than V̇CO2 (SP5) [109.8 ± 7.1 ml/min vs. 81.7 ± 5.8 ml/min (p < 0.01)] and a 1.3 ml/min difference between V̇CO2 (SP3) and V̇CO2 (SP5) [83.0 ± 4.9 ml/min vs. 81.7 ± 5.8 ml/min (p = 0.01)].
Evolution of CO2 flow in the extracorporeal blood circuit during NaCl 0.9% postdilution. Sample point 4 was not included as it is not situated in the extracorporeal blood circuit and is not suited to represent evolution of V̇CO2 in the blood. When difference between data was statistical significant different, this was marked with an asterisk
No statistical analysis was performed when bicarbonate was used as postdilution fluid as it only consisted of 3 data sets. A 21.7 ml/min difference was noted between V̇CO2 at SP5 (116.1 ± 9.7 ml/min) and “expected V̇CO2 at SP5” (94.4 ± 9.9 ml/min) (Fig. 3).
Comparison of tCO2
Results are given in Fig. 4. tCO2 at SP2 (25.5 ± 2.8 mmol/l), SP3 (25.0 ± 2.6 mmol/l) and SP4 (25.1 ± 2.6 mmol/l) were not statistically different (p = 0.51). tCO2 decreased significantly between SP1 and SP2 from 30.6 ± 2.3 mmol/l to 25.5 ± 2.8 mmol/l (p < 0.01). At all SP, tCO2 consisted of CO2 in gas form (pCO2) and HCO3- (Fig. 5).
Effect of citrate vs. no-citrate predilution on transmembrane tCO2
Patients in whom citrate could not be withdrawn were excluded from analysis. ΔtCO2 between SP2 and SP3 was not different in the citrate vs no-citrate group (p = 0.21) (Fig. 6).
Comparison of V̇O2
V̇O2 at SP1, SP2, SP3 and SP5 was respectively 10.6 ± 3.7 ml/min,10.9 ± 3.9 ml/min, 10.3 ± 3.8 ml/min, and 10.9 ± 3.7 ml/min. V̇O2 at SP4 was 0 ml/min as effluent contains no Hb. V̇O2 (SP1) and V̇O2 (SP5) were not different (p = 0.33) (Fig. 7).
Development of formulas
The above findings allow to propose following formulas:
As tCO2 is similar at SP2, SP3 and SP4, the equation becomes:
By assuming that
When [tCO2]SP2 is substituted in the above formula [*], it becomes
Discussion
We present the first study that prospectively evaluated CO2 and O2 behavior in patients undergoing CVVH. The main finding was that a substantial amount of 26.0 ml/min CO2 was removed in the effluent. This represents approximately 14% of the average expired V̇CO2 measured in ICU patients [8] and thus could be clinically relevant. Furthermore, CO2 removal during CVVH was found to be 80% lower than previously observed in an in vitro hemodialysis model. This is explained by the almost threefold higher blood flow rate used in this model as compared to our CVVH setting [7]. V̇CO2 before and after predilution (Δ V̇CO2 between SP1 and SP2) was statistically different, probably because the set CVVH fluid flow at these SP did not correspond with real fluid flow [9]. Blood analysis also depended on “snapshot” sampling which might not exactly reflect average flow. However, this difference did not seem clinically relevant compared with average expired V̇CO2 (< 1%). CO2 flow was then divided between the effluent and the blood running to the de-aeration chamber (SP3 and SP4). The CO2 flow in the effluent correlated with V̇CO2 loss in the blood after passing the filter [\( \dot{\mathrm{V}}{\mathrm{CO}}_{2\left(\mathrm{SP}2\right)}-\dot{\mathrm{V}}{\mathrm{CO}}_{2\left(\mathrm{SP}3\right)}=\dot{\mathrm{V}}{\mathrm{CO}}_{2\left(\mathrm{SP}4\right)} \)].
In patients receiving postdilution NaCl 0.9%, 1.3 ml/min of CO2 was removed in the de-aeration chamber. This is a very small quantity compared to the average V̇CO2 in ICU patients [8]. Thus, CO2 removal was almost entirely determined by transmembrane filtering and measurable in the effluent. However, when postdilution bicarbonate was used, the expected V̇CO2 did not correspond with the calculated V̇CO2 in the blood before it re-entered the body. Several assumptions may explain this observation. First, measurements may be incorrect when CO2 fails to enter red blood cells after being infused in the postdilution fluid into the extracorporeal circuit. Second, tCO2 was calculated and not measured. Formulas for these calculations may not be applicable in a non-physiological state of bicarbonate-induced blood alkalinization. Studies measuring blood tCO2 are needed to elucidate this problem.
As suggested by in vitro hemodialysis, CO2 is removed in the effluent in gas form and as HCO3- [7]. The CO2 concentration or tCO2 is the driving force for this removal as it remains constant in effluent and in the blood passing through the filter [tCO2(SP2) = tCO2(SP3) = tCO2(SP4)]. By adding predilution fluid, tCO2 decreased between SP1 and SP2.
Citrate anticoagulation did not influence tCO2 extraction. Only the short term effect of citrate upon CO2 removal was evaluated as an influencer of acid-base homeostasis. Over a longer time period, citrate could possibly affect CO2 clearance because it preserves membrane porosity better than heparin. tCO2 in blood passing through the CVVH circuit decreased as it was diluted by bicarbonate-free solutions. CVVH had no impact on V̇O2 because values remained constant at the different SP.
Based on previous findings, different formulas were constructed to calculate CO2 removal by CVVH in a clinical setting with the use of only one blood gas analysis in the extracorporeal circuit at a preexisting sample point. As these are the first data that were acquired in a CVVH setting, formulas could not be compared to data from other articles [7]. Further studies need to confirm our findings.
