Abstract
Background
Delayed antifungal therapy for candidemia leads to increased mortality. Differentiating bacterial infection from candidemia in systemic inflammatory response syndrome (SIRS) patients is complex and difficult. The Delta Neutrophil Index (DNI) has recently been considered a new factor to distinguish infections from non-infections and predict the severity of sepsis. We aimed to assess if the DNI can predict and provide a prognosis for candidemia in SIRS patients.
Methods
A matched case-control study was conducted from July 2016 to June 2017 at Kangdong Sacred Heart Hospital. Among patients with a comorbidity of SIRS, those with candidemia were classified as the case group, whereas those with negative blood culture results were classified as the control group. The matching conditions included age, blood culture date, and SIRS onset location. Multivariate logistic regression was performed to evaluate DNI as a predictive and prognostic factor for candidemia.
Results
The 140 included patients were assigned to each group in a 1:1 ratio. The DNI_D1 values measured on the blood culture date were higher in the case group than in the control group (p < 0.001). The results of multivariate analyses confirmed DNI_D1 (odds ratio [ORs] 2.138, 95% confidential interval [CI] 1.421–3.217, p < 0.001) and Candida colonization as predictive factors for candidemia. The cutoff value of DNI for predicting candidemia was 2.75%. The area under the curve for the DNI value was 0.804 (95% CI, 0.719–0.890, p < 0.001), with a sensitivity and specificity of 72.9 and 78.6%, respectively. Analysis of 14-day mortality in patients with candidemia showed significantly higher DNI_D1 and DNI_48 in the non-survivor group than in the survivor group.
Conclusions
DNI was identified as a predictive factor for candidemia in patients with SIRS and a prognostic factor in predicting 14-day mortality in candidemia patients. DNI, along with clinical patient characteristics, was useful in determining the occurrence of candidemia in patients with SIRS.
Similar content being viewed by others
Background
The number of candidemia cases has gradually been increasing due to the development of immunosuppressive treatments and invasive procedures. However, despite advances in medical science, the mortality rate of candidemia remains high, at 35–60% [1]. The primary factor in reducing candidemia-induced mortality rates is the early administration of appropriate antifungal therapy (AAT) [2]. Delayed AAT results in increased mortality. Thus, various markers and testing methods have been developed to predict candidemia to enable early-stage antifungal therapy [3,4,5]. However, there are cases in which these testing methods cannot be easily applied in actual treatment or are difficult to be commonly used.
Moreover, studies reported varying degrees of sensitivity for the developed clinical scores or testing methods [3, 6]. Several recent studies proposed the delta neutrophil index (DNI) as a promising predictive and prognostic marker for sepsis [7,8,9]. Thus, we evaluated DNI as a predictive marker of candidemia in patients with systemic inflammatory response syndrome (SIRS) and as a prognostic marker for patients with candidemia.
Methods
Study setting and patients
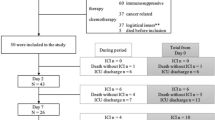
This study was performed at Kangdong Sacred Heart Hospital, a university-affiliated hospital with 640 beds, including 40 beds in three intensive care units (ICUs), located in Seoul, South Korea. The Institutional Review Board of Kangdong Sacred Heart Hospital approved this study (2019–04-003). Informed consent was not required by the board because of the retrospective design of the study. Between July 2016 and June 2018, we performed a retrospective review of patient medical records and microbiology laboratory databases. The case group included patients aged 20 years or older who developed candidemia during the study period. In cases of duplicate candidemia, only the initial episode was enrolled in the study. The matched control group included patients aged 20 years or older displaying SIRS during the same period with negative blood culture results. Case and control patients were matched at a 1:1 ratio using the following matching criteria: 1) blood culture procedure dates within ±3 days, 2) age ± 3 years, and 3) same patient location at SIRS onset (ICU vs. general ward). Patients with hematologic malignancies except for lymphoma, neutropenia, and those who received granulocyte colony-stimulating factor were excluded from this study because these conditions may affect hematologic parameters.
DNI measurement and other laboratory methods
The blood samples for DNI measurement were transferred to the laboratory department in ethylenediaminetetraacetic acid (EDTA) tubes. The DNI was determined within 1 h of blood sampling.
