Abstract
Background
It is now well-known that some antimalarials such as primaquine may induce severe hemolytic anemia in people with G-6-PD deficiency. Antimalarial drug prescriptions must, therefore take into account the patient’s G-6-PD status in malaria endemic areas such as Burkina Faso, where the prevalence of this genetic abnormality is relatively high. Although great clinical heterogeneity is observed depending on the molecular nature of the deficiency and the residual enzyme activity in the red blood cell, there is very poor data on the prevalence of G-6-PD deficiency and the distribution of involved genetic variants in Burkina Faso. In this systematic review, we present a synthesis of the various studies carried out on the G-6-PD deficiency in Burkina Faso in order to determine its prevalence, probable distribution of the genetic variants involved and their clinical implications for a national systematic screening policy among the groups most vulnerable to malaria.
Methods
A systematic review was carried out to analyze available published data on the prevalence, phenotypes and mutations responsible for G-6-PD deficiency in Burkina Faso. The key words used were “G-6-PD deficiency AND Burkina Faso” or “Déficit en G-6-PD AND Burkina Faso” in French. To identify the relevant articles, two independent reviewers reviewed the titles, abstracts and the full text of the selected papers.
Results
An average prevalence of 16.6% (183/1100; CI 95%: 0.145–0.190) and 6.5% (69/1066; CI 95%: 0.051–0.081) of G-6-PD deficiency was found respectively in men and women in this systematic review. Although the predominance (99.8% of G-6-PD deficient cases) of 202A/376G G-6-PD A- variant, the Santamaria and Betica Selma variants were identified in Burkina Faso. Independently of the method used, the enzymatic deficiency was significantly higher in males (2.5–20.5%) compared to females (3.3–12.3%).
Conclusion
This systematic review suggests that despite the ubiquity of the 202A/376G G-6-PD A- variant in Burkina Faso, it will be necessary to consider the Santamaria and Betica Selma variants although their frequencies remain to be specified. A systematic screening of the G-6-PD deficiency is also needed to prevent the occurrence of iatrogenic hemolytic accidents.
Résumé
Contexte
Il est. actuellement bien connu que certains antipaludiques comme la primaquine, peuvent induire des crises d’anémie hémolytique graves chez les personnes présentant un déficit en G-6-PD. Les prescriptions de médicaments antipaludiques doivent donc tenir compte du statut G-6-PD du patient dans les zones d’endémie du paludisme comme le Burkina Faso où la prévalence de cette anomalie génétique est. relativement élevée. En dépit d’une grande hétérogénéité clinique observée selon la nature moléculaire du déficit et l’activité résiduelle de l’enzyme dans le globule rouge, il existe très peu de données sur la prévalence du déficit en G-6-PD et la distribution des variants génétiques en cause au Burkina Faso. Dans cette revue de la littérature nous présenterons la synthèse des différents travaux réalisés sur le déficit en G-6-PD au Burkina Faso afin de déterminer sa prévalence, la distribution probable des variants génétiques en cause et leurs implications cliniques en vue d’une politique nationale de dépistage systématique au sein des groupes les plus vulnérables au paludisme.
Méthodes
Une revue systématique a été réalisée pour analyser les données publiées disponibles sur la prévalence, les phénotypes et les mutations du déficit en G-6-PD au Burkina Faso Les mots clés utilisés étaient « G6PD deficiency AND Burkina Faso » en anglais ou « Déficit en G6PD AND Burkina Faso en français ». Pour identifier les articles pertinents, deux examinateurs indépendants ont examiné les titres, les résumés et le texte intégral des articles retenus.
Résultats
Une prévalence moyenne de 16,6% (183/1100; IC 95%: 0,145–0,190) et 6,5% (69/1066; IC 95%: 0,051–0,081) du déficit en G-6-PD a été observée respectivement chez les hommes et les femmes dans cette revue systématique. Malgré la prédominance (99,8% des cas de déficients en G-6-PD) du variant G-6-PD A- 202A/376G, les variants Santamaria et Betica Selma ont été identifiées au Burkina Faso.
Indépendamment de la méthode utilisée, la prévalence du déficit enzymatique était significativement plus élevée chez les hommes (2,5–20,5%) comparativement aux femmes (3,3–12,3%).
