Abstract
Background
Praziquantel (PZQ) is the standard treatment for Schistosomiasis in sub-Saharan Africa. However, there is evidence suggesting praziquantel treatment failure in Schistosome infections with associated potential renal impairment. The objective of this study was to determine the effect of three monthly doses of 60 mg/kg/day PZQ on schistosome egg count, liver and renal function during the treatment of urinary schistosomiasis in Ghana.
Methods
A nested case-control study was designed from a cohort screened for schistosomiasis; 28 schistosomiasis positive cases by microscopy matched with 53 healthy controls by age and gender. The study population was urban dwellers from the Asokwa sub-metropolitan area, Kumasi in Ghana. Participants were within the age range of 6 to 30 years. We assessed Schistosoma haematobium egg counts in urine and its associated impact on liver and renal function at baseline, treatment and post-treatment phases using serum.
Results
Of the 28 cases and 53 controls, 78.6% and 81.1% were males respectively. Globulin levels before treatment was higher in cases [36.7 (32.8, 40.1) vrs 30.5 (22.4, 33.8), p = 0.005] at pre-treatment but not at post-treatment [35.8 (31.2, 39.1) vrs 37.4 (29.7, 43.0), p = 0.767]. Estimated cure rate was 42.9, 46.4 and 96.4% after first, second and third dose respectively. Schistosome egg counts dropped significantly (p = 0.001) from before second dose to post-treatment. Similarly, levels of alanine aminotransferase (p = 0.001), aspartate aminotransferase (p = 0.028) and gamma glutamyl transferase (p = 0.001) significantly declined towards post-treatment. Estimated glomerular filtration rate significantly improved from before second dose to post-treatment using both the Chronic Kidney Disease Epidemiology Program (p = 0.001) and 4-variable Modification of Diet in Renal Disease (p = 0.002) equations.
Conclusion
Treatment of urinary Schistosoma hematobium infections with a repeated high monthly dose of 60 mg/kg of praziquantel for 3 months is safe and effective.
Similar content being viewed by others
Background
Praziquantel (PZQ) is virtually the sole treatment regimen for Schistosomiasis in sub-Saharan Africa [1]. This oral schistosomicidal agent is constituted of a racemate mixture, with activity both in vivo and in vitro [2, 3]. Evidence from studies conducted on Schistosoma (S.) mansoni and S. japonicum although not very clear, indicate the mode of action of PZQ is the targeting of calcium channels and antigen exposure rendering the worm susceptible to elimination by antibodies [1, 4].
After oral administration, PZQ is rapidly absorbed, metabolized and excreted by the kidney. Metabolism of PZQ is primarily via the cytochrome P450 system leading to the production of toxic metabolic intermediates, which are potentially harmful to hepatocytes [5]. Plasma levels of PZQ are also reported to be reduced by inducers but elevated by inhibitors of cytochrome P450 activity [6, 7].
Several studies, predominantly in Asian populations, where S. japonicum infections are endemic, state conflicting findings on hepatotoxicity associated with PZQ treatment against the helminth [8, 9]. PZQ treatment is reported to be associated with elevated serum concentrations of liver aminotransferase [8]. However, in a large retrospective study from China, there was insignificant (less than 1%) incidence of hepatotoxicity among populations treated for S. japonicum with PZQ [9].
Therapy for Schistosomiasis in sub-Saharan Africa has mainly been documented based on intestinal S. mansoni infections [1]. As a result, there is paucity of data on urinary S. haematobium and its associated drug metabolism effects on organs involved in metabolizing and excretion of PZQ. This leaves a gap in knowledge about the protective or destructive effect of metabolizing the drug in S. haematobium infection. It has further been shown that varied degrees of reduction in incidence and infection rates of S. haematobium are reported with mostly single PZQ dosage of 40 mg/kg/day in both children and adults [1]. There are also indications of drug resistance to single doses of PZQ for treating schistosomiasis [10]. This heightens the need to probe the outcome of repeated PZQ treatment on urinary schistosome counts against its implication on liver and renal function.