The DNI is included as part of the routine complete blood count (CBS) test at our hospital. The DNI was calculated using an automatic cell analyzer (ADVIA 2120 Hematology System, Siemens Healthcare Diagnostics, Forchheim, Germany) [10]. This cell analyzer counts white blood cells (WBCs) in independent myeloperoxidase (MPO) and nuclear lobularity channels. The formula for calculating DNI is as follows: DNI (%) = (neutrophil and eosinophil subfractions measured in the MPO channel by a cytochemical MPO reaction)-(polymorphonuclear neutrophil [PMN] subfraction measured in the nuclear lobularity channel by a reflected light beam). Blood cultures were ordered at the discretion of the primary physician based on the presence of the signs and symptoms of SIRS. Each set of blood samples was inoculated into one aerobic and anaerobic bottle and immediately loaded into a BacT/ALERT 3D Microbial Detection System (bioMerieux, Inc., Durham, NC, USA). Candida species identification was done using the automated Vitek 2 Yeast Biochemical Card (bioMerieux, Inc.).
Study variables and definitions
Clinical data extracted from the patients’ medical records and entered into a database included leukocyte counts, C-reactive protein (CRP) level, procalcitonin level, Candida score, site of infection, comorbidities, epidemiological setting at the time of blood culture, severity of illness, presence of severe sepsis or septic shock, and 14-day mortality. The severity of infection was assessed using the Pitt bacteremia score at the time of blood culture [11]. The severity of comorbid conditions was assessed using McCabe classification [12].
Candidemia was defined as a minimum of one candida-positive blood culture in patients with SIRS. The time of candidemia onset was defined as the time of sampling for the first positive blood culture. The time to positivity (TTP) was determined as the time interval between the start of incubation and the detection of yeast in blood, as documented using an automated monitoring system. The time to antifungal therapy (TAT) was described as the hours elapsed between the first blood culture sample obtained and antifungal agent administration. The candidemia clearance period was defined as the duration from the first candidemia-positive blood culture to negative conversion.
Candida colonization was considered unifocal when Candida species were isolated from one focus and multifocal when Candida species were isolated simultaneously from various non-contiguous foci. The Candida score (CS) for a cut-off value of 3 was as follows: total parenteral nutrition × 1, plus surgery × 1, plus multifocal Candida colonization × 1, plus severe sepsis × 2 [3].
SIRS was defined based on the presence of two or more of the following conditions: body temperature > 38 °C or < 36 °C; heart rate > 90 beats/min; respiratory rate > 20 breaths/min or PaCo2 < 32 mmHg; and WBC > 1200 cells/mm, < 4000 cells/mm, or > 10% immature forms. Severe sepsis was defined as one or more clinical signs of organ dysfunction. Severe sepsis, septic shock, and infection type were defined according to standard criteria [13, 14]. Day 1 (D1) was defined as the first day of blood culture collection, with initial blood cultures collected within 24 h of SIRS onset in study patients. DNI_D1 and DNI_48 were defined as the DNI measured on the initial blood culture date and 2–3 days later, respectively. The site of infection was determined by physicians based on clinical evaluation. Steroid use was defined as daily use of at least 20 mg of prednisone for at least 2 weeks. Steroid administration in patients with SIRS was defined as the administration of steroids for more than 2 weeks within 3 weeks of showing signs and symptoms of SIRS. Patients with immunosuppression included those who were on immunosuppressive therapy (chemotherapeutic agents, immunosuppressive agents, or radiation therapy) within 2 weeks of showing SIRS.
Statistical analysis
Normally distributed continuous variables are reported as means ± standard deviation (SD) and compared using Student’s t-tests. Non-normally distributed continuous variables are reported as medians with interquartile ranges (IQRs) and compared using Mann–Whitney U-tests. Categorical variables are reported as percentages and compared using chi-squared or Fisher’s exact tests, as appropriate. To measure the sensitivity and specificity of DNI values at different cutoffs, a conventional receiver operating characteristic (ROC) curve was generated and the area under the curve (AUC) was calculated to quantify the accuracy of DNI values as predictor and prognostic markers for candidemia. The cutoff values were selected to maximize the sensitivity and specificity of the DNI values. An AUC of 0.5 was considered to be no better than expected by chance, whereas a value of 1.0 signified a perfect marker. Spearman’s method was used to analyze the correlations between the time of antifungal therapy and 14-day mortality and between other factors.
