Abstract
Background
Podocyte phospholipase A2 receptor (PLA2R) is a major target antigen in idiopathic adult membranous nephropathy (MN). Histological PLA2R staining in the renal tissue has proven to be useful for the detection of idiopathic MN. However, glomerular PLA2R deposits have also been recently observed in several patients with secondary MN, such as hepatitis B virus-associated, hepatitis C virus-associated, and neoplasm-associated MN. Certain inflammatory environments have been suggested to lead to abnormal expression of PLA2R epitopes, with the resulting production of PLA2R autoantibodies.
Case presentation
We report two patients diagnosed with anti-neutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis with MN-lesions, in whom ANCA titers for myeloperoxidase (MPO) were persistently positive. The first patient was a 52-years-old man who presented with interstitial pneumonitis. Microscopic hematuria and proteinuria were found when the interstitial pneumonitis became more severe. Renal biopsy findings yielded a diagnosis of ANCA-associated glomerulonephritis (mixed class) with MN-lesions. The second patient was a 63-years-old woman who had been treated for relapsing polychondritis. Her renal tissue showed evidence of focal ANCA-associated glomerulonephritis with MN-lesions. Interestingly, both MPO and PLA2R were detected in the glomerular subepithelial deposits of both patients. Immunoglobulin G (IgG) 1 and IgG2 were positive in the glomeruli of patient 2, and all subclasses of IgGs were positive in patient 1.
Conclusion
The present cases suggest that ANCA-associated glomerulonephritis could expose PLA2R, leading to the development of MN-lesions.
Similar content being viewed by others
Background
In 2009, podocyte phospholipase A2 receptor (PLA2R) was reported as a major target antigen in idiopathic adult membranous nephropathy (MN) [1]. Subsequently, the presence of PLA2R antibodies in the serum has been shown to have high sensitivity and specificity for differentiating idiopathic MN from secondary MN [2]. In addition, histological PLA2R staining in renal tissue has been shown to be equally useful for the detection of idiopathic MN [3]. However, glomerular PLA2R deposits have also been observed in several patients with secondary MN [4]. For example, 64% of patients with hepatitis B virus (HBV)-associated MN were positive for renal PLA2R, overlapping with hepatitis B surface (HBs) antigen [5].
MN rarely occurs as a complication of anti-neutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis, and the pathological processes of the two diseases are generally thought to occur concurrently [6]. However, the pathogenesis of such disease and the involvement of PLA2R remain unclear.
We herein report two patients with microscopic polyangiitis (MPA) in whom ANCA-associated glomerulonephritis with MN-lesions developed. Although the levels were low, the ANCA titers for MPO were persistently positive in both patients. Interestingly, MPO and PLA2R were both detected in the glomerular subepithelial deposits of the two patients.
Case presentation
Patient 1
A 52-years-old man showing worsening of interstitial pneumonitis and presenting with microscopic hematuria and proteinuria was referred to our department. His interstitial pneumonitis was diagnosed 11 years ago, and since then, he had shown persistent serological positivity for MPO-ANCA. MPA was therefore suspected, and he was carefully followed-up without any medications.
After the referral, his proteinuria progressed to nephrotic syndrome. Physical examination showed bilateral fine crackles and pitting edema in the feet. His urinary protein excretion was 15.9 g/g urinary creatinine. Urinary microscopic examination showed massive erythrocytes. The results of blood examination were as follows: white blood cell count, 12.4 × 103/μL; hemoglobin, 13.5 g/dL; platelet count, 529 × 103/μL; serum creatinine, 1.99 mg/dL; urea nitrogen, 17 mg/dL; total protein/albumin (TP/Alb), 6.4/2.5 g/dL; total cholesterol, 245 mg/dL; immunoglobulin G (IgG), 1103 mg/dL; and IgA/M, 416/89 mg/dL. The C-reactive protein level was 1.2 mg/dL, and hypocomplementemia was absent. The ANCA titer for MPO was 19.4 U/mL, and the proteinase 3 (PR3) titer was within the normal range. Viral antibodies for HBV, HCV, and human immunodeficiency virus (HIV) were negative. His chest X-ray suggested exacerbation of interstitial pneumonitis. Computed tomography scans did not show any evidence of malignant tumors.
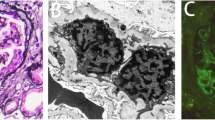
We diagnosed the patient with MPA clinically, and renal biopsy was performed. Light microscopy observations showed crescents in 13 of 28 glomeruli (Fig. 1a) as well as global glomerulosclerosis in 4 of 28 glomeruli. The biopsy also showed diffuse and global spike formation of the glomerular capillary walls (Fig. 1b). Immunofluorescence staining showed granular 2+ deposition of IgG (Fig. 1c) and complement C3 and ± deposition of IgM and complement C1q on the glomerular capillary walls. Electron microscopy showed subepithelial electron-dense deposits, spike formation of the glomerular basement membrane throughout the deposits, and effacement of the podocyte foot processes (Fig. 1d).
