Abstract
Background
Severe malarial anemia (SMA) is a leading cause of malaria-related morbidity and mortality in children. The genetic factors that influence development of SMA and inefficient erythropoiesis, a central pathogenic feature of SMA, are only partially understood.
Methods
We performed a pilot Genome-wide Association Study (GWAS) on children with Plasmodium falciparum. The GWAS was performed using the Illumina® Infinium® HD Super Assay in conjunction with Illumina’s® Human Omni2.5-8v1 BeadChip (with > 2.45 M markers). Data were analyzed using single SNP logistic regression analysis with an additive model of inheritance controlling for covariates. Results from our pilot global genomics study identified that variation in interleukin (IL)-7 was associated with enhanced risk of SMA. To validate this finding, we investigated the relationship between genotypes and/or haplotypes of two single nucleotide polymorphisms (SNPs) in IL7 [72194 T/C and − 2440 A/G] and susceptibility to both SMA and inefficient erythropoiesis [i.e., reticulocyte production index (RPI) < 2.0 in anemic children (Hb < 11.0 g/dL). Children presenting with P. falciparum malaria (< 3 years, n = 883) were stratified into two groups: Uncomplicated malaria (UM, n = 718) and SMA (n = 165).
Results
Regression modeling, controlling for anemia-related confounders, revealed that carriage of the TC genotype at position 72194 T/C was associated with enhanced susceptibility to inefficient erythropoiesis (OR = 1.90; 95% CI 1.09–3.30; P = 0.02) as was homozygous CC (OR 5.14; 95% CI = 1.20–21.99; P = 0.03). Consistent with this finding, individuals with the CA (72194C/−2440A) haplotype had an increased risk of inefficient erythropoiesis (OR = 1.90; 95% CI = 1.10–3.30; P = 0.02), whereas TA haplotype carriers had marginal protection against inefficient erythropoiesis (OR = 0.24; 95% CI = 0.06–1.21; P = 0.05). These observations were supported by Cochran-Armitage trend test for inefficient erythropoiesis (CA > TA > CG; P < 0.01). Although none of the genotype and/or haplotypic variants were significantly associated with SMA, the direction of the risk profiles were consistent with the erythropoiesis results.
Conclusion
Taken together, variation in IL7 is associated with erythropoietic responses in children with falciparum malaria, a central physiological feature contributing to development of SMA.
Similar content being viewed by others
Background
The majority of malaria-related deaths occur in Sub-Saharan Africa as a consequence of infection with Plasmodium falciparum [1]. Severe malarial anemia [SMA, hemoglobin, (Hb<5.0 g/dL), with any density parasitemia], is the most prevalent life-threatening complication of severe malaria occurring in infants and young children in holoendemic P. falciparum transmission areas [1]. The etiology of SMA involves direct and indirect destruction of parasitized and non-parasitized red blood cells (RBCs), and reduced erythropoiesis [2]. We previously demonstrated that genetic variation in immune response genes (i.e., cytokines, chemokines, and effector molecules) influence susceptibility to SMA by altering the production of inflammatory mediators [3,4,5,6,7,8]. To identify novel targets that influence susceptibility to SMA, we utilized a global genomic approach [i.e., Genome-wide Association Study (GWAS)] which identified interleukin (IL)-7 as a potential candidate locus for further validation (unpublished data).
IL-7 is a hematopoietic growth factor which modulates both T- and B-cell development and T-cell homeostasis [9]. The effects of IL-7 are mediated through binding to both the IL-7 receptor alpha (IL-7Rα) and common cytokine gamma chain (γc) which is shared with members of the IL-2 cytokine family (i.e., IL-2, IL-4, IL-9, IL-15, and IL-21) [10]. Production of IL-7 is primarily from non-hematopoietic cells such as stromal cells in the bone marrow and thymus, dendritic cells, hepatocytes, keratinocytes, and epithelial cells [11, 12]. IL-7 induces differentiation of multipotent (pluripotent) hematopoietic stem cells into lymphoid progenitor cells, promotes proliferation, differentiation, and survival of naïve and mature T cells, and stimulates the production of myelopoietic factors (e.g., IL-3 and GM-CSF) [11, 12]. Additionally, IL-7 up-regulates T-cell-dependent activation of monocytes/macrophages which initiates the production of tumor necrosis factor (TNF)-α, IL-1, IL-6, IL-8, and macrophage inflammatory protein-1β (MIP-1β) [9, 13]. Importantly, IL-7 also mediates cell-mediated immune responses to human pathogens, and induces tissue destruction in inflammatory diseases [9, 13,14,15,16,17].
Human IL7 is located on chromosome 8q12–13 and contains 6 exons distributed over more than 33-kbp [18]. In non-African populations, variation in the IL7 locus has been shown to influence susceptibility to inflammatory diseases, including multiple sclerosis and osteoarthritis [19,20,21]. In addition, a targeted genetic-association study in pregnant women from Mozambique revealed that IL7 was strongly associated with susceptibility to placental malaria [22]. A role for IL-7 in malaria is also suggested by studies in murine models in which malarial anemia was associated with suppression of erythropoietic-related cytokines [i.e., granulocyte colony stimulating factor (G-CSF), GM-CSF, IL-7, and IL-17], and elevation of IL-10 and TNF-α [23]. These results are similar to our previous findings showing that levels of circulating IL-7 are suppressed in children with SMA [24].