Univariate and multivariate multiple logistic regression analyses were conducted to assess predictors of candidemia and prognostic factors for 14-day mortality. Variables with p < 0.05 in the univariate analyses and clinically significant factors were included in the multivariate analysis. Odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated. All reported p-values were two-tailed, and p < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 24.0 (IMB Corp., Armonk, NY, USA).
Results
Patient characteristics
The baseline clinical characteristics of the 140 patients included in the study are summarized in Table 1. The mean patient age was 65.98 ± 14.63 years and no significant difference in age was observed between the two groups. Following matching, there was no significant difference in patient locations at the time of SIRS occurrence between the case and control groups. At SIRS onset in both groups, 27 (38.6%) and 43 (61.4%) patients were in the ICU and general ward, respectively.
Among all patients included in this study, the most common underlying disease was solid cancer, followed by neurological disorders and diabetes. Assessment of co-morbidity severity using the McCabe classification showed a greater number of rapidly fatal cases in the case group than in the control group (5.7% vs. 0%, p = 0.007). There was also a clear difference in the cause of infection between the two groups (Table 1). The most common cause of candidemia in the case group was catheter-related infection (CRI), which accounted for 48 of the 70 cases (68.6%). CRIs were followed by primary bacteremia (28.6%, 20/70 cases). In contrast, the major cause of SIRS in the control group was pneumonia, which accounted for 42.9% (31/70) of the cases. However, the severity of infection assessed by the Pitt bacteremia score, severe sepsis, or septic shock did not differ significantly between the two groups (Table 1). To measure the CS, the presence of Candida colonization, total parenteral nutrition (TPN), and receipt of surgery were examined. The proportion of patients with Candida colonization and the proportion of patients who underwent surgery while hospitalized were significantly higher in the case group than in the control group (Table 1). All patients were administered antibiotics at the time of participation in this study. The overall 14-day mortality was 10.7% (15/140) and was significantly higher in the case group than in the control group (18.6% vs. 2.9%, p = 0.005).
Comparison of DNI and other indicators as predictive markers of candidemia
To evaluate DNI as a predictive factor for candidemia, DNI, CRP level, procalcitonin level, leukocyte count, and CS > 3 were compared between the case and control groups (Table 2). The DNI_D1 in the case group was significantly higher than that in the control group (3.5%, [0.5–3.3] vs. 1.3% [0.1–2.4], p < 0.001). The DNI_48 value was also significantly higher in the case group (2.0%) than in the control group (1.0%) (p < 0.001). The procalcitonin level was also higher in the case group than in the control group (Table 2). However, CS > 3, a known predictive factor for candidemia, did not differ significantly between the two groups.
As our control group included five non-infectious SIRS patients, we also compared the DNI values between patients with clinically documented infections and non-infectious SIRS in the control group. The DNI_D1 values were 1.3% (0.2–2.4) in patients with clinically documented infection and 0.0% (0.0–1.0) in those with non-infectious SIRS. While the patients with clinically documented infection tended to show higher DNI_D1 value, the difference was not statistically significant (p = 0.304). A similar trend was observed for DNI_48 values (1.0% vs. 0.2%, p = 0.178). Comparison of the case and control groups after excluding patients with non-infectious SIRS showed that the clinical characteristics did not differ from those of the entire control group. The DNI_D1 value in the case group (3.5%, 0.5–3.3) was significantly higher than that in the control group after excluding patients with non-infectious SIRS (1.3%, 0.2–2.4) (p < 0.001). The DNI_48 value was also significantly higher in the case group (2.0%) than in the control group after excluding patients with non-infectious SIRS (1.0%) (p = 0.010).
The results of multivariate analyses to determine the independent predictive factors for candidemia in the case and control groups are summarized in Table 3. The factors that differed significantly in univariate analysis, CS > 3 used for predicting candidemia, and clinically important factors exhibiting insignificant differences in univariate analysis were included in the multivariate analysis.
The multivariate analyses identified DNI_D1 (OR, 2.183, 95% CI, 1.421–3.217, p < 0.001) and Candida colonization (OR, 7.361, 95% CI, 1.717–31.553, p = 0.007) as useful indices for predicting candidemia (Table 3). Multivariate analyses of the control group, including only patients with clinically documented infection, also identified DNI_D1 (OR, 2.139, 95% CI, 1.424–3.213, p < 0.001) and Candida colonization (OR, 7.638, 95% CI, 1.732–33.676, p = 0.007) as useful indicators for predicting candidemia.