Conclusion
Cette revue systématique suggère qu’en dépit de l’ubiquité du variant G-6-PD A- 202A/376G au Burkina Faso, il est. nécessaire de prendre en compte les variants Santamaria et Betica Selma, bien que leurs fréquences restent à préciser. Un dépistage systématique de la déficience en G-6-PD est. également nécessaire pour prévenir la survenue d’accidents hémolytiques iatrogènes notamment chez les populations les plus vulnérables au paludisme.
Similar content being viewed by others
Background
Glucose-6-phosphate dehydrogenase (G-6-PD) is a key enzyme (EC 1.1.1.49) of the pentose phosphate pathway for the production of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) and ribose-5-phosphate [1,2,3]. It plays a very important role in oxidative stress control in red blood cells without nucleus and mitochondria. A dysfunction of this enzyme, therefore makes the erythrocyte vulnerable to oxidative damage [4]. The clinical presentation of G-6-PD deficiency, however, depends on the level of enzyme deficiency and the intensity of oxidative stress within the erythrocytes [5]. G-6-PD deficiency is a human genetic abnormality with high frequency in malaria endemic areas. G-6-PD gene is highly polymorphic with many allelic variants responsible for different levels of enzymatic deficiency and causing various clinical manifestations [6,7,8]. The different variants responsible for the G-6-PD deficiency were grouped into five (5) classes according to the level of the erythrocyte enzyme activity (≤ 1 to 150%) and the importance of the clinical manifestations [9,10,11]. The most common deficient haplotype or G-6-PD A- in sub-Saharan Africa has two mutations in cis. These are the G202A (rs1050828) and A376G (rs1050829) mutations with a high linkage disequilibrium [8]. Other alleles responsible for the G-6-PD deficiency with frequencies that are over 1% have also been reported in West Africa.
The latter are represented respectively by T968C (rs76723693) and A542T (rs5030872) substitutions [8, 12,13,14]. G-6-PD deficiency is the most common enzymopathy affecting about 7% of the world population [15]. Former studies have suggested that the geographic distribution of G-6-PD deficiency, which is highly correlated with the distribution of current or past malaria endemicity, is not a matter of chance but reflects the only significant selective advantage conferred to carriers of deficient alleles: a resistance against malaria progression to severe forms [16,17,18,19]. Analyses of association between the different G-6-PD genotypes and malaria showed that high levels of G-6-PD deficiency are associated with a decreased risk of cerebral malaria and an increased risk of severe malarial anemia [8, 20].
In Burkina Faso, malaria is highly endemic with an increase in transmission during the rainy season [21]. During this season, it is estimated that more than half of all cases of fever are attributable to malaria [22]. In 2013, malaria remained the leading cause of consultations (46.5%), hospitalizations (61.5%) and deaths (30.5%) in health facilities in Burkina Faso [23]. Under the malaria pressure, the country has a relatively high frequency of G-6-PD deficiency. A particular attention should, therefore be paid to G-6-PD deficiency, which is an X-linked genetic disorder with variable clinical expressions in heterozygous women, which may present serious problems, particularly during malaria treatment [11, 24, 25]. The use of certain antimalarials such as primaquine, consumption of certain foods (fava beans), and a variety of infections (hepatitis, typhoid fever, malaria) induce hemolytic anemia in G-6-PD deficient individuals with various intensity and severity, sometimes requiring emergency blood transfusions [3, 26]. The country should, therefore be informed about the actual prevalence of this enzymatic disorder at the national level as well as the distribution of the genetic variants involved and their clinical implications.
This will allow for safe and appropriate national decisions on the use of potentially dangerous drugs for individuals with G-6-PD deficiency. The aim of this systematic review is to assess the prevalence of G-6-PD deficiency in Burkina Faso and the distribution of the genetic variants involved and their clinical implications for a national systematic screening particularly among the groups most vulnerable to malaria; children under five and pregnant women.
Methods
A systematic review was carried out to analyze available published data on the prevalence, phenotypes and mutations responsible for G-6-PD deficiency in Burkina Faso. Potentially relevant articles in English or French were searched for in PubMed, Google Scholar and Science Direct for a full-text review. The key words used were “G-6-PD deficiency AND Burkina Faso” or “Déficit en G-6-PD AND Burkina Faso” in French. Additional articles were obtained through the follow-up of quotations from journals/opinion articles and original documents. The relevant papers search strategy is presented in Fig. 1. To identify the relevant articles, two independent reviewers reviewed the titles, abstracts and the full text of the selected papers. Prevalences were calculated by plotting the number of people with G-6-PD deficiency in the different studies on the total number of people screened. Confidence Intervals were calculated using the R software version 3.3.3. The haplotypic frequencies of G-6-PD B, A and A- variants were calculated from data from three studies [14, 27, 28]. The populations of these three studies were conform to Hardy-Weinberg equilibrium and these data were used for Inverse Distance weighted (IDW) interpolation of G-6-PD deficiency allele frequency in Burkina Faso using QGIS 2.18.14 software.