We postulate that reduced peripheral levels of IL-7 are contributing to inefficient erythropoietic responses. This hypothesis is consistent with results in mice infected with P. yoelii showing that levels of erythropoietic-related cytokines, including IL-7, G-CSF, GM-CSF and IL-17 were reduced in the context of malarial anemia, relative to anemia by other causes [23]. Based on the potentially important role of IL-7 in malaria, results presented here examined the relationship between two IL7 variants identified in the pilot genomic wide experiment (72194 T/C, rs2583759 and − 2440 A/G, rs7007634) and susceptibility to SMA in Kenyan children. In addition, we explored the association between the IL7 variants and inefficient erythropoiesis [defined by a reticulocyte production index (RPI), < 2.0].
Methods
Study participants
Children with falciparum malaria (aged < 3 years, n = 883) presenting at Siaya County Referral Hospital (SCRH) for their first documented visit for acute malaria were recruited. Siaya County is a rural area in western Kenya with holoendemic P. falciparum transmission largely inhabited by Luo ethnic tribe (> 96%), thus, providing a homogeneous population for genetic-based studies. The study site for the current investigation is described in detail in our previous report [25]. Written informed consent in the language of choice (Dholuo, Swahili or English) was sought from the parent/guardian of each child participating in the study. A questionnaire was used to collect demographic and clinical information (Additional file 3). Children were excluded from the study if they had a positive blood smear with non-P. falciparum species; any previous hospitalization; and/or a diagnosis of cerebral malaria. Hemoglobin (Hb) concentrations were used to stratify parasitemic children into SMA (Hb < 5.0 g/dL; n = 165) and uncomplicated malaria [UM (Hb ≥ 5.0 g/dL; n = 718)] [1].
Since nearly all children in holoendemic regions, such as Siaya, develop repeated episodes of malaria prior to reaching naturally-acquired immunity, and it is unclear what disease outcome will occur in healthy children once they acquire malaria, UM was selected as the control group in the study. All children were tested for absence/presence of HIV-1 and bacteremia since we have previously shown that these co-infections impact on malarial anemia severity [8, 26]. Pre- and post-test HIV counselling was provided to the parents/guardians of the study participants. The study was approved by the Scientific Ethics and Research Committee (SERU) of the Kenya Medical Research Institute (KEMRI) and University of New Mexico Institutional Review Board. All samples were collected prior to the administration of any treatment regimen. Patients were treated according to the Ministry of Health (MOH)-Kenya guidelines.
Laboratory procedures
Venous blood samples (<3.0 mL) were collected in EDTA-containing vacutainer tubes. Trophozoites were counted against 300 leukocytes in peripheral blood smears stained with 10% Giemsa for 15 minutes. Parasite density was estimated using the formula: parasite density/μL=white blood cell (WBC) count/μL*trophozoites/300. Complete hematological parameters were determined using a Beckman Coulter® AcT diff2™ (Beckman–Coulter Corporation). Reticulocytes were stained using new methylene blue on thin blood films and then counted. The reticulocyte production index (RPI) was calculated as follows: RPI=reticulocyte index (RI)/maturation factor (MF), where RI=(reticulocyte count [%] x hematocrit [Hct]/0.36) and MF=b+(m)(x), where b=1, m=0.05, and x=(average normal population Hct - patient’s Hct) [27]. Typing for the common African 3.7-kb α-globin deletion (α-thalassemia) was carried out by PCR as previously described [8]. Hemoglobin phenotypes were determined by cellulose acetate electrophoresis, while glucose-6-phosphate dehydrogenase (G6PD) deficiency was determined through the fluorescent spot test (Sigma-Aldrich) as previously described [4, 8]. HIV infection was assessed by HIV-1 proviral DNA PCR testing according to our published methods [26]. Bacteremia was determined using Wampole Isostat Pediatric 1.5 system (Wampole Laboratories). API biochemical galleries (bioMerieux, Inc.) and/or serology were used for identification of bacterial isolates [28].
High-throughput genotyping
A pilot GWAS case-control study was performed in children infected with falciparum malaria to identify novel genes/gene pathways involved in the pathogenesis of SMA. Children were stratified into polarized phenotypes: SMA (cases, avg. Hb = 4.1 g/dL, n = 70) and UM (controls, avg. Hb = 10.8, n = 74) to enrich the signals in the tails (extremes) of the disease distribution. Selected individuals were from a cohort of children infected with P. falciparum (n = 1218), and matched according to age, sex, and peripheral parasite density, excluding children with HIV-1, bacteremia, sickle-cell disease (SSD), and G6PD deficiency. The GWAS was performed using the Illumina® Infinium® HD Super Assay in conjunction with Illumina’s® Human Omni2.5-8v1 BeadChip (with > 2.45 M markers). Data were analyzed using single SNP logistic regression analysis with an additive model of inheritance, while controlling for covariates. Analysis was performed using SNP & Variation Suite v8.6 (Golden Helix, Inc., Bozeman, MT, http://www.goldenhelix.com).
Transcriptional factor binding sequence analysis
Polymorphic variation in the promoter regions can functionally affect the transcriptional activity of genes [29]. To determine either gain or loss of binding for (potential) transcription factors (TFs) due to SNP variation, we performed in-silico transcription factor binding site (TFBSs) analyses on the region encompassing the promoter variant using TRANSFAC [30, 31]. Specifically, analysis of IL7–2440 A/G (rs7007634)] was carried out to identify potential changes in TFBS.