Histological features of the renal biopsy in patient 1. a Cellular crescent and endocapillary proliferation in the glomerulus by light microscopy (Periodic acid-Schiff staining). Duplication of the glomerular capillary walls was also suggested where prominent endocapillary proliferation was detected (arrows in inset). b Spike formation of the glomerular basement membrane (arrows) (Periodic acid silver-methenamine stain). c Positive immunofluorescence staining for immunoglobulin G on glomerular capillary walls. d Subepithelial electron-dense deposits, spike formation of the glomerular basement membrane, and effacement of the podocyte foot processes were shown on electron microscopy. e Immunoperoxidase staining for myeloperoxidase (MPO; Nichirei Biosciences, Tokyo, Japan) on paraffin-embedded tissue showed that the glomerular capillary walls and infiltrating neutrophils were positive for MPO. MPO staining was negative where the crescent was formed (arrow). f Indirect immunofluorescence staining for phospholipase A2 receptor (PLA2R; Sigma-Aldrich, St. Louis, MO) labeled with Alexa Fluor 488 (Thermo Fisher Scientific, Waltham, MA) on frozen tissue. The glomerular capillary walls were positive for PLA2R. Original magnification: a, c, e, f: 200×; b: 1000 ×
All of the IgG subclasses were positive on the glomerular capillary walls. Immunoperoxidase staining showed that the glomerular capillary walls were positive for MPO (Fig. 1e). In addition, although serum anti-PLA2R antibody level without any medications, measured by enzyme-linked immunosorbant assay at the day of renal biopsy, was negative, glomerular PLA2R was clearly detected (Fig. 1f). PLA2R staining was completely negative in the negative control (without the primary antibody, data not shown). According to these findings, the patient was diagnosed with ANCA-associated glomerulonephritis (mixed class) [7] with MN-lesions.
Patient 2
A 63-years-old woman who had been treated with steroid therapy for relapsing polychondritis for 11 years was referred to our department because of continuous hematuria and proteinuria. The hematuria appeared 5 years ago, and the proteinuria appeared 1 year ago. Persistently positive ANCA titers for MPO were detected in her serum for 10 years.
Laboratory test findings were as follows: serum creatinine, 0.73 mg/dL; urea nitrogen, 8 mg/dL; TP/Alb, 6.6/4.4 g/dL; IgG, 793 mg/dL; and IgA/M, 208/115 mg/dL. Levels of complements and C-reactive protein were within the normal ranges. The ANCA titer for MPO was 25.6 U/mL, and the PR3 titer was negative. Viral antibodies for HBV, HCV, and HIV were negative. Urinalysis showed urinary protein excretion of 1.6 g/g urinary creatinine, and 10–19 erythrocytes were detected per high-power field.
Renal biopsy showed two crescentic glomeruli (Fig. 2a) and two globally sclerotic glomeruli in light microscopy sections containing 19 glomeruli. Diffuse and global spike formation of the glomerular capillary walls was also present. Immunofluorescence staining showed granular 1+ deposition of IgG and complement C3 on the glomerular capillary walls. Electron microscopy revealed subepithelial and intramembranous deposits of high electron density (Fig. 2b). Immunofluorescence staining of IgG subclasses showed deposition of IgG1 and IgG2 but was negative for IgG3 and IgG4. Immunoperoxidase staining showed that the glomerular capillary walls were positive for MPO (Fig. 2c). Immunofluorescence staining of PLA2R was positive along the glomerular capillary walls (Fig. 2d), although serum PLA2R antibody level at the day of renal biopsy was negative. All findings suggested a diagnosis of MPA, and the renal lesion was diagnosed as ANCA-associated glomerulonephritis (focal class) [7] with MN-lesions.
Representative light microscopy, immunofluorescence, and electron microscopy on the biopsy specimen from patient 2. a Fibrocellular crescent and thickening and duplication of the glomerular capillary walls (inset) (Periodic acid-Schiff stain). b Electron-dense deposits and widespread effacement of podocyte foot processes on electron microscopy. The electron-dense deposits were mainly detected in the subepithelial area, but some were surrounded by the glomerular basement membrane (inset). c Immunoperoxidase staining for myeloperoxidase (MPO) showed that the glomerular capillary walls were granularly positive for MPO. d Indirect immunofluorescence staining for phospholipase A2 receptor (PLA2R) showed that the glomerular capillary walls were positive for PLA2R. Original magnification: a, c, d: 200 ×
Discussion
Although MN and crescentic glomerulonephritis associated with levamisole-adulterated cocaine-induced ANCA-associated vasculitis have been reported [8], ANCA-associated glomerulonephritis and MN are generally considered independent pathologies, and coexistence of the two diseases is thought to be due to chance [9]. However, the common histological findings of the present patients with no history of drug abuse, i.e., both MPO and PLA2R in glomerular deposits, suggested that the coexistence of ANCA-associated glomerulonephritis and MN-lesions reflected mutually related diseases rather than a coincidence. There is one reported case of both MPO-ANCA and anti-PLA2R antibody positivity in the serum [10], and serum anti-PLA2R antibodies and glomerular deposits were simultaneously detected in some patients with ANCA-associated glomerulonephritis combined with MN [11]. To the best of our knowledge, however, these are the first reported cases with ANCA-associated glomerulonephritis and MN-lesions in which both MPO and PLA2R were detected in the glomerular deposits.