Several limitations of our study must be emphasized. First, despite the high number of analyses per patient, the sample size remains small and future studies in more patients are needed to confirm our results. Second, assumptions were made based on “snapshot” blood gas analysis. Continuous monitoring would be more precise. Third, fluid flows as set on CVVH may not correlate with real flow [13]. In-circuit flow measurements may be a better option. Finally, it remains to be determined whether the removed CO2 influences expired CO2.
Conclusion
A significant amount of CO2, both as gas and bicarbonate and measurable in the effluent, is removed during CVVH under citrate anticoagulation. Pre-filter tCO2 is the major determinant for CO2 removal. Citrate does not influence CO2 elimination. To a certain extent, bicarbonate fluids influence blood gases but data are too limited to permit relevant conclusions. Oxygen flow is not influenced by CVVH. CO2 removal by CVVH in bicarbonate-free conditions can be calculated by multiplying effluent or blood flow with CO2 content at a preexisting sample point. Their clinical relevance requires confirmation.
Change history
08 August 2019
.
Abbreviations
- CO2 :
-
Carbon dioxide
- CRRT:
-
Continuous renal replacement therapy
- CVVH:
-
Continuous veno-venous hemofiltration
- Hb:
-
Hemoglobin
- ICU:
-
Intensive care unit
- IHD:
-
Intermittent hemodialysis
- n:
-
Amount of substance of gas
- O2 :
-
Oxygen
- p:
-
Pressure of the gas
- Q:
-
Fluid flow
- R:
-
Gas constant
- RRT:
-
Renal replacement therapy
- SP:
-
Sample point
- T:
-
Absolute temperature of the gas
- tCO2 :
-
Total CO2
- tO2 :
-
Oxygen content
- V:
-
Volume of gas
- V̇CO2 :
-
CO2 flow
- V̇O2 :
-
O2 flow
References
Boron WF. Transport of oxygen and carbon dioxide in the blood. In: Boron WF, Boulpaep EL, editors. Medical physiology, updated edition; 2005. p. 654–68.
Taccone FS, Malfertheiner MV, Ferrari F, et al. Extracorporeal CO2 removal in critically ill patients: a systematic review. Minerva Anestesiol. 2017;83(7):762–72.
Bosch JP, Glabman S, Moutoussis G, Belledonne M, von Albertini B, Kahn T. Carbon dioxide removal in acetate hemodialysis: effects on acid base balance. Kidney Int. 1984;25(5):830–7.
Nosé Y, Malchesky PS. Therapeutic membrane plasmapheresis. Ther Apher. 2000;4(1):3–9.
Sherlock JE, Yoon Y, Ledwith JW, Letteri JM. Respiratory gas exchange during hemodialysis. Proc Clin Dial Transplant Forum. 1972;2:171–4.
Gille JP, Saunier C, Schrijen F, Hartemann O, Tousseul B. Metabolic CO2 removal by dialysis: THAM vs NaOH infusion. Int J Artif Organs. 1989;12(11):720–7.
May AG, Sen A, Cove ME, Kellum JA, Federspiel WJ. Extracorporeal CO2 removal by hemodialysis: in vitro model and feasibility. Intensive Care Med Exp. 2017;5(1):20.
De Waele E, Opsomer T, Honore PM, et al. Measured versus calculated resting energy expenditure in critically ill adult patients. Do mathematics match the gold standard. Minerva Anestesiol. 2015 Mar;81(3):272–82.
Case J, Khan S, Khalid R, et al. Epidemiology of acute kidney injury in the intensive care unit. Crit Care Res Pract. 2013:479730.
Uchino S, Bellomo R, Kellum JA, et al. Patient and kidney survival by dialysis modality in critically ill patients with acute kidney injury. Int J Artif Organs. 2007;30(4):281–92.
Lins RL, Elseviers MM, Van der Niepen P, et al. Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: results of a randomized clinical trial. Nephrol Dial Transplant. 2009;24(2):512–8.
Schneider A, Journois D, Rimmele T, et al. Complications of regional citrate anticoagulation: accumulation or overload? Crit Care. 2017;21(1):281.
Baldwin I, Bellomo R, Koch B. A technique for the monitoring of blood flow during continuous hemofiltration. Intensive Care Med. 2002;28(9):1361–4.
Acknowledgments
The authors thank Walter Moeyersons, RN, for expert technical assistance.
Funding
A Baxter grant was received for this trial. It was a investigation initiated research project with no interference during the study design or during the collection, analysis and interpreting of the data or during the writing of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
JJ and EDW were responsible for the study design and background search. JJ, EDW and JD collected and assembled the data. JJ, HS, AD, JD, MD, OC, KL, TO, PMH, MM and EDW participated in analysis and interpretation of data and manuscript writing. JJ, HS, AD, JD, MD, OC, KL, TO, PMH, MM and EDW approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethical Committee of the University Hospital Brussels (reference BUN 143201731636). Written informed consent for participation was obtained from the patient or a legal representative.
Consent for publication
Not applicable.
Competing interests
Dr. Jonckheer and Prof. Dr. De Waele have received a grant from Baxter as a replacement fee and for logistic support.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional information
The original version of this article was revised: the name of one of the authors had been spelled incorrectly. It should be Patrick M. Honoré, not Patrick Honoré.
Additional files
Additional file 1:
Inclusion and exclusion criteria that were used during the study. (DOCX 15 kb)
Additional file 2:
CVVH settings and postdilution fluid per patients. (DOCX 14 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jonckheer, J., Spapen, H., Debain, A. et al. CO2 and O2 removal during continuous veno-venous hemofiltration: a pilot study. BMC Nephrol 20, 222 (2019). https://doi.org/10.1186/s12882-019-1378-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-019-1378-y