Interleukin-7 genotyping
Genomic DNA was extracted using the MasterAmp™ Buccal swab DNA extraction kit (Epicentre Biotechnologies, Madison, WI), and amplified using the Genomiphi DNA amplification kit (GE Health, Life Sciences, Amersham). IL7 (72194 T/C, rs2583759 and − 2440 A/G, rs7007634) was genotyped by a TaqMan 5′ allelic discrimination Assay-By-Design method according to the manufacturer’s instructions (Assay ID: C_9168017_10 for 72194 T/C and Assay ID: C_30930344_10 for − 2440 A/G; Applied Biosystems, Inc.). PCR was performed in a total volume of 10 μL with the following amplification protocol: initial denaturation (60 °C for 30s and 95 °C for 10 min.) followed by 40 cycles of (95 °C for 15 s and 60 °C for 1 min.) and a final extension (− 60 °C for 30s). Genotypes were then determined using allele-specific fluorescence on the StepOnePlus™ Real-Time PCR Systems. StepOne™ Software Version 2.3 was used for allelic discrimination (Applied Biosystems, Inc.). Genotypes were then used to generate haplotypes in HPlus software (Fred Hutchinson Cancer Research Center, Seattle, WA, USA).
Statistical analyses
Statistical analyses were performed using SPSS (version 19.0). Differences between proportions and comparisons of genotype and haplotypic frequencies of IL7 polymorphisms between the UM and SMA groups were performed using the chi-square (χ2) tests. Mann-Whitney U test was used for comparisons of demographic, clinical, and laboratory characteristics between the clinical groups. The relationship between IL7 genotypes/haplotypes, SMA, and inappropriate erythropoiesis were determined using bivariate logistic regression in a model that controlled for potential confounding variables [age, gender, HIV-1 and bacteremia status, Hb phenotypes, G6PD deficiency, and α-thalassemia variants]. Statistical significance was set at P≤0.05. As secondary analyses, multiple linear regression modeling was performed with RPI as the outcome variable. For this model, identical covariates as those used in the bivariate models were entered into block 1, while and genotypes/haplotypes were entered into block 2. In addition, the Cochran-Armitage trend test was used to establish trends in disease outcomes among the genotypes/haplotypes.
Results
Characteristics of the study participants
The demographic, clinical, and laboratory characteristics of the study participants are presented in Table 1. Gender was comparable between the two clinical categories (P=0.72). Children with UM and SMA differed by age, with the UM group being older than children with SMA (P=0.01). Axillary temperature differed between the two clinical groups (P<0.01). As expected based on the a priori grouping, hemoglobin (Hb) concentrations and red blood cell counts (RBC) were lower in SMA patients in relation to the UM group (P<0.01 and P<0.01, respectively). Red cell distribution width (RDW), mean corpuscular hemoglobin concentration (MCHC), and reticulocyte production index (RPI) were comparable between the clinical groups (P=0.48, P=0.79 and P=0.42, respectively). Inefficient erythropoiesis (RPI<2.0) was marginally lower in children with SMA (P=0.08). The RPI is used as marker in the diagnosis of anemia due to altered erythropoietic responses. This standard measure corrects for both the degree of anemia, and the early release of reticulocytes from the bone marrow in anemic patients [27]. We have previously shown that children with malarial anemia have reduced erythropoiesis (RPI<2.0) which increases with anemia severity [26,27,28, 32]. Comparison of the UM and SMA groups also revealed that white blood cell (WBC) counts were marginally elevated in children with SMA (P=0.08). Parasite density was significantly lower in children with SMA (P=0.03), while the proportions of children with high-density parasitemia (HDP: ≥10,000 parasites/μL) did not differ between the groups (P=0.11). The proportion of children with α-thalassemia (α-3.7/α-3.7), G6PD deficiency, and sickle-cell trait (HbAS) were comparable between the groups (P=0.42, P=0.45, and P=0.62, respectively).
IL-7 pilot GWAS and transcription factor binding site analysis
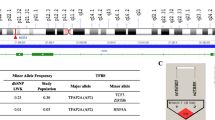
From the pilot GWAS, IL7 emerged as a gene of interest based on statistical significance, and the fact that IL-7 is a hematopoietic growth factor which is largely unexplored in the pathogenesis of SMA [9]. Although the GWAS contained 32 SNPs encompassing the IL7 haploblock (10.8 kb), we selected two SNPs (72194 T/C, rs2583759 and − 2440 A/G, rs7007634) for validation that contained high rankings on the p-value, MAFs (> 10%) in African populations, and contained variation that could impart functional changes based on in-silico analysis.
In-silico analysis of the − 2440 locus revealed that there is a potential TFBS that could potentially impact on the binding of TFs, and consequently transcriptional activity. In the simulated presence of the major allele A at the − 2440 locus, there is proposed binding for interferon regulatory transcription factor (IRF-3), c-E-twenty six transformation-specific-2 (c-Ets-2), activating enhancer binding protein 2 alpha (AP-2alphaA), and Nuclear Factor of Activated T Cells 1 (NF-AT1), while the simulated presence of the minor G allele, indicates binding for Early gene expression factor (Elk-1) and CCAAT/enhancer-binding protein beta (C/EBP beta) (Additional file 1: Table S1).
An A to G transition at the IL7 -2440 locus results in loss of the NFAT1 binding site. NFAT1 is a transcription factor that induces expression of cytokines in T cells (e.g., IL-2, IL-3, IL-4, TNF-α and GM-CSF). Upon T-cell activation, NFAT1 is dephosphorylated by calcineurin and translocates to the nucleus [33].