Optimal cutoff value of DNI for predicting candidemia
ROC curve and AUC analyses were conducted to determine the DNI_D1 cutoff value for predicting candidemia (Fig. 1). The optimal cutoff value was 2.75% and the AUC of DNI_D1 was 0.804 (95% CI, 0.719–0.890, p < 0.001). The sensitivity and specificity in predicting candidemia for a cutoff DNI value of 2.75% were 72.9 and 78.6%, respectively, and the positive and negative predictive values were 77.3 and 74.3%, respectively. For DNI_D1 > 2.75%, the OR for the presence of candidemia was 9.842 (95% CI, 4.562–21.402, p < 0.001).
In comparison with the control group, including only patients with clinically documented infection, the cutoff value was 2.75%, and the AUC of DNI_D1 was 0.796 (95% CI, 0.721–0.871, p < 0.001). The sensitivity, specificity, positive predictive, and negative predictive value were 72.9, 76.9, 77.3, and 72.5%, respectively. The OR for the predicting candidemia was 8.947 (95% CI, 4.096–19.544, p < 0.001).
Factors associated with 14-day mortality in patients with candidemia
To determine the prognostic factors for mortality in patients with candidemia, various factors were comparatively analyzed according to 14-day mortality. Among 70 patients with candidemia, 13 died within 14 days of candidemia onset. There were no differences in age, sex, or patient location at the time of candidemia onset (p = 0.210) between the survivor and non-survivor groups. The underlying diseases and site of infection also did not differ significantly between the survivor and non-survivor groups. The non-survivor group showed a significantly higher Pitt bacteremia score (4.0 [1.0–5.0] vs. 0.0 [0.0–2.0], p < 0.001) and septic shock rate (61.5% vs. 7.5%, p = 0.003) than those in the survivor group. The percentage of patients with CS ≥3 was higher in the non-survivor group than in the survivor group (46.2% vs. 19.3%, p = 0.042). DNI_D1 and DNI_48 values were also significantly higher in the non-survivor group than in the survivor group (7.4% [4.0–22.0] and 6.1% [2.1–14.4], respectively. The TTP in all patients with candidemia was 48 h (32.0–73.0, range 10–120 h). Among patients with candidemia, the most common Candida species was C. albicans (33/70, 47.1%) followed by C. tropicalis (16/70, 22.9%), C. parasilopsis (10/70, 14.3%), and C. glabrata (9/70, 12.7%). The TTPs for the four most frequently identified Candida species were 68 h (47.0–78.0), 30.5 h (26.5–33.5), 47.0 h (34.0–58.0), and 72.0 h (50.5–93.0), respectively.
The TTP in the non-survivor group was shorter than that in the survivor group, but the difference was not statistically significant (31.0 h [26.5–53.0] vs. 48.0 h [34.0–72.0], p = 0.066).
The non-survivor group showed a significantly shorter TAT than did the survivor group (36 h [12.5–42.0] vs. 60 h [42.0–96.0] p = 0.013), showing that antifungal therapies were administered earlier to the non-survivor group. As shown in Table 4, no significant difference was observed in TAT between the survivor and non-survivor groups in the multivariate analysis. However, antifungal therapy tended to be administered faster, with earlier Candida detection from blood cultures (r = 0.56 p = 0.044). In addition, TAT and 14-day mortality showed an inverse correlation (r = − 0.33, p = 0.011).
The duration until the negative conversion of candidemia also did not differ between the survivor and non-survivor groups (Table 4).
Multivariate analysis also confirmed that DNI_D1 (OR, 1.156, 95% CI, 1.039–1.287, p = 0.008) and the occurrence of septic shock were reliable prognosis factors for 14-day mortality. The DNI_D1 cutoff value for predicting 14-day mortality was 3.95%, and the AUC was 0.769 (95% CI, 0.624–0.914, p = 0.001).
Discussion
The results of this study confirmed the DNI to be a predictive marker of candidemia in patients with SIRS and a prognostic marker for patients with candidemia.