The aim of this study was to assess the effect of PZQ on schistosome egg count, liver and renal function after 3 doses of 60 mg/kg/day (PZQ60) in three months for treating urinary S. haematobium infection.
Methods
This was a nested-Case Control study conducted among children and adults of ages 6–30 years from the urban Asokwa District in Kumasi, Ghana (see plate 1). This was part of a larger study to assess plasmodium transmission in persons infected with Schistosomiasis (NCT02769013). Ethical approval was obtained from the Committee for Human Research Publication and Ethics, School of Medical Sciences, Kwame Nkrumah University of Science and Technology, Ghana. All participants were required to sign an informed consent. For minors below 16 years, a signed assent form from the participant and an informed consent from a parent or guardian were obtained. Cases were respondents diagnosed to have S. haematobium by routine microscopic examination of urine samples. Controls from the same communities, without laboratory or clinical detection of urinary schistosomiasis infection were age and sex matched with cases.
Study area
Apromase, Deduako, Emena and Kokoben were the study communities in the urban Asokwa District with a population of 140,161 inhabitants in 36, 183 households (Fig. 1) [11]. These communities are located between latitude 6°30′ and 7°00′ North and longitude 1°30′ and 2°00 West of Kumasi, the capital city of the Ashanti Region of Ghana. The four communities have Saman (Kokoben and Apromase), Oda (Deduako) and Subin (Emena) as names of three rivers running through it.
Climatic conditions are tropical with temperatures varying from 20.2 °C to 37.1 °C. Rainfall pattern is seasonally bimodal with major rains extending from late April to August with a minor one from September to October [12]. The average annual rainfall for the area is 6.25 mm with peaks of 214.3 mm and 16.2 mm in June and September respectively. The dry season (harmattan) is from November to March with humidity ranging between 53 and 93%.
Screening and enrolment
A census of the selected communities was conducted with the ages and number of inhabitants per building collected along with corresponding GPS coordinates using Personal Digital Assistants (PDAs). Households within the buildings were selected and their members asked for written informed consent to be screened in the study. Twenty millilitres (20 ml) of urine samples were collected once, from consenting participant into well-labelled 30 ml urine containers. The urine samples were collected within the hours of 6:00 am and 12 noon. Subsequently, the samples were kept in cold boxes at temperature of 4–6 °C until transported to the laboratory at Kumasi Centre for Collaborative Research in Tropical Medicine (KCCR) about 15–20 min drive from the study sites.
Asymptomatic schistosomiasis positive (SP) cases and schistosomiasis-negative (SN) controls based on screening results were invited to participate in the study.
Sampling procedures
A total of 1258 participants were screened for schistosomiasis out of which 104 were positive. All 104 schistosome positive participants (Fig. 2) were placed on PZQ60 treatment. Controls were selected from the same communities and matched in a 2: 1 ratio with cases by sex and age. Out of the 104 positive cases, 32 started the treatment phase with 28 successfully completing the course with samples analyzed. On the other hand, 53 controls had all samples analyzed.
Design of Experiment
Biochemical parameters and schistosome counts were analysed before and after treatment for both cases and controls (Fig. 3). In between pre- and post-treatment, the biochemical and schistosome counts were monitored before the 2nd and 3rd dosages of PZQ60 for cases.
Laboratory processes
Processing of urine and S. haematobium quantification
Freshly voided urine was collected between 6:00 am and 12:00 pm in a sterile wide-mouthed screw-top plastic container (30 ml) and transported to the laboratory on ice at 4 °C to 8 °C. Urine processing and quantification were done as described by Cheesebrough [13]. Briefly, blunt-ended forceps were utilized to place a polycarbonate membrane filter of pore size of 12.0 μm (Whatmann Nuclepore) on the filter-support of a filter-holder (Swinnex 25 mm support chamber). The filter holder was re-assembled and attached to a 10 ml syringe filled with well-mixed urine which was filtered with the aid of the plunger. The filter was carefully removed and transferred with the face upwards to a clean glass-slide. A drop of physiological saline was added, covered with a cover-slip, and examined by two independent expert microscopists using the 10X objective (Carl Zeiss Microscope) with the condenser iris closed sufficiently to give good contrast. The entire filter was examined systematically for the presence of S. haematobium eggs. The number of the eggs counted per 10 ml of urine was recorded and the average of the two counts was calculated. A slide was declared negative when no parasites were detected.