Distribution of IL7 genotypes and haplotypes in the clinical groups
We assessed the distribution of the IL7 genotypes and the derived haplotypes between children presenting with UM and those with SMA. The overall genotypic frequencies at the 72194 T/C locus were; TT (n=391/883, 44.30%), TC (n=399/883, 45.20%) and CC (n=93/883, 10.50%) (Table 2). There was no significant departure from the Hardy Weinberg Equilibrium (HWE) observed for this variant in the overall population (χ2=0.04, P=0.83). For IL7 (72194 T/C) there were 391 participants inheriting the homozygous wild type genotype (TT), of which 318 (81.30%) had UM (uncomplicated malaria), while 73 (18.70%) were in the SMA group. In addition, 399 study participants were heterozygous TC, of which 325 (81.50%) were in the UM and 74 (18.50%) in the SMA group. For this locus, the homozygous mutant CC, was inherited by 93 participants. These were distributed as follows; 75 (80.60%) were in UM while 18 (19.40%) were in the SMA groups, respectively. The genotypes in this locus were comparable between the two clinical categories (P=0.98). However, there was a significant departure from HWE at the IL7 72194 variant in both the UM (χ2=26.55, P<0.01) and SMA groups (χ2=6.78, P<0.01).
The overall genotypic frequencies at the − 2440 A/G locus were: AA (n = 733/883, 83.00%), AG (n = 142/883, 16.10%), and GG (n = 8/883, 0.90%). No significant departure from Hardy Weinberg Equilibrium (HWE) was observed for this variant in the overall population (χ2 = 0.01, P = 0.81). The distribution in the IL7 (− 2440 A/G) was as follows: homozygous wild type (AA) was inherited by 733 study participants distributed as 601 (82.00%) in UM and 132 (18.00%) in the SMA group. The heterozygous AG was inherited by 142 participants, of which 111 (78.20%) and 31 (21.80%) were in UM and SMA groups, respectively. In addition, the homozygous mutant was inherited by 8 study participants, of which 6 (75.00%) were in UM and 2 (25.00%) were in SMA clinical category. The distribution of the genotypes at this locus was comparable between UM and SMA (P = 0.51). There was significant departure from HWE at the IL7–2440 A/G locus in both the UM (χ2 = 26.60, P < 0.01) and SMA (χ2 = 6.60, P = < 0.01) groups.
Following analyses of the individual variants, haplotypes of 72194 T/C and − 2440 A/G locus were constructed using HPlus software (version 2.5). The following haplotypes were derived: TA, TG, CA and CG. The TA haplotype was inherited by 784 study participants, of which, 639 (81.50%) were in UM and 145 (18.50%) were in the SMA group. The distribution of this haplotype was comparable between UM and SMA groups (P = 0.68). The TG haplotype was inherited by 62 participants, 44 (71.00%) were in UM and 18 (29.00%) were in SMA group. The proportion of TG haplotype was higher in the UM relative to the SMA clinical category (P = 0.03). The CA haplotype was inherited by 415 participants, of these, 336 (81.00%) was in the UM while 79 (19.00%) was in the SMA group. The proportion of CA haplotype was comparable between the clinical categories (P = 0.80). The CG haplotype was inherited by 91 study participants, 76 (83.5%) was in UM and 15 (16.5%) was in SMA group. The proportion of CG haplotype was comparable between the two clinical categories (P = 0.57). There was a weak magnitude of linkage disequilibrium (LD) for the IL7 SNPs 72194/− 2440 (D’ = 0.11; r2 < 0.01).
Susceptibility to SMA and inefficient erythropoiesis (RPI < 2.0)
To determine the impact of the IL7 variants (genotypes and haplotypes) on susceptibility to SMA and inefficient erythropoiesis, we performed binary logistic regression analyses in a model controlling for the potential confounding effects of age, gender, HIV-1 and bacteremia status, G6PD deficiency, Hb phenotypes, and α-thalassemia status. These results are summarized in Table 3. Relative to 72194 TT carriers, neither TC (OR = 0.93; 95% CI = 0.61–1.42; P = 0.73) nor CC (OR = 1.34; 95% CI = 0.69–2.58; P = 0.39) influenced susceptibility to SMA. Similarly, relative to − 2440 AA inheritance, AG (OR = 1.24; 95% CI = 0.74–2.09; P = 0.41) and GG carriers (OR = 0.80; 95% CI = 0.09–7.13; P = 0.84) did not have altered susceptibility to SMA, suggesting that potential ablation of the NFAT1 binding site through carriage of the G allele did not functionally influence disease outcomes. In addition, we determined the association between the derived haplotypes and susceptibility to SMA. Our results show that relative to non-haplotype carriers, TA (OR = 0.70; 95% CI = 0.38–1.29; P = 0.26), TG (OR = 1.29; 95% CI = 0.61–2.71; P = 0.51), CA (OR = 1.04; 95% CI = 0.69–1.56; P = 0.86), and CG (OR = 0.94; 95% CI = 0.48–1.85; P = 0.86) haplotypic carriage had no impact on susceptibility to SMA.
Since altered erythropoietic responses are central to the pathogenesis of SMA [34], we examined the association between genotypes/haplotypes and inefficient erythropoiesis (RPI < 2.0) in the overall population. Inheritance of the 72194 TC (OR = 1.90; 95% CI = 1.09–3.30; P = 0.02) and CC (OR = 5.14; 95%CI = 1.20–21.99; P = 0.03) genotypes enhanced susceptibility to inefficient erythropoiesis. However, relative to − 2440 AA carriage, inheritance of the AG (OR = 1.39; 95% CI = 0.63–3.03; P = 0.42) genotype did not significantly influence susceptibility to inefficient erythropoiesis. The sample size in the clinical groups for determining susceptibility to inefficient erythropoiesis in the GG group was too small for logistic regression analysis. The relationship between haplotypes and inefficient erythropoiesis was also determined. Inheritance of the TA haplotype was associated with moderate protection against inefficient erythropoiesis (OR = 0.24; 95% CI = 0.06–1.21; P = 0.05), whereas individuals with the CA haplotype were at an increased risk of inefficient erythropoiesis (OR = 1.90; 95% CI = 1.10–3.30; P = 0.02). Carriage of the TG (OR = 1.17; 95% CI = 0.40–3.44; P = 0.78) and CG (OR = 1.66; 95% CI = 0.57–4.80; P = 0.35) haplotypes were not associated with altered susceptibility to inefficient erythropoiesis. These observations were supported by Cochran-Armitage trend test for inefficient erythropoiesis (CA > TA > CG; P < 0.01).
