Abstract
Background
Egr4 is expressed in primary and secondary spermatocytes in adult mouse testes and has a crucial role in regulating germ cell maturation. The functional loss of Egr4 blocks spermatogenesis, significantly reducing the number of spermatozoa that are produced. In this study, we examined whether EGR4 variants are present in Korean men with impaired spermatogenesis.
Methods
A total 170 Korean men with impaired spermatogenesis and 272 normal controls were screened. The coding regions including exon-intron boundaries of EGR4 were sequenced by PCR-direct sequencing method.
Results
We identified eight sequence variations in the coding region and 3′-UTR regions of the EGR4 gene. Four were nonsynonymous variants (rs771189047, rs561568849, rs763487015, and rs546250227), three were synonymous variants (rs115948271, rs528939702, and rs7558708), and one variant (rs2229294) was localized in the 3′-UTR. Three nonsynonymous variants [c.65_66InsG (p. Cys23Leufs*37), c.236C > T (p. Pro79Leu), c.1294G > T (p. Val432Leu)] and one synonymous variant [c.1230G > A (p. Thr410)] were not detected in controls. To evaluate the pathogenic effects of nonsynonymous variants, we used seven prediction methods. The c.214C > A (p. Arg72Ser) and c.236C > T (p. Pro79Leu) variants were predicted as “damaging” by SIFT and SNAP2. The c.65_66insG (p. Cys23Leufs*37) variants were predicted as “disease causing” by Mutation Taster, SNPs &GO and SNAP2. The c.867C > G (p. Leu289) variants were predicted as “disease causing” only by Mutation Taster.
Conclusion
To date, this study is the first to screen the EGR4 gene in relation to male infertility. However, our findings did not clearly explain how nonsynonymous EGR4 variations affect spermatogenesis. Therefore, further studies are required to validate the functional impact of EGR4 variations on spermatogenesis.
Similar content being viewed by others
Background
The early growth response (EGR; MIM# 128992) proteins are a family of zinc finger transcription factors that moderate the regulation of gene expression in response to receptor ligand binding [1]. The EGR family consists of EGR1 (NGFI-A), EGR2 (Krox20), EGR3, and EGR4 (NGFI-C, pAT133) [2, 3]. The zinc finger motifs bind to a specific 9 base pair (bp) consensus sequence (−GCGGGGGCG-) within the promoter regions of downstream genes for transcriptional activation [4, 5]. Egr knock-out mice have provided insights into the biological functions of Egr. For example, Egr1 regulates luteinizing hormone (LH) β expression, and female Egr1-knockout mice are infertile [6]. Egr2- and Egr3-null mice also have specific abnormalities. Moreover, human EGR2 mutations have been identified in patients with congenital hypomyelinating neuropathy or type 1 day Charcot-Marie-Tooth disease [7].
Egr4 is known to be ubiquitously expressed in the central nervous system. However, Tourtellotte et al. found low levels of EGR4 expression in maturing male germ cells. Egr4 expression was detected in primary and secondary spermatocytes in adult mouse testes and had a crucial role in spermatogenesis by regulating germ cell maturation during early-mid pachytene. The functional loss of Egr4 blocked spermatogenesis, leading to a significant reduction in spermatozoa production [8]. In another study, Hogarth et al. also reported Egr4 expression in murine testes and suggested that it may regulate multiple stages of spermatogenesis [9].
To date, only a few studies have investigated the function of the EGR4 gene in humans. Matsuo et al. suggested that EGR4 may regulate bone metastasis and the proliferation of small cell lung cancer cells [10]. EGR4 also regulates the secretion of LH and has a role in the fertility of cryptorchidism patients [11]. However, the relationship between EGR4 and impaired spermatogenesis has not been studied. In this study, we examined for the first time whether sequence variations in the EGR4 gene are present in men with idiopathic non-obstructive azoospermia.
Methods
Subjects
A total of 170 Korean men with impaired spermatogenesis [51 with oligozoospermia aged 33.9 ± 5.00 (age in years, ± standard deviation)) and 119 with azoospermia (aged 34.3 years ± 5.28)] and 272 normal controls (aged 34.5 years ± 4.61) were recruited from the CHA Gangnam Medical Center at CHA University between January 2010 and December 2012. Patients with tubule obstruction, chromosome abnormality, or a microdeletion in the AZF region of the Y chromosome were excluded. Normal controls had a normal sperm count and no history of infertility. Semen was analyzed according to the 1999 World Health Organization criteria.