Flow diagram showing the method for the papers selection. The database search according to the search strategy described in the methodology section was clean up to exclude duplicates. Titles and abstract were initially screened to include all relevant studies describing the prevalence and/or genetic variants of the G-6-PD deficiency in Burkina Faso. Review articles, articles without abstract or without full text as well as those that did not meet the inclusion criteria were then excluded during the full-text review. Seven (7) research relevant articles and one (1) conference paper were finally selected for this review of the literature
Results
Prevalence of G-6-PD deficiency in Burkina Faso
The prevalence of G-6-PD deficiency observed in the various studies is presented in Table 1. In all selected studies for this systematic review, an average prevalence of 16.6% (183/1100; CI 95%: 0.145–0.190) and 6.5% (69/1066; CI 95%: 0.051–0.081) of G-6-PD deficiency was found respectively in men and women.
According to the methodology used, the prevalence of the deficiency ranged from 15.1 to 20.5% with an average of 17.3% (130/750; CI 95%: 0.147–0.202) in men, against 7.0–12.3% with an average of 9.2% (52/564; CI 95%: 0.070–0.119) in women for enzymatic activity assays. However, this prevalence ranged from 14.3 to 15.4% with an estimated average of 15.1% (53/350; CI 95%: 0.115–0.193]) in men and 3.3–6.0% with an average of 4.4% (17/384; CI 95%: 0.026–0.070) in women for the genotyping studies (PCR method).
Depending on the genus and independently of the method used, the enzymatic deficiency was significantly higher in males (2.5–20.5%) compared to females (3.3–12.3%) (Table 1).
Genetic variant involved in G-6-PD deficiency in Burkina Faso
It should be noted that the most studied deficient variant in Burkina Faso is 202A/376G G-6-PD A- variant. In the study carried out by Meissner et al. [29] in the health district of Nouna, twenty-five over thirty (25/30) of G-6-PD deficiency cases observed, were confirmed carriers of this G-6-PD A- variant. Among the 1136 samples from different genotyping studies identified, 739 samples (Table 2) were screened for mutations 376G, 202A, 542 T, 680 T and 968C with the predominance (99.8% of G-6-PD deficient cases) of 202A/376G G-6-PD A- variant.
The study carried out by Modiano et al. [30] only screened for the 202A/376G G-6-PD A- variant whose frequency varied according to ethnic groups. In the latter study, the lowest frequency (0.069) of this variant was observed among the Fulani compared to the Mossi and the Rimaibe (0.19). However, Santamaria (376G/542T) and Betica Selma (376G/968C) variants were identified in the study carried out by Ouattara et al. [28]. G-6-PD genotypes and B, A and A- alleles frequencies are shown in Table 2.
Discussion
It should be noted that there are very poor data on the prevalence and especially the distribution of G-6-PD deficiency genetics variants in Burkina Faso despite the context of malaria endemicity and self-medication [31,32,33] that would contribute to an increase in malaria mortality due to iatrogenic accidents in G-6-PD-deficient individuals. Indeed, self-medication involves risks such as maladjustment between medication and pathology, wrong dosage or drug interaction that can lead to an increase in oxidative stress.
Prevalence of G-6-PD deficiency in Burkina Faso
The prevalence of G-6-PD deficiency was estimated to be between 9.2 and 17.0% in the various studies carried out or approximately 1,751,164 to 3,235,847 of people affected by G-6-PD deficiency in Burkina Faso. Therefore, this genetic abnormality is a public health problem requiring special attention from the country authorities in charge of health. These disparities in the prevalence could be explained by the fact that the different surveys were carried out in different parts of the country and by methodologies, which differ from one study to another.
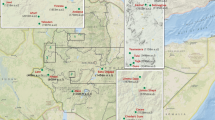
These few studies cited with various methodologies [28, 34, 35] however, do not cover the whole national territory (Fig. 2). The prevalence of G-6-PD deficiency in Burkina Faso, therefore deserves to be determined following a national study with a standard methodology. Indeed, the variation of the methodologies in the various studies carried out greatly influences the results observed. For example, in the enzymatic studies which were the object of this systematic review, it was noted as a diagnostic technique for G-6-PD deficiency detection: the modified paper fluorescence test (NFP Test) [34], the BinaxNOW G-6-PD test [35], or spectrophotometric assay of enzymatic activity [36]. Genotyping studies are also limited by the phenotypic status of deficient heterozygous women, which are not taken into account in the prevalence of G-6-PD deficiency [14, 28].