In addition, results from the hierarchical linear regression analyses revealed that only the 72194 T/C genotype significantly predicted RPI. Block 1 was R2 = 0.01, P = 0.15 and block 2 was R2 = 0.02, P = 0.04. The 72194 T/C genotype had a β-weight of 0.11, semipartial r2 of 0.09, and P = 0.02.
Discussion
Severe malarial anemia (SMA) is a major cause of morbidity and mortality in western Kenya [2]. The pathogenesis of SMA in holoendemic regions is multifactorial and includes lysis of infected and uninfected RBCs, splenic sequestration of RBCs, dyserythropoiesis and bone marrow suppression, co-infections with bacteremia, HIV-1, and hookworm, and chronic transmission of malaria [28, 35,36,37].
Our previous investigations demonstrated that variations in immune mediator genes, such as IL-1β, IL-12, IL-10, IL-13, IL-18, MIF, and SCGF, influence susceptibility to SMA [3,4,5,6,7,8, 38]. Although the candidate gene approach can be a viable means of uncovering the molecular pathways that influence malaria disease outcomes, unbiased approaches, such as global genomic characterization can identify both novel and known genetic variants associated with complex human phenotypes. As such, we performed a GWAS and whole-transcriptome profiling in a case-control study of children infected with falciparum malaria that were stratified into polarized phenotypes: SMA (cases, avg. Hb = 4.10 g/dL) and UM (controls, avg. Hb = 10.80). Among a prioritized list of genes for further investigation, IL7 emerged as a target for further evaluation (unpublished). On the basis of these findings, we selected variants in IL7 (72194 T/C, located in the intronic region and − 2440 A/G located in promoter region) and explored their association with SMA and inefficient erythropoiesis.
The importance of IL7 in conditioning inflammatory disease outcomes has been previously described [19,20,21]. To the best of our knowledge, this is the first pediatric study to examine the association between the IL7 polymorphisms and susceptibility to these severe disease outcomes in malaria. First, we compared the variant frequencies of the two loci separately and found that the prevalence of individual genotypes at both loci were comparable between the UM and SMA groups. In addition, the single-locus minor allele frequencies (0.33 for 72194C and 0.09 for -2440G) in this population were the same as those for the Yoruban Nigerian population in the HapMap (i.e., 0.33 and 0.09, respectively). These results suggest that the investigated alleles remain largely unchanged over time in different African ethnic groups. However, since the overall genotypic frequencies for the 72194 T/C and -2440A/G SNPs in the UM and SMA groups departed from HWE, it is feasible that population selection pressures from malaria may be responsible for this observation.
To date, only one study has reported an association between IL7 genetic variation and malaria. This study, in pregnant women from Mozambique, revealed an association between IL7 SNPs (rs2583764 and rs2583762, 33 kb apart) and increased susceptibility to placental malaria [22]. These two SNPs are in close proximity to SNP 72194 T/C (rs2583759) genotyped in the present study. This particular SNP (72194 T/C), along with additional SNPs in the same haploblock (rs2583760, rs2583764 and rs6993386), were associated with susceptibility to osteoarthritis in the Chinese Han population [20]. Although we observed significant associations between genotypes and SMA in our GWAS pilot data, this was not replicated in the validation sample. Thus, the signal for SMA in the pilot study was a false-positive and may be attributed to the relatively small sample size in the pilot GWAS study. Alternatively, the pilot study examined children with extreme phenotypes. By including children in the validation study that were parsed into two groups above and below Hb of 5.0 g/dL, there are many children with low Hbs, yet slightly above 5.0 g/dL in the UM group. This may have weakened the ability to identify a significant signal, and is consistent with the reality that there is not a clear-cut clinical difference between Hb levels of 6.0 vs. 5.0 g/dL. Results presented here indicate that carriage of the C allele at IL7 72194 loci significantly increases susceptibility to inefficient erythropoiesis (i.e., RPI < 2.0) in both heterozygous and homozygous carriers. In addition, the trend of reduced susceptibility (TT < TC < CC) in cases with inefficient erythropoiesis was maintained. However, variation at IL7 72194 was not significantly associated with susceptibility to SMA, although carriage of the CC allele trended towards increased risk. This finding suggests that the association between IL7 72194 C allele carriage and erythrocyte production are more closely linked than the phenotypic expression of SMA in which reduced Hb concentrations are a central feature. This result may not be unexpected since inefficient erythropoiesis is an overlapping feature of both UM (89.50%) and SMA (94.40%), albeit slightly higher in SMA. Although a primary cause of SMA in the region is suppression of erythropoiesis, hemolysis of parasitized and non-parasitized RBCs also contributes to the reduced Hb levels [2]. Consistent with the importance of inefficient erythropoiesis in SMA, there was a significant relationship between RPI<2.0 and SMA (Additional file 2: Table S2). Further, we did not find any significant association between IL7–2440A/G and either SMA or inefficient erythropoiesis.