PLA2R staining in the renal tissue was originally reported to be useful for differentiating idiopathic MN from secondary MN [4]. However, glomerular PLA2R deposition has since been reported in some patients with secondary MN, such as HBV-, HCV-, and neoplasm-associated MN [12]. Glomerular PLA2R was also found to overlap with HBs antigen in patients with HBV-associated MN [5]. In accordance with these findings, the present patients with ANCA-associated glomerulonephritis exhibited glomerular PLA2R deposits in MN-lesions.
Matsumoto et al. [13] first reported granular MPO deposition along the glomerular capillary walls in a patient with MPO-ANCA-associated glomerulonephritis complicated by MN-lesions. Hanamura et al. [14] also reported that MPO was detected within electron-dense deposits in patients with MPO-ANCA-associated glomerulonephritis and MN-lesions, and suggested that highly cationic MPO released from activated neutrophils could be trapped by the glomerular basement membrane, thereby forming immune complexes and MN-lesions. This same pathogenic mechanism may be at play in our patients with MN-lesions with persistent positive ANCA titers for MPO. However, it should be kept in mind that because serum anti-PLA2R antibodies and tissue PLA2R antigen were not examined in the previous report [14], it cannot be absolutely assumed that MPO is responsible for the MN-lesions.
Anti-PLA2R antibodies in the serum have mainly been detected in IgG4, and PLA2R and IgG4 are colocalized in the glomerular immune deposits of patients with MN [1]. In addition, IgG1 and IgG2 have been reported to be positive in MPO-ANCA-associated glomerulonephritis and MN-lesions [14]. The finding of positive IgG subclass staining in both patient 1 (all IgG subclasses) and patient 2 (IgG1 and IgG2) was consistent with these previous reports. However, the glomeruli of some patients showed negative IgG4 staining but positive PLA2R staining, and the imbalance in the amounts of the corresponding antigens was assumed to influence the results [3]. This explanation could also account for the IgG subclass staining results in patient 2.
Certain inflammatory environments have been suggested to lead to the abnormal expression of PLA2R epitopes, resulting in the production of PLA2R autoantibodies [12]. Although further studies will be required to elucidate the specific mechanism, we speculate that persistent stimulation by MPO and MPO-ANCA may induce podocyte PLA2R expression. Indeed, in patients with both ANCA and anti-glomerular basement membrane (GBM) antibody, it has been suggested that glomerular damage caused by ANCA could expose the target antigen and trigger the production of anti-GBM antibody [15, 16]. However, both of the present cases were negative for serum anti-PLA2R antibodies, although the intensity and pattern of PLA2R immunostaining were adequate for interpretation as real positivity; PLA2R antigen is reportedly expressed on the normal podocyte cytoplasm [2], but was strongly and granularly concentrated within the subepithelial areas in the renal tissue in the present cases (Figs. 1f and 2d). Notably, Debiec and Ronco [17] reported that 10 of 42 patients with primary MN showed positive glomerular PLA2R deposition and negative serum PLA2R antibodies. As a potential explanation for the discordance between the presence of glomerular PLA2R deposition and the absence of serum anti-PLA2R antibodies, the authors proposed that the circulating anti-PLA2R antibodies are initially deposited in the glomeruli and then rapidly cleared from the blood [17]. Indeed, the rate of patients with positive glomerular PLA2R antigens was reported to be much higher than that of patients with positive serum antibodies [17, 18]. It could be also possible that in the present cases without serum anti-PLA2R antibodies and IgG4-restricted glomerular deposits, the trapping theory [14] could explain the pathogenesis, and PLA2R was only a bystander. In addition, the timing of serum PLA2R antibody level measurement could have affected the result, as patient 2 had already been treated with steroid therapy.
Conclusion
In this report, we described the first cases of individuals diagnosed with ANCA-associated glomerulonephritis with MN-lesions in which both MPO and PLA2R were detected in the glomerular deposits. These findings suggest that ANCA-associated glomerulonephritis could expose PLA2R, leading to the development of MN-lesions.