Candidemia is associated with one of the highest rates of mortality of any bloodstream infection (BSI) [15]. To reduce mortality due to candidemia, AAT needs to be administered as early as possible. The current initiation of antifungal therapy upon identification of the yeast from the blood culture results in delayed treatment. However, avoiding delays in the treatment of patients with candidemia is difficult. As the risk factors currently known to be associated with Candidemia are also related to drug-resistant bacterial infection, they do not help differentiate candidemia from a bacterial infection in patients manifesting SIRS [16]. Various scoring systems and markers have been developed to distinguish candidemia, such as the CS or Candida colonization index [3,4,5]. However, their sensitivity for differentiating invasive Candida infection and colonization is only around 60% [6]. As markers such as Β-D-glucan are generally not used by all hospitals, they have limits in their application. The administration of empirical antifungal therapy can be considered in patients suspected of sepsis but can cause problems like inappropriate administration of antifungal therapy and increased resistance.
The DNI is a novel index reflecting a circulating fraction of immature granulocytes (IGs) [17, 18]. Previous studies identified the DNI as a factor that distinguishes infection from non-infection and a prognostic factor for severity in patients with sepsis [19,20,21,22]. Although studies on the differentiation between bacterial infection and non-infectious condition have been reported, there are no studies on invasive Candida infection, candidemia prediction, or candidemia prognosis assessment. The DNI can be calculated automatically while measuring CBC. The CBC is routinely and frequently evaluated in patients with SIRS at a substantially lower cost than other laboratory markers. The DNI can be easily calculated and reported without an additional cost. Using samples collected from patients at SIRS onset in our study of 70 patients with candidemia and 70 patients without candidemia, a DNI_D1 > 2.75% had a sensitivity of 72.9% and a specificity of 78.6% for predicting candidemia. In SIRS patients, Candida colonization was also identified as a predictor of candidemia, along with the DNI_D1. The cause of infection was mostly CRI or primary bacteremia in patients with candidemia. Among patients with suspected SIRS and sepsis, empirical antifungal therapy can be restrictively considered for patients with Candida colonization and potential CRI or primary bacteremia due to the absence of clear infection site based on a DNI cutoff of 2.75%. In this study, the median Candida detection time was 48 h (32–73). The preemptive administration of antifungal therapy only to selected patients may permit economical early administration of antifungal therapy compared to the administration of empirical antifungal agents to all patients. Based on the results of previous studies reporting that short-term fluconazole administration was not associated with increased fluconazole resistance in patients with candidemia [23, 24], preemptive antifungal therapy can be administered to patients with SIRS, Candida colonization, ambiguous primary infection sites, and increased DNI values. Alternatively, in patients with SIRS with DNI < 2.75% and clinically clear sites of infection, we may consider discontinuing empirical antifungal therapy. Using DNI in this way contributes to antifungal stewardship in empirical antifungal therapy.
DNI_D1 and DNI_48 were identified as independent, predicting factors for 14-day mortality in patients with candidemia. Many patients in the non-survivor group also had septic shock. Although it is generally known that mortality can be reduced if antifungal therapy is administered earlier, the results of this study demonstrated earlier administration of antifungal therapy in the non-survivor group compared to that in the survivor group (36 h [12.5–42.0] vs. 60 h [42.0–96.0]). TTP was also administered earlier in the non-survivor group than in the survivor group. As previously reported, the TTP of blood culture is associated with the microbial burden in the blood [25, 26].. In other words, a shorter TTP indicates a higher microbial burden. The shorter TTP (36 h vs. 60 h, p = 0.066) in the non-survivor group suggested a higher Candida burden in the non-survivor group, thus leading to severe infection. Also, higher DNI values in the non-survivor group suggested that the DNI reflected the severity of candidemia. As in the case of bacterial infection, increased infection severity leads to increased DNI values [8, 19, 21].
To date, antifungal therapy for candidemia generally begins when yeast is detected in the blood culture. This explains why antifungal therapy began earlier in the non-survivor group in this study. In the non-survivor group, the median TAT and TTP were 36 and 31 h respectively, suggesting antifungal therapy was initiated after the detection of yeast from the blood culture. Earlier administration of antifungal therapy based on the DNI may increase the survival rate of candidemia patients, especially those with severe sepsis or septic shock.