Since haplotypic carriage can provide additional insight, beyond analysis of the individual loci, we constructed IL7 haplotypes from the two SNPs (72194 T/C and -2440A/G). Our previous results demonstrated that haplotypes are highly informative allelic markers for identifying associations with disease outcomes, not identifiable with single polymorphisms [3,4,5,6,7, 39]. Findings from this study show that carriage of the TA haplotype (72194 T/−2440A) was significantly associated with a reduced risk of inefficient erythropoiesis. This finding reflects the fact that carriage of the wild-type alleles at the two loci each trended towards better erythropoietic responses. Conversely, the relationship between carriage of the C allele at IL7 72194 and inefficient erythropoiesis was reflected in the haplotypes in which the CA haplotype was significantly associated with an RPI < 2.0, and the CG haplotype was associated with a non-significant increase in the risk of inefficient erythropoiesis. Similar to the findings for the individual genotypes, none of the haplotypes were associated with susceptibility to SMA. However, the directionality of the risk profiles for SMA and inefficient erythropoiesis were consistent. Since there were not enough children in the current study for which circulating IL-7 levels were available, we could not explore the functional association between the IL7 variants and cytokine production. Previous investigations from our laboratory demonstrated that circulating levels of IL-7 were reduced in children with SMA, but were not significant predictors of Hb concentrations [24]. Moreover, IL-7 promotes T cell-dependent activation of monocyte/macrophages which, in turn, release a number of cytokines (i.e., IL-1, IL-3, IL-6, IL-8, GM-CSF, MIP-1β, TNF-α, and IFN-γ) whose dysregulation is associated with SMA [2, 24, 40,41,42,43]. Additionally, since IL-7 induces erythropoiesis, reduced levels in children with SMA may contribute to delayed and/or inappropriate erythroid development [42].
This study presents the first report on the association between genotypes and haplotypes of IL7 (72194 T/C and -2440A/G) and disease outcomes in children with severe malaria: profoundly low Hb levels and inefficient erythropoiesis. Results presented here suggest that the selected variants are associated with erythropoietic responses. Additional studies are required to determine if the explored IL7 variants functionally alter IL-7 production. It will also be important to determine if products derived from malarial parasite (e.g., antigens, soluble parasitic molecules, and hemozoin) can alter IL-7 production. Investigations in our laboratory are currently exploring these additional experiments in the context of IL-7 and other inflammatory mediators.
We, however, want to note the following limitations of this study. There was a small sample size in the pilot GWAS (n = 144) and moderate sample size in the validation set (n = 883). Given the relatively low frequency of the G allele at the − 2440 locus, the haplotype analyses for this locus may be underpowered.
Conclusion
Results presented here suggest that variation in IL7 is associated with altered erythropoietic responses in children with uncomplicated vs. severe falciparum malaria. These findings have relevance to the development of SMA in children with malaria since inappropriate erythropoiesis is a central physiological feature contributing to development of SMA.
Availability of data and materials
The datasets used and/or analyzed in the current manuscript are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- G-CSF:
-
Granulocyte colony stimulating factor
- GM-CSF:
-
Granulocyte Monocyte colony stimulating factor
- GWAS:
-
Genome-wide Association Study
- Hb:
-
Hemoglobin
- HWE:
-
Hardy Weinberg Equilibrium
- MIP-1β:
-
Macrophage inflammatory protein-1β
- OR:
-
Odds ratio
- RBCs:
-
Red blood cells
- RPI:
-
Reticulocyte production index
- SCRH:
-
Siaya County Referral Hospital
- High school:
-
Severe malarial anemia
- SNPs:
-
Single nucleotide polymorphisms
- SSD:
-
Sickle-cell disease
- TFBS:
-
Transcription factor binding site
- TNF-α:
-
Tumor necrosis factor alpha
References
WHO, World Malaria report, (2017).
Perkins DJ, Were T, Davenport GC, Kempaiah P, Hittner JB, Ong'echa JM. Severe malarial anemia: innate immunity and pathogenesis. Int J Biol Sci. 2011;7:1427–42.
Anyona SB, Kempaiah P, Raballah E, Ouma C, Were T, Davenport GC, Konah SN, Vulule JM, Hittner JB, Gichuki CW, Ong'echa JM, Perkins DJ. Functional promoter haplotypes of interleukin-18 condition susceptibility to severe malarial anemia and childhood mortality. Infect Immun. 2011;79:4923–32.
Awandare GA, Hittner JB, Kremsner PG, Ochiel DO, Keller CC, Weinberg JB, Clark IA, Perkins DJ. Decreased circulating macrophage migration inhibitory factor (MIF) protein and blood mononuclear cell MIF transcripts in children with plasmodium falciparum malaria. Clin Immunol. 2006;119:219–25.
Okeyo WA, Munde EO, Okumu W, Raballah E, Anyona SB, Vulule JM, Ong'echa JM, Perkins DJ, Ouma C. Interleukin (IL)-13 promoter polymorphisms (−7402 T/G and -4729G/a) condition susceptibility to pediatric severe malarial anemia but not circulating IL-13 levels. BMC Immunol. 2013;14:15.
Ouma C, Davenport GC, Awandare GA, Keller CC, Were T, Otieno MF, Vulule JM, Martinson J, Ong'echa JM, Ferrell RE, Perkins DJ. Polymorphic variability in the interleukin (IL)-1beta promoter conditions susceptibility to severe malarial anemia and functional changes in IL-1beta production. J Infect Dis. 2008;198:1219–26.