DNA extraction
Genomic DNA was extracted from peripheral blood samples using the QuickGene DNA blood kit (Fujifilm, Japan) according to the manufacturer’s instructions. DNA yield was quantified using a NanoDrop™ spectrophotometer (Thermo Scientific, Maryland, USA). Extracted DNA was stored at−80 °C until further analysis.
Sequence analysis of EGR4
The coding regions of EGR4 were screened by PCR and direct sequencing. PCR primers for two exons and their intron boundaries were designed using Primer 3 (http://bioinfo.ut.ee/primer3-0.4.0/). The locations and sequences of primer sets are presented in Fig. 1 and Table 1. Because of their large size, the exons were divided: exon 1 was divided into two and exon 2 was divided into six overlapping fragments. The GC-rich PCR system (Roche Diagnostics, Mannheim, Germany) was used for exon 1, and the Hotstart Taq PCR premix (Bioneer, Daejeon, Republic of Korea) was used for exon 2. Thermal cycling conditions were as follows: initial denaturation at 94 °C for 5 min, 30–35 cycles for 30 s at 94 °C, 30 s at 60 °C, and 30 s at 72 °C, with a final extension at 72 °C for 10 min. PCR products were loaded on a 2% agarose gel and then purified with ExoSAP-IT (USB Corporation, Cleveland, OH). The sequencing reaction was performed using BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Austin, TX) according to the manufacturer’s instructions. After the sequencing reaction, 55 μl of BigDye® X-Terminator™ (Applied Biosystems, Bedford, MA) solution was added directly to the sequencing reaction plate well and vortexed for 30 min at 1800 rpm. After vortexing, the reaction plate was briefly centrifuged, and the supernatant was loaded onto an ABI 3130XL Genetic Analyzer using the BigDye® X-Terminator run module. All sequence reactions were performed in forward and reverse directions to eliminate error.
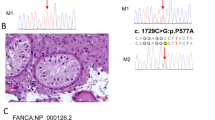
Identification of variants in the EGR4 gene. a Schematic representation of the EGR4 locus containing the two exons that were sequenced. Coding regions are indicated by a black box. Four nonsynonymous variations (c.65_66InsG, c.214C > A, c.236C > T, and c.1294G > T) identified in Korean patients with oligo/azoospermia are illustrated. b Electropherograms showing the variant sequences. Variants identified in the azoo-/oligozoospermia group (c.65_66InsG, c.214C > A, c.236C > T, and c.1294G > T) are shown in the upper panel. Wild-type sequences from the control group are shown in the lower panel. Red stars indicate the variant locations
Statistical analysis and database search
For each sequence variation, data were statistically analyzed using Statistical Package for Social Sciences (SPSS) version 22 software (Chicago, IL, USA). To evaluate the association between patient and control groups, the odds ratio (OR), 95% confidence interval (CI) and applied p values were calculated using the chi-square test and Fisher’s exact test (two-tailed). An applied p value of less than 0.05 was considered statistically significant. SIFT [12, 13], PolyPhen-2 [14, 15], Mutation Taster [16, 17], fathmm [18], Mutation assessor [19], SNPs &GO [20, 21] and SNAP2 [22] databases were used to predict potentially damaging effects of the identified sequence variations.
Results
We identified eight sequence variations in the coding region and 3′-UTR of the EGR4 gene in our Korean population. The locations, types, and allele frequencies of the variations are presented in Fig. 1 and Table 2. Four were nonsynonymous variants (rs771189047, rs561568849, rs763487015, and rs546250227), three were synonymous variants (rs115948271, rs528939702, and rs7558708), and one (rs2229294) was localized in the 3′-UTR. Three nonsynonymous variants [c.65_66InsG (p. Cys23Leufs*37), c.236C > T (p. Pro79Leu), c.1294G > T (p. Val432Leu)] and one synonymous variant in exon 2 (c.1230G > A) were detected only in patients (Fig. 1). The c.214C > A (p. Arg72Ser) and c.867C > G (p. Leu289) variants were identified in both patients and controls. The genotype frequencies of EGR4 c.65_66InsG, c.214C > A, c.236C > T, c.867C > G, c.1230G > A, c.1294G > T, and c.1488C > T variants were not significantly different between the patient and the control groups (Table 3). We evaluated the pathogenic effects of the nonsynonymous variants using 7 programs by PolyPhen-2, SIFT, Mutation Taster, fathmm, Mutation assessor, SNPs &GO and SNAP2 (Additional file 1: Table S1). The c.214C > A (p. Arg72Ser) and c.236C > T Pro79Leu) variants were predicted as “damaging” by SIFT and SNAP2. The c.65_66insG (p. Cys23Leufs*37) variants were predicted as “disease causing” by Mutation Taster, SNPs &GO and SNAP2. The c.867C > G (p. Leu289) variants were predicted as “disease causing” by only Mutation Taster.