G-6-PD deficiency genetics variants and allelic frequency in Burkina Faso. The different colored areas (blue and dark green) represent the provinces or cities with data on the prevalence of the G-6-PD deficiency according to Table 1. The different genetic variants identified are represented by colored triangles (ref. [28]). Only data from dark green areas (shown in Table 2) were used for Inverse Distance weighted interpolation of the allelic frequency of G-6-PD deficiency in Burkina Faso because data from other areas did not allow the evaluation of the allelic frequency. There were no data on the G-6-PD genetics variants in Banfora because the prevalence was determined by measuring G-6-PD activity [Source: AKO]
The type of population also influences the prevalence of G-6-PD deficiency. Indeed, the frequency of this genetic abnormality will be relatively lower in groups with clinical malaria due to the mechanisms of protection against the infection progression towards a clinical form or a severe form as described by Ouattara et al. [14]. In their study, Badoum et al. [35] also showed that the prevalence of hemoglobin abnormalities and G-6-PD deficiency (6.9%) was relatively lower in children affected by Plasmodium falciparum symptomatic malaria. Moreover, in the different studies carried out in Burkina Faso, the highest prevalence of G-6-PD deficiency was observed among groups of people without clinical symptoms of malaria [30, 36].
Distribution of G-6-PD gene variants in Burkina Faso
The distribution of the variants involved in G-6-PD deficiency in Burkina Faso is hard to determine. Indeed, not only the numbers of genotyping studies are poor and localized in regions, but most of all, the majority have focused on single 202A/376G G-6-PD A- variant considered as the most common in Africa [30, 34].
However, an analysis of the results of the few genotyping studies carried out in Burkina Faso and West Africa allows us to make some hypotheses. In the few studies conducted in Burkina Faso that looked for known polymorphic variants in the West African area, 202A/376G G-6-PD A- is unequivocally the most predominant variant in Burkina Faso [14, 27,28,29]. However, the Santamaria (376G/542T) and Betica Selma (376G/968C) variants have recently been identified Ouattara et al. [28].
In the latter study, the Santamaria variant (376G/542T) was identified particularly in an Ivorian from the Gouro ethnic group who simultaneously carried the 202A/376G variant with very low parasitemia. This demonstrates the allelic heterogeneity of the G-6-PD deficiency in West Africa and probably a relatively high frequency of the Santamaria (376G/542T) variant in Central-West Ivory Coast, mainly within the Gouro ethnic group. In view of these results, we suggest a distribution of this variant in the South-West area of Burkina Faso with a relatively higher prevalence compared to the others regions due to the community of history between certain ethnic groups (Mandingo) of this area with the Gouro (also Mandingo ethnic group) from Ivory Coast.
However, it is necessary to confirm and specify the frequency of this variant within the Gouro ethnic group in Ivory Coast. The Betica Selma (376G/968C) variant was identified in an individual of Mossi descent in the study conducted by Ouattara et al. [28]. However, we suggest a high prevalence of this variant in the northern region of Burkina Faso, mainly within the Fulani ethnic group. Indeed, the study of Modiano et al. [30] reported a low prevalence of 202A/376G variant within the Fulani ethnic group compared to the Mossi and Rimaibe despite their low susceptibility to malaria.
In addition, Maiga et al. [37] in Mali reported a high frequency of the Betica Selma (376G/968C) variant among the Fulani (6.1%) compared to the Dogon (0.0%), hence the hypothesis of a high frequency of this variant among the Fulani of Burkina Faso.
Different genotyping studies have reported three (3) G-6-PD polymorphic variants in Burkina Faso. These variants are G-6-PDB, G-6-PDA and G-6-PDA-. The present systematic review reports allelic frequencies of 0.563, 0.268 and 0.129, respectively, for the G-6-PDB, G-6-PDA and G-6-PDA- alleles in Burkina Faso. Frequencies of the last two alleles averaged around 0.39 and 0.15 respectively for the G-6-PD A allele and the G-6-PD A- allele in sub-Saharan Africa [3, 8, 18].