Ouma C, Davenport GC, Were T, Otieno MF, Hittner JB, Vulule JM, Martinson J, Ong'echa JM, Ferrell RE, Perkins DJ. Haplotypes of IL-10 promoter variants are associated with susceptibility to severe malarial anemia and functional changes in IL-10 production. Hum Genet. 2008;124:515–24.
Ouma C, Keller CC, Davenport GC, Were T, Konah S, Otieno MF, Hittner JB, Vulule JM, Martinson J, Ong'echa JM, Ferrell RE, Perkins DJ. A novel functional variant in the stem cell growth factor promoter protects against severe malarial anemia. Infect Immun. 2010;78:453–60.
Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99:3892–904.
Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, Durum SK. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–33.
Kittipatarin C, Khaled AR. Interlinking interleukin-7. Cytokine. 2007;39:75–83.
Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–62.
Churchman SM, El-Jawhari JJ, Burska AN, Parmar R, Goeb V, Conaghan PG, Emery P, Ponchel F. Modulation of peripheral T-cell function by interleukin-7 in rheumatoid arthritis. Arthritis Res Ther. 2014;16:511.
Damas JK, Waehre T, Yndestad A, Otterdal K, Hognestad A, Solum NO, Gullestad L, Froland SS, Aukrust P. Interleukin-7-mediated inflammation in unstable angina: possible role of chemokines and platelets. Circulation. 2003;107:2670–6.
Hartgring SA, van Roon JA, Wenting-van Wijk M, Jacobs KM, Jahangier ZN, Willis CR, Bijlsma JW, Lafeber FP. Elevated expression or interleukin-7 receptor in inflamed joints mediates interleukin-7-induced immune activation in rheumatoid arthritis. Arthritis Rheum. 2009;60:2595–605.
Kreft KL, Verbraak E, Wierenga-Wolf AF, van Meurs M, Oostra BA, Laman JD, Hintzen RQ. Decreased systemic IL-7 and soluble IL-7Ralpha in multiple sclerosis patients. Genes Immun. 2012;13:587–92.
Sieling PA, Sakimura L, Uyemura K, Yamamura M, Oliveros J, Nickoloff BJ, Rea TH, Modlin RL. IL-7 in the cell-mediated immune response to a human pathogen. J Immunol. 1995;154:2775–83.
Lupton SD, Gimpel S, Jerzy R, Brunton LL, Hjerrild KA, Cosman D, Goodwin RG. Characterization of the human and murine IL-7 genes. J Immunol. 1990;144:3592–601.
Ghavimi R, Pourhossein M, Ghaedi K, Alesahebfosoul F, Honardoost MA, Maracy MR. Genetic association of rs1520333 G/a polymorphism in the IL7 gene with multiple sclerosis susceptibility in Isfahan population. Adv Biomed Res. 2014;3:238.
Zhang HX, Wang YG, Lu SY, Lu XX, Liu J. Identification of IL-7 as a candidate disease mediator in osteoarthritis in Chinese Han population: a case-control study. Rheumatology (Oxford). 2016;55:1681–5.
Zuvich RL, McCauley JL, Oksenberg JR, Sawcer SJ, De Jager PL, C. International Multiple Sclerosis Genetics, Aubin C, Cross AH, Piccio L, Aggarwal NT, Evans D, Hafler DA, Compston A, Hauser SL, Pericak-Vance MA, Haines JL. Genetic variation in the IL7RA/IL7 pathway increases multiple sclerosis susceptibility. Hum Genet. 2010;127:525–35.
Sikora M, Laayouni H, Menendez C, Mayor A, Bardaji A, Sigauque B, Netea MG, Casals F, Bertranpetit J. A targeted association study of immunity genes and networks suggests novel associations with placental malaria infection. PLoS One. 2011;6:e24996.
Xu L, Zheng X, Berzins K, Chaudhuri A. Cytokine dysregulation associated with malarial anemia in plasmodium yoelii infected mice. Am J Transl Res. 2013;5:235–45.
Ong'echa JM, Davenport GC, Vulule JM, Hittner JB, Perkins DJ. Identification of inflammatory biomarkers for pediatric malarial anemia severity using novel statistical methods. Infect Immun. 2011;79:4674–80.
Ong'echa JM, Keller CC, Were T, Ouma C, Otieno RO, Landis-Lewis Z, Ochiel D, Slingluff JL, Mogere S, Ogonji GA, Orago AS, Vulule JM, Kaplan SS, Day RD, Perkins DJ. Parasitemia, anemia, and malarial anemia in infants and young children in a rural holoendemic plasmodium falciparum transmission area. Am J Trop Med Hyg. 2006;74:376–85.
Otieno RO, Ouma C, Ong'echa JM, Keller CC, Were T, Waindi EN, Michaels MG, Day RD, Vulule JM, Perkins DJ. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS. 2006;20:275–80.
Were T, Hittner JB, Ouma C, Otieno RO, Orago AS, Ong'echa JM, Vulule JM, Keller CC, Perkins DJ. Suppression of RANTES in children with plasmodium falciparum malaria. Haematologica. 2006;91:1396–9.
Were T, Davenport GC, Hittner JB, Ouma C, Vulule JM, Ong'echa JM, Perkins DJ. Bacteremia in Kenyan children presenting with malaria. J Clin Microbiol. 2011;49:671–6.
Cookson W, Liang L, Abecasis G, Moffatt M, Lathrop M. Mapping complex disease traits with global gene expression. Nat Rev Genet. 2009;10:184–94.
TFBSanalysis, http://www.cbil.upenn.edu/cgi-bin/tess/tess. Accessed June 2018.
Splice/enhancers, http://www.fruitfly.org/seq_tools/splice.html. Accessed June 2018.