Discussion
In this study, we identified sequence variations in the EGR4 gene of patients with idiopathic non-obstructive spermatogenetic impairment. Spermatogenesis is followed by the differentiation of primordial germ cells into motile spermatozoa. This process is controlled by numerous factors [23, 24], and disruption of these factors may affect the quality and quantity of spermatozoa production and fertility in males. Many genes have been associated with spermatogenesis, but the pathophysiological mechanisms of these genes have not been elucidated. EGR4 is expressed in germ cells and has a crucial role in spermatogenesis by regulating germ cell maturation during pachytene. In addition, the functional loss of EGR4 blocks spermatogenesis, thereby reducing spermatozoa production [8].
We identified eight variants in the EGR4 gene of Korean males with impaired spermatogenesis. However, these variants were all listed in the NCBI Single Nucleotide Polymorphisms (SNP) database [25], and the allele frequencies of the variants alleles were not significantly different between patients and controls. Therefore, the significance of these variations in spermatogenesis is not clear. The allele frequency of each variant was very low (less than 0.01), and variants not found in the controls were only identified in one patient. We were also unable to elucidate the biochemical and physiological significance of each variant. Instead, we evaluated the pathological significance of the variants using 7 computational prediction algorithms: PolyPhen-2, SIFT, Mutation Taster, fathmm, Mutation assessor, SNPs &GO and SNAP2.
Four variants were not identified in the controls, and three were nonsynonymous. The c.65_66InsG (p. Cys23Leufs*37) variant was detected in only one patient with oligozoospermia. This insertion shifted the reading frame and generated a premature stop codon. It was predicted as “damaging” by the Mutation Taster and SNAP2 database. Haploinsufficiency is a well-known mechanism of many genetic diseases [26,27,28]. In null mouse models, hemizygotes (+/−) may show a normal phenotype because a deleted allele may not affect its phenotype. However, a gene product of a variant allele may interfere with a normal gene product. The allele frequency of the insertion was very rare and may have been responsible for the reduced sperm count in the patient.
The c.236C > T (p. Pro79Leu) variant was detected in only one patient. The variant was also identified by the 1000 genomes project with a MAF of 0.0003. Proline and leucine are nonpolar amino acids, so the mutability of this variant should be negligible. However, this type of substitution could affect protein function. Computational analyses predicted the variant as both “damaging” (SIFT) and “benign” (PolyPhen-2); therefore, its pathological significance is unclear. The nonsynonymous variant c.1294G > T (p. Val432Leu) was also only identified in one patient and had already been identified by the 1000 genomes project with a MAF of 0.0016. Valine and leucine are structurally similar; therefore, nonpolar amino acids of this variant should be insignificant. The synonymous c.1230G > A (p. Thr410) variant was also only found in one patient with impaired spermatogenesis and had also previously been identified by the 1000 genomes project with a MAF of 0.0008.
The remaining four variants, including one nonsynonymous variant, were identified in both patients and controls. The c.214C > A (p. Arg72Ser) variant had a MAF of 0.009, and the c.867C > G (p. Leu289) variant had a MAF of 0.023 in our Korean population. These frequencies were much higher than those reported in the NCBI SNP database. The c.867C > G (p. Leu289) variant was predicted to be “disease causing” by Mutation Taster. The substitution may affect a splice site [16, 17]. However, this variant occurred more frequently in controls and is considered to be a normal variation in the Korean population. Two of our identified variants (c.1488C > T and c2373 + 52C > T) are considered common variants; c.1488C > T has been reported in European (MAF: 0.0636) and African (MAF: 0.4554) populations and was also identified by the 1000 genomes project (MAF: 0.2845). Here, we found that the MAF for this variant in a Korean population is similar to that previously reported in an African population. The c.2373 + 52C > T variant was located in the 3′-UTR and was tightly linked to the c.1448C > T variant in our Korean population; the C allele of the c1448C > T variant was coupled to the C allele of the c.2373 + 52C > T variant. The haplotype frequencies did not differ between the two groups.