Clinical manifestations of G-6-PD deficiency
The different mutations on the G-6-PD gene affect both the stability and the catalytic activity of the enzymatic protein [6, 15]. These different mutations determine haplotypes or deficient variants [38, 39], which has been classified into five (5) WHO categories according to the severity of clinical manifestations [9, 11]. Among the three deficient variants identified in Burkina Faso, the G-6-PD A- (202A/376G) and Betica Selma (376G/968C) variants have class III phenotype, while the Santamaria (376G/542T) variant has WHO class II phenotype. The class III phenotype confers moderate to mild enzyme deficiency of between 10 and 60% of the normal enzyme activity against 2-3% residual G-6-PD activity for the WHO class II phenotype [11, 26].
G-6-PD class III phenotype is associated with hemolytic anemia following oxidative stress while, the class II variants cause severe enzyme deficiency associated with acute hemolytic anemia [8, 40]. A measure of the enzymatic activity associated with these different G-6-PD variants in the context of Burkina Faso is needed for more precision on G-6-PD variants and clinical manifestation at the national level.
Indeed, the T968C allele showed a lower enzymatic activity than the other variants in a study conducted in Mexico [41]. Genetic and environmental factors may, therefore influence the clinical manifestation of G-6-PD deficiency.
G-6-PD deficiency is associated with some protection against severe Plasmodium falciparum malaria infections, but also with increased susceptibility to oxidant hemolysis [42, 43]. Primaquine, which in the treatment of Plasmodium falciparum malaria is highly effective in reducing the transmissibility of gametocytes, has concerns about its safety [43, 44]. This drug, like methylene blue, induces dose-dependent acute hemolytic anemia in individuals with G-6-PD deficiency [42,43,44]. Although when given at a single low dose of 0.25 mg base/kg body weight, primaquine is well tolerated regardless of the patient’s G-6-PD status, a wrong dosage through self-medication could be dangerous for G-6-PD deficient individuals [45]. Indeed, due to the absence of systematic screening, the G-6-PD status of the patient is rarely known and self-medication against malaria remains a reality for a large part of the population [31,32,33]. Methylene blue in combination with chloroquine for the treatment of malaria has been tested effectively in G-6-PD deficient individuals in Burkina Faso. The authors reported that this combination at a given dose was effective in fighting malaria without inducing hemolytic anemia in people with G-6-PD deficiency [34, 46, 47]. Such studies are necessary for the reduction of malaria mortality in Burkina Faso. A systematic screening of the G-6-PD deficiency and increased awareness of the use of antimalarials on medical prescriptions would therefore contribute to a considerable reduction in malaria mortality in Burkina Faso.
Conclusion
Burkina Faso is a country where malaria is endemic with a high frequency of G-6-PD deficiency. The prevalence of this genetic disorder is estimated to average of about 15.0% in the various studies carried out. Despite the ubiquity of the 202A/376G G-6-PD A- variant in all regions of the country, it will be necessary to consider the Santamaria and Betica Selma variants whose frequencies remain to be specified in the different areas of the country. A national study with a standardized method combining genotyping and phenotyping is, therefore more than necessary to determine the actual prevalence and distribution of the different genetic variants involved. Such an approach will make it possible, on the one hand, to establish a national policy for the systematic screening of the G-6-PD deficiency, at least in the groups most at risk, namely children under five and pregnant women in order to prevent the occurrence of iatrogenic hemolytic accidents. On the other hand, it will contribute to reducing malaria mortality through the adequate management of G-6-PD-deficient individuals.
Abbreviations
- CERBA:
-
Pietro Annigoni Biomolecular Research Center
- EC:
-
Enzyme classification
- G-6-PD:
-
Glucose-6-phosphate dehydrogenase
- LABIOGNE:
-
Laboratory of Molecular Biology and Molecular Genetics
- WHO:
-
World Health Organization
References
Beutler E, Duparc S, Group GPDW. Glucose-6-phosphate dehydrogenase deficiency and antimalarial drug development. Am J Trop Med Hyg. 2007;77(4):779–89.
McDonagh EM, Thorn CF, Bautista JM, Youngster I, Altman RB, Klein TE. PharmGKB summary: very important pharmacogene information for G6PD. Pharmacogenet Genomics. 2012;22(3):219–28.
Howes RE, Battle KE, Satyagraha AW, Baird JK, Hay SI. G6PD deficiency: global distribution, genetic variants and primaquine therapy. Adv Parasitol. 2013;81:133–201.