Blood sampling and processing
Blood samples were collected from the antecubital vein with the aid of a tourniquet and the puncture site cleaned with 70% alcohol prep. Blood drawn into separator gel tubes (5 ml) were centrifuged at 1780 x g for 10 min at 4 °C to obtain the sera which were subsequently stored at -80 °C.
Biochemical analysis
Assays for the liver function; albumin, globulin, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma glutamyl transferase (GGT) and renal function (urea and creatinine), were conducted using a chemistry analyzer (HumaStar 200, Human, Germany). Elevated levels of AST, ALT and GGT are known indications of liver damage which may be caused by drug metabolism, infections and alcohol consumption. Increase in the levels of globulin is also found to correlate with infection or an inflammatory state. Aliquots of the serum were dispensed into cuvettes after thawing and placed at pre-programmed positions in the auto-analyzer and analysis done in batches. Due to the limited strength of serum urea and creatinine as markers of renal function, renal insufficiency based on estimated glomerular filtration was used as a more robust indicator. Renal insufficiency was assessed based on the 4-variable Modification of Diet in Renal Disease (4v-MDRD) and the Chronic Kidney Disease Epidemiology Program (CKDEPI) equations [14]. The 4v-MDRD estimates the glomerular filtration rate based on serum creatinine concentration, age, sex and race whiles the CKDEPI uses variations of an equation based on cut-offs for serum creatinine concentrations and sex.
Statistical analysis
Data were entered into excel and analyzed with Stata V.12 (StataCorp, USA). Continuous variables were reported as median with interquartile ranges and categorical variables as proportions. The Wilcoxon signed rank test was used to assess differences in continuous variables between groups with statistical significance set at p < 0.05.
Results
Characteristics of study population before treatment
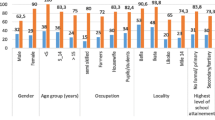
Majority of the study population were males [78.6 (62.4, 94.8) for schistosome positives and 81.1 (70.2, 92.0) for controls] with no significant statistical difference in age for the two groups (p = 0.547). Serum albumin levels were higher (p = 0.003) in controls [55.9 (52.1, 61.5)] compared with schistosome cases [51.9 (48.5, 53.0)]. On the contrary, serum globulin levels were elevated (p = 0.005) in cases 36.7 (32.8, 40.1) compared with controls 30.5 (22.4, 33.8). No significant difference was reported between schistosome cases and controls for liver (ALT, AST and GGT) and kidney (serum creatinine and urea) markers. Similarly, no significant difference (p = 0.600) was reported for estimated glomerular filtration rate (eGFR) between schistosome positive [96.0 (80.0, 116.0)] and negative [101.0 (88.0, 127.0)] groups using the CKDEPI equation before treatment (Table 1). Furthermore, the 4v-MDRD renal equation also showed no difference in levels between schistosome positive and negative groups at pre-treatment (p = 0.776).
Effect of treatment of cases with 3 doses of PZQ60 on liver, renal function and S. haematobium egg count
After the first dose, ALT levels increased significantly (p = 0.001) and gradually declined after the second dose and then significantly (p = 0.001) towards post-treatment stage (Fig. 4). AST levels were elevated (p = 0.006) after the first dose and significantly (p = 0.001) declined before the third dose. However, there was no statistically significant difference (p = 0.577) in AST levels from before the third dose to post-treatment. GGT levels increased sharply (p = 0.001) after the first dose and declined significantly (p = 0.001) after the second dose. Schistosome egg count decreased significantly (p = 0.001) after the third dose towards post-treatment.