This study has several limitations. First, this study was performed at a single center and the results were analyzed retrospectively. Thus, despite matching, selection bias is possible. Second, DNI elevation is not specific to infection and may be observed in various other conditions, including hematologic malignancy, acute hemorrhage, and chronic inflammatory diseases [18]. Third, this study classified patients with candidemia into the case group and patients without bacteremia into the control group. We cannot exclude the possibility that these selection criteria might contribute to differences in DNI values between the two groups. For these reasons, further prospective analyses with larger populations are needed to confirm the DNI as a prediction and prognostic marker of candidemia.
Conclusions
The results of this study confirmed the DNI as a predictive and prognosis factor for candidemia in patients with SIRS. With recent advancements in testing tools, the DNI can be obtained easily and quickly. Although prospective studies with larger numbers of patients are needed, the ability to use DNI values and clinical characteristics (Candida colonization or site of infection) to screen patients at high risk for candidemia in real clinical settings may contribute to a decrease in candidemia mortality.
Availability of data and materials
The datasets used and analyzed during the current study are publicly available from the corresponding author on reasonable request and permission of the Institutional Review Board of Kangdong Sacred Heart Hospital in Seoul, South Korea.
Abbreviations
- AAT:
-
Appropriate antifungal therapy
- DNI:
-
Delta neutrophil index
- SIRS:
-
Systemic inflammatory response syndrome
- ICU:
-
Intensive care units
- IRB:
-
Institutional Review Board
- CBC:
-
Complete blood count
- WBC:
-
White blood cell
- MPO:
-
Myeloperoxidase
- PMN:
-
Polymorphonuclear neutrophil
- CRP:
-
C-reactive protein
- TTP:
-
Time to positivity
- TAT:
-
Time to antifungal therapy
- CS:
-
Candida score
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- ORs:
-
Odds ratio
- CI:
-
Confidence interval
- IG:
-
Immature granulocyte
References
Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang CH, Webster KM. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48(12):1695–703.
Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43(1):25–31.
Leon C, Ruiz-Santana S, Saavedra P, Galvan B, Blanco A, Castro C, Balasini C, Utande-Vazquez A, Gonzalez de Molina FJ, Blasco-Navalproto MA, et al. Usefulness of the "Candida score" for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: a prospective multicenter study. Crit Care Med. 2009;37(5):1624–33.
Posteraro B, De Pascale G, Tumbarello M, Torelli R, Pennisi MA, Bello G, Maviglia R, Fadda G, Sanguinetti M, Antonelli M. Early diagnosis of candidemia in intensive care unit patients with sepsis: a prospective comparison of (1-->3)-beta-D-glucan assay, Candida score, and colonization index. Crit Care. 2011;15(5):R249.
Ruiz-Ruigomez M, Duenas C, Hernandez C, Vinuesa D, Coronado-Alvarez NM, Portillo-Tunon V, Cardozo C, Munoz-Medina L, Cabo-Magadan R, Luna JD, et al. Clinical predictors of candidemia in medical non-neutropenic, non-ICU patients. The CaMed score. Int J Clin Pract. 2018;72(12):e13275.
Leon C, Ruiz-Santana S, Saavedra P, Almirante B, Nolla-Salas J, Alvarez-Lerma F, Garnacho-Montero J, Leon MA. A bedside scoring system ("Candida score") for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med. 2006;34(3):730–7.
Cha YS, Lee KH, Lee JW, Kwon W, Lee SJ, Kang KS, Kim HI, Kim OH, Cha KC, Kim H, et al. The usefulness of the Delta neutrophil index for predicting superimposed pneumonia in patients with acute decompensated heart failure in the emergency department. PLoS One. 2016;11(9):e0163461.
Park BH, Kang YA, Park MS, Jung WJ, Lee SH, Lee SK, Kim SY, Kim SK, Chang J, Jung JY, et al. Delta neutrophil index as an early marker of disease severity in critically ill patients with sepsis. BMC Infect Dis. 2011;11:299.
Kim YC, Song JE, Kim EJ, Choi H, Jeong WY, Jung IY, Jeong SJ, Ku NS, Choi JY, Song YG, et al. A simple scoring system using the red blood cell distribution width, Delta neutrophil index, and platelet count to predict mortality in patients with severe Sepsis and septic shock. J Intensive Care Med. 2019;34(2):133–139.
Nahm CH, Choi JW, Lee J. Delta neutrophil index in automated immature granulocyte counts for assessing disease severity of patients with sepsis. Ann Clin Lab Sci. 2008;38(3):241–6.