SNPs and other structural variants have been associated with impaired spermatogenesis in different populations, but the same variants have not yet been reported in more than one population [29,30,31,32]. Chuncheng et al. performed the largest genetic association study in patients with non-obstructive azoospermia and identified SNPs associated with five potentially related genes. They suggested that these SNPs act as cofactors rather than directly affecting spermatogenesis because they were also present at a lower frequency in fertile men. These variants may cause mild impairment of spermatogenesis, but this could be worsened in the presence of other cofactors [33]. Tourtellotte et al. suggested that the EGR4 gene can compensate for the function of EGR1 in regulating LH during steroidogenesis [34]. This supports the idea that EGR1 has a dominant role in maintaining male fertility, while EGR4 can compensate for the functional loss of EGR1 in germ cells.
Conclusions
Eight variations were detected in the EGR4 gene of Korean men with idiopathic spermatogenetic impairment. To the best of our knowledge, this is the first screening of the EGR4 gene in relation to male infertility. Our findings did not fully elucidate how the identified variants affect spermatogenesis.
Our results found no difference in mutation frequency between cases and controls, and there is no evidence that heterozygous EGR4 variations are associated with infertility in humans.
Nevertheless, further studies are required to validate whether these variants affect EGR4 gene function and increase the risk of male infertility associated with other genetic changes, such as EGR1 mutations.
References
Gómez-Martín D, Díaz-Zamudio M, Galindo-Campos M, Alcocer-Varela J. Early growth response transcription factors and the modulation of immune response: implications towards autoimmunity. Autoimmun Rev. 2010;9:454–8.
O’Donovan KJ, Tourtellotte WG, Millbrandt J, Baraban JM. The EGR family of transcription regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci. 1999;22:167–73.
Crosby SD, Veile RA, Donis-Keller H, Baraban JM, Bhat RV, Simburger KS, et al. Neural specific expression, genomic structure, and chromosomal localization of the gene encoding the zincfinger transcription factor NGFI-C. Proc Natl Acad Sci U S A. 1992;89:6663.
Christy B, Nathans D. DNA binding site of the growth factor-inducible protein Zif268. Proc Natl Acad Sci U S A. 1989;86:8737–41.
Nardelli J, Gibson TJ, Vesque C, Charnay P. Base sequence discrimination by zinc-finger DNA binding domains. Nature. 1991;349:175–8.
Lee SL, Sadovsky Y, Swirnoff AH, Polish JA, Goda P, Gavrilina G, et al. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science. 1996;273:1219–21.
Shiga K, Noto Y, Mizuta I, Hashiguchi A, Takashima H, Nakagawa M. J Peripher A novel EGR2 mutation within a family with a mild demyelinating form of Charcot-Marie-Tooth disease. J Peripher Nerv Syst. 2012;17:206–9.
Tourtellotte WG, Nagarajan R, Auyeung A, Mueller C, Milbrandt J. Infertility associated with incomplete spermatogenic arrest and oligozoospermia in Egr4-deficient mice. Development. 1999;126:5061–71.
Hogarth CA, Mitchell D, Small C, Griswold M. EGR4 displays both a cell- and intracellular-specific localization pattern in the developing murine testis. Dev Dyn. 2010;239:3106–14.
Matsuo T, Le Dat T, Komatsu M, Yoshimaru T, Daizumoto K, Sone S, et al. Early growth response 4 is involved in cell proliferation of small cell lung cancer through transcriptional activation of its downstream genes. PLoS ONE. 2014;9(11), e113606.
Hadziselimovic F, Hadziselimovic NO, Demougin P, Krey G, Hoecht B, Oakeley EJ. EGR4 is a master gene responsible for fertility in cryptorchidism. Sex Dev. 2009;3:253–63.
Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–81.
SIFT nonsynonymous single nucleotide variants [database on the Internet]. Available from: http://siftdna.org/www/Extended_SIFT_chr_coords_submit.html. Accessed 26 Nov 2015
PolyPhen-2 prediction of functional effects of human nsSNPs [database on the Internet]. Available from: http://genetics.bwh.harvard.edu/pph2/. Accessed 15 Feb 2012
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9.
Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11:361–2.
MutationTaster [database on the Internet]. Available from: http://www.mutationtaster.org/. Accessed 31 July 2014.
Shihab HA, Julian G, Cooper DN, Stenson PD, Barker GLA, Edwards KJ, Day INM, Gaunt TR. Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Hum Mutat. 2013;34:57–65.
Reva B, Antipin Y, Sander C. Determinants of protein function revealed by combinatorial entropy optimization. Genome Biol. 2007;8(11):R232.