Zhang Z, Chen X, Jiang C, Fang Z, Feng Y, Jiang W. The effect and mechanism of inhibiting glucose-6-phosphate dehydrogenase activity on the proliferation of plasmodium falciparum. Biochim Biophys Acta. 2017;1864(5):771–81.
Luzzatto L, Seneca E. G6PD deficiency: a classic example of pharmacogenetics with on-going clinical implications. Br J Haematol. 2014;164(4):469–80.
Masson P. Presentation. Enzymes: the long pathway from genes to drugs. Ann Pharm Fr. 2007;65(2):95–7.
Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371(9606):64–74.
Clarke GM, Rockett K, Kivinen K, Hubbart C, Jeffreys AE, Rowlands K, Jallow M, Conway DJ, Bojang KA, Pinder M, et al. Characterisation of the opposing effects of G6PD deficiency on cerebral malaria and severe malarial anaemia. elife. 2017;6. doi:10.7554/eLife.15085.
WHO. Glucose-6-phosphate dehydrogenase deficiency. WHO working group. Bull World Health Organ. 1989;67(6):601–11.
Frank JE. Diagnosis and management of G6PD deficiency. Am Fam Physician. 2005;72(7):1277–82.
Luzzatto L, Nannelli C, Notaro R. Glucose-6-phosphate dehydrogenase deficiency. Hematol Oncol Clin North Am. 2016;30(2):373–93.
De Araujo C, Migot-Nabias F, Guitard J, Pelleau S, Vulliamy T, Ducrocq R. The role of the G6PD AEth376G/968C allele in glucose-6-phosphate dehydrogenase deficiency in the seerer population of Senegal. Haematologica. 2006;91(2):262–3.
Clark TG, Fry AE, Auburn S, Campino S, Diakite M, Green A, Richardson A, Teo YY, Small K, Wilson J, et al. Allelic heterogeneity of G6PD deficiency in West Africa and severe malaria susceptibility. Eur J Hum Genet. 2009;17(8):1080–5.
Ouattara AK, Bisseye C, Bazie BV, Diarra B, Compaore TR, Djigma F, Pietra V, Moret R, Simpore J. Glucose-6-phosphate dehydrogenase (G6PD) deficiency is associated with asymptomatic malaria in a rural community in Burkina Faso. Asian Pac J Trop Biomed. 2014;4(8):655–8.
Cunningham AD, Colavin A, Huang KC, Mochly-Rosen D. Coupling between protein stability and catalytic activity determines pathogenicity of G6PD variants. Cell Rep. 2017;18(11):2592–9.
Allison AC. Glucose-6-phosphate dehydrogenase deficiency in red blood cells of East Africans. Nature. 1960;186:531–2.
Tishkoff SA, Varkonyi R, Cahinhinan N, Abbes S, Argyropoulos G, Destro-Bisol G, Drousiotou A, Dangerfield B, Lefranc G, Loiselet J, et al. Haplotype diversity and linkage disequilibrium at human G6PD: recent origin of alleles that confer malarial resistance. Science. 2001;293(5529):455–62.
Allahverdiyev AM, Bagirova M, Elcicek S, Koc RC, Ates SC, Baydar SY, Yaman S, Abamor ES, Oztel ON. Glucose-6-phosphate dehydrogenase deficiency and malaria: a method to detect primaquine-induced hemolysis in vitro. In: Canuto RA, editor. Dehydrogenases. Rijeka: InTech; 2012. Ch. 04.
Howes RE, Dewi M, Piel FB, Monteiro WM, Battle KE, Messina JP, Sakuntabhai A, Satyagraha AW, Williams TN, Baird JK, et al. Spatial distribution of G6PD deficiency variants across malaria-endemic regions. Malar J. 2013;12:418.
Malaria Genomic Epidemiology N. Reappraisal of known malaria resistance loci in a large multicenter study. Nat Genet. 2014;46(11):1197–204.
Diboulo E, Sie A, Vounatsou P. Assessing the effects of malaria interventions on the geographical distribution of parasitaemia risk in Burkina Faso. Malar J. 2016;15:228.
Bisoffi Z, Sirima SB, Menten J, Pattaro C, Angheben A, Gobbi F, Tinto H, Lodesani C, Neya B, Gobbo M, et al. Accuracy of a rapid diagnostic test on the diagnosis of malaria infection and of malaria-attributable fever during low and high transmission season in Burkina Faso. Malar J. 2010;9:192.