Rhee JY, Kwon KT, Ki HK, Shin SY, Jung DS, Chung DR, Ha BC, Peck KR, Song JH. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: a comparison of the Pitt bacteremia score and the acute physiology and chronic health evaluation II scoring systems. Shock. 2009;31(2):146–50.
Reilly JS, Coignard B, Price L, Godwin J, Cairns S, Hopkins S, Lyytikainen O, Hansen S, Malcolm W, Hughes GJ. The reliability of the McCabe score as a marker of co-morbidity in healthcare-associated infection point prevalence studies. J Infect Prev. 2016;17(3):127–9.
Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–32.
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–55.
Harbarth S, Ferriere K, Hugonnet S, Ricou B, Suter P, Pittet D. Epidemiology and prognostic determinants of bloodstream infections in surgical intensive care. Arch Surg. 2002;137(12):1353–9 discussion 1359.
Eggimann P, Garbino J, Pittet D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis. 2003;3(11):685–702.
Seebach JD, Morant R, Ruegg R, Seifert B, Fehr J. The diagnostic value of the neutrophil left shift in predicting inflammatory and infectious disease. Am J Clin Pathol. 1997;107(5):582–91.
Cornbleet PJ. Clinical utility of the band count. Clin Lab Med. 2002;22(1):101–36.
Shin DH, Kim EJ, Kim SJ, Park JY, Oh J. Delta neutrophil index as a marker for differential diagnosis between acute graft pyelonephritis and acute graft rejection. PLoS One. 2015;10(8):e0135819.
Pyo JY, Kim DS, Jung SM, Song JJ, Park YB, Lee SW. Clinical significance of delta neutrophil index in the differential diagnosis between septic arthritis and acute gout attack within 24 hours after hospitalization. Medicine (Baltimore). 2017;96(30):e7431.
Seok Y, Choi JR, Kim J, Kim YK, Lee J, Song J, Kim SJ, Lee KA. Delta neutrophil index: a promising diagnostic and prognostic marker for sepsis. Shock. 2012;37(3):242–6.
Lee CH, Kim J, Park Y, Park YC, Kim Y, Yoon KJ, Uh Y, Lee KA. Delta neutrophil index discriminates true bacteremia from blood culture contamination. Clin Chim Acta. 2014;427:11–4.
Piarroux R, Grenouillet F, Balvay P, Tran V, Blasco G, Millon L, Boillot A. Assessment of preemptive treatment to prevent severe candidiasis in critically ill surgical patients. Crit Care Med. 2004;32(12):2443–9.
Lopez J, Pernot C, Aho S, Caillot D, Vagner O, Dalle F, Durnet-Archeray MJ, Chavanet P, Bonnin A. Decrease in Candida albicans strains with reduced susceptibility to fluconazole following changes in prescribing policies. J Hosp Infect. 2001;48(2):122–8.
Haimi-Cohen Y, Vellozzi EM, Rubin LG. Initial concentration of Staphylococcus epidermidis in simulated pediatric blood cultures correlates with time to positive results with the automated, continuously monitored BACTEC blood culture system. J Clin Microbiol. 2002;40(3):898–901.
Kim SH, Yoon YK, Kim MJ, Sohn JW. Clinical impact of time to positivity for Candida species on mortality in patients with candidaemia. J Antimicrob Chemother. 2013;68(12):2890–7.
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding
This work was supported by the Hallym University Research Fund 2019 (grant number HURF-2019-19). The funder had no role in the design of the study and collection, analysis, and interpretation of data, in writing the manuscript, and in the decision to submit the report for publication.
Author information
Authors and Affiliations
Contributions
SYP carried out data screening and statistical analysis and participated in the study design and manuscript writing. JSL participated in the study concept and design and data interpretation. JO carried out data screening and acquisition. JYP participated in data analysis and interpretation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was provided by the Institutional Review Board of Kangdong Sacred Heart Hospital (IRB No 2019–04-003). The requirement for informed consent was waived because this study was a retrospective medical chart review study with no personal information for each participant.
Consent for publication
Not applicable.
Competing interests
The authors have disclosed no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Park, S.Y., Lee, J.S., Oh, J. et al. Delta neutrophil index as a predictive and prognostic factor for Candidemia patients: a matched case-control study. BMC Infect Dis 20, 396 (2020). https://doi.org/10.1186/s12879-020-05117-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-020-05117-0