Remo C, Emidio C, Piero F, Pier Luigi M, Rita C. Functional annotations improve the predictive score of human disease-related mutations in proteins. Hum Mutat. 2009;30:1237–44.
Emidio C, Remo C, Piero F, Pier Luigi M, Altman RB, Rita C. WS-SNPs&GO: a web server for predicting the deleterious effect of human protein variants using functional annotation. BMC Genomics. 2013;14(3):S6.
Bromberg Y, Rost B. SNAP: predict effect of non-synonymous polymorphisms on function. Nucleic Acids Res. 2007;35:3823–35.
Matzuk MM, Burns KH. Genetics of mammalian reproduction: modeling the end of the germline. Annu Rev Physiol. 2012;74:503–28.
Ge SQ, Grifin J, Liu LH, Aston KI, Simon L, Jenkins TG, Emery BR, Carrell DT. Associations of single nucleotide polymorphisms in the Pygo2 coding sequence with idiopathic oligospermia and azoospermia. Genet Mol Res. 2015;14:9053–61.
dbSNP Short Genetic Variations [database on the Internet] 2016. Available from: https://www.ncbi.nlm.nih.gov/projects/SNP/. Accessed 2 Mar 2016.
Choi Y, Jeon S, Choi M, Lee MH, Park M, Lee DR, Jun KY, Kwon Y, Lee OH, Song SH, Kim JY, Lee KA, Yoon TK, Rajkovic A, Shim SH. Mutations in SOHLH1gene associate with nonobstructive azoospermia. Hum Mutat. 2010;31(7):788–93.
Tewes AC, Ledig S, Tüttelmann F, Kliesch S, Wieacker P. DMRT1 mutations are rarely associated with male infertility. Fertil Steril. 2014;102(3):816–20.
Takasaki N, Tachibana K, Ogasawara S, Matsuzaki H, Hagiuda J, Ishikawa H, Mochida K, Inoue K, Ogonuki N, Ogura A, Noce T, Ito C, Toshimori K, Narimatsu H. A heterozygous mutation of GALNTL5 affects male infertility with impairment of sperm motility. Proc Natl Acad Sci U S A. 2014;111(3):1120–5.
Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197–213.
Okada H, Tajima A, Shichiri K, Tanaka A, Tanaka K, Inoue I. Genome-wide expression of azoospermia testes demonstrates a specific profile and implicates ART3 in genetic susceptibility. PLoS Genet. 2008;4, e26.
Aston KI, Krausz C, Laface I, Ruiz-Castane E, Carrell DT. Evaluation of 172 candidate polymorphisms for association with oligozoospermia or azoospermia in a large cohort of men of European descent. Hum Reprod. 2010;25:1383–97.
Navarro-Costa P, Goncalves J, Plancha CE. The AZFc region of the Y chromosome: at the crossroads between genetic diversity and male infertility. Hum Reprod Update. 2010;16:525–42.
Chuncheng Lu XM, Wang R, Qin Y, Wang Y, Wu W, et al. Pathogenic variants screening in five non-obstructive azoospermia-associated genes. Mol Hum Reprod. 2014;20:178–83.
Tourtellotte WG, Nagarajan R, Bartke A, Milbrandt J. Functional compensation by Egr4 in Egr1-dependent luteinizing hormone regulation and Leydig cell steroidogenesis. Mol Cell Biol. 2000;20:5261–8.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: H14C0106020014).
Availability of data and materials
All relevant data are available within the manuscript.
Authors’ contributions
Funding for this study was obtained by SHS. SHS and SRS drafted the manuscript, and SHS, SRS, SHS and KMK designed the experiments. SRS, KMK, YJS, and YJN carried out the molecular genetics studies, JEP participated in the analysis, and SHS, SRS, DHC, JTS and TKY prepared the publication. All authors read and approved the final manuscript.
Competing interests
All authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All patients recruited from the CHA Gangnam Medical Center at CHA University between January 2010 and December 2012 provided written informed consent. The study was approved by the Institutional Review Board of CHA Gangnam Medical Center, CHA University.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Table S1.
Summary of variants identified in the EGR4 gene. (DOCX 16 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sung, S.R., Song, S.H., Kang, K.M. et al. Sequence variations of the EGR4 gene in Korean men with spermatogenesis impairment. BMC Med Genet 18, 47 (2017). https://doi.org/10.1186/s12881-017-0408-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12881-017-0408-5