Ministère de la Santé BF. Tableau de bord 2014 des indicateurs de santé. 2014. http://cns.bf/IMG/pdf/sante_tableau_de_bord_2014.pdf. Accessed 17 Mar 2017.
Lyon MF. Gene action in the X-chromosome of the mouse (Mus Musculus L.). Nature. 1961;190:372–3.
Beutler E, Yeh M, Fairbanks VF. The normal human female as a mosaic of X-chromosome activity: studies using the gene for C-6-PD-deficiency as a marker. Proc Natl Acad Sci U S A. 1962;48:9–16.
Mura M, Saidi R, Wolf A, Moalic JL, Oliver M. Congenital hemolytic anemia due to glucose-6-phosphate dehydrogenase deficiency. Med Trop (Mars). 2009;69(6):551–5.
Carter N, Pamba A, Duparc S, Waitumbi JN. Frequency of glucose-6-phosphate dehydrogenase deficiency in malaria patients from six African countries enrolled in two randomized anti-malarial clinical trials. Malar J. 2011;10:241.
Ouattara AK, Yameogo P, Diarra B, Obiri-Yeboah D, Yonli A, Compaore TR, Soubeiga ST, Djigma FW, Simpore J. Molecular heterogeneity of glucose-6-phosphate dehydrogenase deficiency in Burkina Faso: G-6-PD Betica Selma and Santamaria in people with symptomatic malaria in Ouagadougou. Mediterr J Hematol Infect Dis. 2016;8(1):e2016029.
Meissner PE, Coulibaly B, Mandi G, Mansmann U, Witte S, Schiek W, Muller O, Schirmer RH, Mockenhaupt FP, Bienzle U. Diagnosis of red cell G6PD deficiency in rural Burkina Faso: comparison of a rapid fluorescent enzyme test on filter paper with polymerase chain reaction based genotyping. Br J Haematol. 2005;131(3):395–9.
Modiano D, Luoni G, Sirima BS, Lanfrancotti A, Petrarca V, Cruciani F, Simpore J, Ciminelli BM, Foglietta E, Grisanti P, et al. The lower susceptibility to plasmodium falciparum malaria of Fulani of Burkina Faso (west Africa) is associated with low frequencies of classic malaria-resistance genes. Trans R Soc Trop Med Hyg. 2001;95(2):149–52.
Pale A, Ladner J. “Street” medication in Burkina Faso: local names, social relationships, and alleged therapeutic effects. Sante. 2006;16(2):113–7.
Ouedraogo LT, Some IT, Diarra M, Guissou IP. Self-medication in the treatment of acute malaria: study based on users of private health drug stores in Ouagadougou, Burkina Faso. Bull Soc Pathol Exot. 2008;101(2):124–7.
Yameogo TM, Kyelem CG, Bamba S, Savadogo LB, Sombie I, Traore AZ, Sanon D, Ouedraogo SM, Guiguemde TG. Management of suspected cases of malaria before admission to a district hospital in Burkina Faso. Med Sante Trop. 2014;24(3):301–6.
Coulibaly B, Eubel JK, Gromer S, Schirmer RH: Biochemistry-based health care research. In: Health research in developing countries: a collaboration between Burkina Faso and Germany. Edited by Becher H, Kouyaté B. Berlin, Heidelberg: Springer Berlin Heidelberg; 2005: 285-292.
Badoum E, Bougouma E, Serme S, Soulama I, Sombie S, Yaro JB, Ouedraogo A, Traore A, Sirima S. Prévalence de l’anomalie de l’hémoglobine et du déficit en Glucose-6-Phosphate- Déshydrogénase chez des enfants ayant un accès palustre à P. falciparum au Burkina Faso. 2014. p. 131. http://www.jssb.org/docs/abstract_book_jssb_2014.pdf. Accessed 25 Mar 2017.
Simpore J, Ilboudo D, Damintoti K, Sawadogo L, Maria E, Binet S, Nitiema H, Ouedraogo P, Pignatelli S, Nikiema JB. Glucose-6-phosphate dehydrogenase deficiency and sickle cell disease in Burkina Faso. Pak J Biol Sci. 2007;10(3):409–14.
Maiga B, Dolo A, Campino S, Sepulveda N, Corran P, Rockett KA, Troye-Blomberg M, Doumbo OK, Clark TG. Glucose-6-phosphate dehydrogenase polymorphisms and susceptibility to mild malaria in Dogon and Fulani, Mali. Malar J. 2014;13:270.