Novelli EM, Hittner JB, Davenport GC, Ouma C, Were T, Obaro S, Kaplan S, Ong'echa JM, Perkins DJ. Clinical predictors of severe malarial anaemia in a holoendemic plasmodium falciparum transmission area. Br J Haematol. 2010;149:711–21.
Hogan PG. Calcium-NFAT transcriptional signalling in T cell activation and T cell exhaustion. Cell Calcium. 2017;63:66–9.
Anyona SB, Kempaiah P, Raballah E, Davenport GC, Were T, Konah SN, Vulule JM, Hittner JB, Gichuki CW, Ong'echa JM, Perkins DJ. Reduced systemic bicyclo-prostaglandin-E2 and cyclooxygenase-2 gene expression are associated with inefficient erythropoiesis and enhanced uptake of monocytic hemozoin in children with severe malarial anemia. Am J Hematol. 2012;87:782–9.
Buffet PA, Safeukui I, Milon G, Mercereau-Puijalon O, David PH. Retention of erythrocytes in the spleen: a double-edged process in human malaria. Curr Opin Hematol. 2009;16:157–64.
Phillips RE, Looareesuwan S, Warrell DA, Lee SH, Karbwang J, Warrell MJ, White NJ, Swasdichai C, Weatherall DJ. The importance of anaemia in cerebral and uncomplicated falciparum malaria: role of complications, dyserythropoiesis and iron sequestration. Q J Med. 1986;58:305–23.
Price RN, Simpson JA, Nosten F, Luxemburger C, Hkirjaroen L, ter Kuile F, Chongsuphajaisiddhi T, White NJ. Factors contributing to anemia after uncomplicated falciparum malaria. Am J Trop Med Hyg. 2001; 65: 614–22.
Phawong C, Ouma C, Tangteerawatana P, Thongshoob J, Were T, Mahakunkijcharoen Y, Wattanasirichaigoon D, Perkins DJ, Khusmith S. Haplotypes of IL12B promoter polymorphisms condition susceptibility to severe malaria and functional changes in cytokine levels in Thai adults. Immunogenetics. 2010;62:345–56.
Ouma C, Davenport GC, Garcia S, Kempaiah P, Chaudhary A, Were T, Anyona SB, Raballah E, Konah SN, Hittner JB, Vulule JM, Ong'echa JM, Perkins DJ. Functional haplotypes of fc gamma (Fcgamma) receptor (FcgammaRIIA and FcgammaRIIIB) predict risk to repeated episodes of severe malarial anemia and mortality in Kenyan children. Hum Genet. 2012;131:289–99.
Park BL, Kim LH, Choi YH, Lee JH, Rhim T, Lee YM, Uh ST, Park HS, Choi BW, Hong SJ, Park CS, Shin HD. Interleukin 3 (IL3) polymorphisms associated with decreased risk of asthma and atopy. J Hum Genet. 2004;49:517–27.
Rafatpanah H, Bennett E, Pravica V, McCoy MJ, David TJ, Hutchinson IV, Arkwright PD. Association between novel GM-CSF gene polymorphisms and the frequency and severity of atopic dermatitis. J Allergy Clin Immunol. 2003;112:593–8.
Aiello FB, Keller JR, Klarmann KD, Dranoff G, Mazzucchelli R, Durum SK. IL-7 induces myelopoiesis and erythropoiesis. J Immunol. 2007;178:1553–63.
Dokter WH, Sierdsema SJ, Esselink MT, Halie MR, Vellenga E. IL-7 enhances the expression of IL-3 and granulocyte-macrophage-CSF mRNA in activated human T cells by post-transcriptional mechanisms. J Immunol. 1993;150:2584–90.
Acknowledgements
We are indebted to the Siaya County Referral Hospital team and the University of New Mexico/KEMRI staff for support and management. We are also grateful to the parents/guardians of the study participants and the children that participated in the study.
Funding
This work was supported by grants from the National Institutes of Health [AI51305-02 (DJP) and TW05884-02 (DJP)]. The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
LEK participated in genotyping experiments, data analyses and manuscript writing. PK participated in the genotyping assays and writing of the manuscript. SBA participated in the analyses and manuscript writing. EOM participated in the analyses and manuscript writing. AOA participated in the analyses and manuscript writing. JMO participated in the analyses and manuscript writing. CGL participated in data analyses and manuscript writing. KC participated in the analyses and manuscript writing. CO participated in the analyses and manuscript writing. DJP conceived the study, participated in the design, implementation, analyses and manuscript writing. ER participated in the design and implementation of the genotyping assays, supervised the study and participated in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Scientific Ethics and Research Committee (SERU) of the Kenya Medical Research Institute (KEMRI) and the Institutional Review Board at the University of New Mexico. Written informed consent in the language of choice (Dholuo, Swahili or English) was sought from the parent/guardian of each child participating in the study.
Consent for publication
Not Applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Transcription factor binding analysis. Transcription factor binding analysis of the IL-72440 A/G (rs7007634)] and amino acid change for the [72194 T/C (rs2583759). (DOCX 16 kb)
Additional file 2:
Table S2. Regression analysis. Relationship between reduced erythropoiesis and SMA. (DOCX 14 kb)
Additional file 3:
Questionnaire. Demographic and clinical data collected at enrollment. (XLS 494 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kisia, L.E., Kempaiah, P., Anyona, S.B. et al. Genetic variation in interleukin-7 is associated with a reduced erythropoietic response in Kenyan children infected with Plasmodium falciparum. BMC Med Genet 20, 140 (2019). https://doi.org/10.1186/s12881-019-0866-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12881-019-0866-z