Abbreviations
- ANCA:
-
Anti-neutrophil cytoplasmic antibody
- IgG:
-
Immunoglobulin G
- MN:
-
Membranous nephropathy
- MPA:
-
Microscopic polyangiitis
- MPO:
-
Myeloperoxidase
- PLA2R:
-
Phospholipase A2 receptor
- PR3:
-
Proteinase 3
References
Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11–21.
VanBeek C, Haas M. Anti-PLA2R-associated membranous nephropathy: a review with emphasis on diagnostic testing methods. Clin Nephrol. 2015;84(1):1–9.
Iwakura T, Ohashi N, Kato A, Baba S, Yasuda H. Prevalence of enhanced granular expression of thrombospondin type-1 domain-containing 7A in the glomeruli of Japanese patients with idiopathic membranous nephropathy. PLoS One. 2015;10(9):e0138841.
Svobodova B, Honsova E, Ronco P, Tesar V, Debiec H. Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol Dial Transplant. 2013;28(7):1839–44.
Xie Q, Li Y, Xue J, Xiong Z, Wang L, Sun Z, et al. Renal phospholipase A2 receptor in hepatitis B virus-associated membranous nephropathy. Am J Nephrol. 2015;41(4):345–53.
Gaber LW, Wall BM, Cooke CR. Coexistence of anti-neutrophil cytoplasmic antibody-associated glomerulonephritis and membranous glomerulopathy. Am J Clin Pathol. 1993;99(2):211–5.
Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010;21(10):1628–36.
Collister D, Sathianathan C, Ryz K, Karpinski M, Bernstein K, Gibson IW. ANCA associated vasculitis secondary to levamisole-adultered cocaine with associated membranous nephropathy: a case series. Am J Nephrol. 2017;45(3):209–16.
Nasr SH, Said SM, Valeri AM, Stokes MB, Masani NN, D'Agati VD, et al. Membranous glomerulonephritis with ANCA-associated necrotizing and crescentic glomerulonephritis. Clin J Am Soc Nephrol. 2009;4(2):299–308.
Surindran S, Ayalon R, Hasan N, Beck LH Jr, Salant DJ, Barisoni L, et al. Coexistence of ANCA-associated glomerulonephritis and anti-phospholipase A2 receptor antibody-positive membranous nephropathy. Clin Kidney J. 2012;5(2):162–5.
Zou R, Liu G, Cui Z, Chen M, Zhao MH. Clinical and immunologic characteristics of patients with ANCA-associated glomerulonephritis combined with membranous nephropathy: a retrospective cohort study in a single Chinese center. Medicine (Baltimore). 2015;94(37):e1472.
Larsen CP, Messias NC, Silva FG, Messias E, Walker PD. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Modern Pathol. 2013;26:709–15.
Matsumoto K, Honda H, Shibata T, Sanada D, Wada Y, Ashikaga E, et al. MPO-ANCA crescentic glomerulonephritis complicated by membranous nephropathy: MPO demonstrated in epimembranous deposits. NDT Plus. 2009;2(6):461–5.
Hanamura K, Tojo A, Kinugasa S, Asaba K, Onozato ML, Uozaki H, et al. Detection of myeloperoxidase in membranous nephropathy-like deposits in patients with anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. Hum Pathol. 2011;42:649–58.
Srivastava A, Rao GK, Segal PE, Shah M, Geetha D, et al. Characteristics and outcome of crescentic glomerulonephritis in patients with both antineutrophil cytoplasmic antibody and anti-glomerular basement membrane antibody. Clin Rheumatol. 2013;32:1317–22.
Almouradi T, Hart P, Muram-Zborovski T, et al. An 80-year-old female with double positive disease: case report and brief review of literature. Am J Case Rep. 2013;14:30–3.
Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med. 2011;364(7):689–90.
Qin HZ, Zhang MC, Le WB, Ren Q, Chen DC, Zeng CH, et al. Combined assessment of phospholipase A2 receptor autoantibodies and glomerular deposits in membranous nephropathy. J Am Soc Nephrol. 2016;27(10):3195–203.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Contributions
KT, TU, TI, HK, and NO analyzed and interpreted the patient data regarding kidney disease. KI analyzed and interpreted the patient data regarding collagen disease. HS and KN performed the histological examination of the kidney. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was received from the two patients for the publication of all personal information contained in this case report and accompanying images.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Tominaga, K., Uchida, T., Imakiire, T. et al. Anti-neutrophil cytoplasmic antibody-associated glomerulonephritis with detection of myeloperoxidase and phospholipase A2 receptor in membranous nephropathy-lesions: report of two patients with microscopic polyangiitis. BMC Nephrol 19, 120 (2018). https://doi.org/10.1186/s12882-018-0922-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-018-0922-5