Minucci A, Moradkhani K, Hwang MJ, Zuppi C, Giardina B, Capoluongo E. Glucose-6-phosphate dehydrogenase (G6PD) mutations database: review of the “old” and update of the new mutations. Blood Cells Mol Dis. 2012;48(3):154–65.
Minucci A, Giardina B, Zuppi C, Capoluongo E. Glucose-6-phosphate dehydrogenase laboratory assay: how, when, and why? IUBMB Life. 2009;61(1):27–34.
Cittadella R, Civitelli D, Manna I, Azzia N, Di Cataldo A, Schiliroa G, Brancati C. Genetic heterogeneity of glucose-6-phosphate dehydrogenase deficiency in south-east Sicily. Ann Hum Genet. 1997;61(3):229–34.
Zamorano-Jimenez CA, Baptista-Gonzalez HA, Bouchan-Valencia P, Granados-Cepeda ML, Trueba-Gomez R, Coeto-Barona G, Rosenfeld-Mann F, Rosa-Mireles LB, Melendez-Ramirez R. Molecular identification of glucose-6-phosphate dehydrogenase (G6PD) detected in neonatal screening. Gac Med Mex. 2015;151(1):34–41.
Youngster I, Arcavi L, Schechmaster R, Akayzen Y, Popliski H, Shimonov J, Beig S, Berkovitch M. Medications and glucose-6-phosphate dehydrogenase deficiency: an evidence-based review. Drug Saf. 2010;33(9):713–26.
WHO. Testing for G6PD deficiency for safe use of primaquine in radical cure of P. vivax and P. ovale. WHO/HTM/GMP/20169. 2016. http://www.who.int/entity/malaria/publications/atoz/WHO-testing-for-G6PD-2016-presentation.pdf?ua=1. Accessed 5 Oct 2017.
Beutler E. Glucose-6-phosphate dehydrogenase deficiency: a historical perspective. Blood. 2008;111(1):16–24.
White NJ, Qiao LG, Qi G, Luzzatto L. Rationale for recommending a lower dose of primaquine as a plasmodium falciparum gametocytocide in populations where G6PD deficiency is common. Malar J. 2012;11(1):418.
Mandi G, Witte S, Meissner P, Coulibaly B, Mansmann U, Rengelshausen J, Schiek W, Jahn A, Sanon M, Wust K, et al. Safety of the combination of chloroquine and methylene blue in healthy adult men with G6PD deficiency from rural Burkina Faso. Tropical Med Int Health. 2005;10(1):32–8.
Meissner PE, Mandi G, Witte S, Coulibaly B, Mansmann U, Rengelshausen J, Schiek W, Jahn A, Sanon M, Tapsoba T, et al. Safety of the methylene blue plus chloroquine combination in the treatment of uncomplicated falciparum malaria in young children of Burkina Faso [ISRCTN27290841]. Malar J. 2005;4:45.
Acknowledgments
The authors are particularly grateful to Cyrille BISSEYE (Ph.D.) for valuable comments on the manuscript and for proofreading. This work was entirely supported (internet resources) by Laboratory of Molecular Biology and Genetics (LABIOGENE) UFR/SVT, University Ouaga I Prof Joseph KI-ZERBO, Burkina Faso. The authors would also like to thank Dr. Serge Alain TOUGOUMA (MD) for his valuable contribution to the realization of the G-6-PD deficiency prevalence map in Burkina Faso.
Funding
There were no funding for this manuscript. Internet resources were provided by LABIOGENE.
Availability of data and materials
All data and materials are included in the manuscript and can be found by following the link at the references section.
Author information
Authors and Affiliations
Contributions
Study concept and design: JS, AKO. Independent research of relevant articles: AKO, PY. Full text review of relevant articles: AKO, PY, LT, BD, MA. Statistical analysis and interpretation of data: AKO, PY, LT. Drafting of the manuscript: AKO, PY, LT, BD, and JS. Critical revision of the manuscript for important intellectual content: AKO, PY, LT, BD, MA, DOY, TRC, STS, FWD, JS. Administrative, technical, and material support: JS, AKO. Study supervision: JS. Manuscript Approval: All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ouattara, A.K., Yameogo, P., Traore, L. et al. Prevalence, genetic variants and clinical implications of G-6-PD deficiency in Burkina Faso: a systematic review. BMC Med Genet 18, 139 (2017). https://doi.org/10.1186/s12881-017-0496-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12881-017-0496-2