Estimated glomerular filtration rates (Fig. 5) decreased significantly after the first dose using both CKDEPI (p = 0.003) and 4v-MDRD (p = 0.004). On the contrary, eGFR increased significantly from before second dose towards post-treatment for both CKDEPI (p = 0.001) and 4v-MDRD (p = 0.002).
Effect of treatment with 3 doses of PZQ60 on cure rate
Estimated cure rate was 42.9% a month after administering the first dose of PZQ60 (Table 2). This increased to 46.4% before administering the third dose and finally to 96.4% post-treatment.
Comparing Schistosome count and biochemical parameters between cases and controls after treatment
At post-treatment, there was no significant difference in mean levels for albumin (p = 0.441) between cases [48.8 (45.4, 52.5)] and controls [52.9 (49.6, 55.4)]. Similarly, mean levels of ALT, AST and GGT did not vary statistically between cases and controls post-treatment (Table 3). Moreover, creatinine and urea levels did not statistically vary between cases and controls. No significant difference (p = 0.753) was recorded using CKDEPI equation at post-treatment (Table 3) between the two groups. Likewise, 4v-MDRD reported no significant difference post-treatment (p = 0.866), between cases and controls.
Discussion
Schistosome egg numbers declined after administration of the second dose of 60 mg/kg praziquantel and almost completely eliminated post-treatment with 96.4% cure rate. The estimated glomerular filtration rate dropped significantly after the first dose of praziquantel but resolved after the third dose. Moreover, levels of liver enzymes increased after the first dose and returned to pre-treatment levels in schistosome positive cases post-treatment.
Several studies assessing the efficacy of praziquantel treatment on S. haematobium has been based on single doses of 40 mg/kg with cure rates ranging from 39.8 to 88.9% in mainly children below 17 years [15,16,17]. In addition, multiple dose regimens have been reported to clear S. haematobium with 53.1 to 100.0% cure rates [18, 19]. We report a final cure rate of 96.4% after a monthly dose of PZQ60 for three months. Contrary to case reports [20, 21] where multiple courses of 40 mg/kg failed in clearing persistent S. haematobium infection, this study finding provides evidence of an efficacious treatment regimen with repeated and higher doses of PZQ60 which can clear Schistosome eggs and eliminate the worm stage of the parasite. This assertion is further supported by an initial increase in schistosome egg count before the second dose which could be attributed to a single dose of PZQ60 having little effect on juvenile worms as concluded in a previous study that persist and perpetuate more eggs [22]. It is worthy of note that although praziquantel is the standard treatment for schistosome infections due to reported efficacy and minimal side effects [23], there are studies that have found cases of drug failure [20, 21]. However, these investigations are not definite on the causes of these treatment failures. It is also plausible that the low cure rates after the first and second dose could be attributed to some level of resistance to PZQ previously reported in S. haematobium infections [24] in some endemic regions and could be now present in Ghana.
With respect to organ damage, many studies have tried to analyze the efficacy of varying concentrations of PZQ on organ injury [25, 26]. However, little is known of its effect to the liver and kidney as a result of repeated and higher doses for treating S. haematobium among sub-Saharan subjects. It is well established that metabolizing of PZQ analogs by cytochrome P450 can lead to highly oxidative intermediates [27] which overwhelm the body’s defense resulting in varied levels of organ damage. This study found a significant increase in liver enzymes after the first dose, which steadily dropped to pre-treatment levels. The initial upsurge in liver enzymes could be explained as due to highly oxidative intermediates of PZQ than can be quenched by the antioxidant activity of superoxide dismutase, glutathione and glutathione S-transferase. Consequently, hepatic cells are disrupted leading to the release of compartmentalized liver enzymes. However, the steady return after two more doses of PZQ to pre-treatment levels could be proof of the body system’s response of mopping up toxic metabolites by inducing higher expression of cells that synthesize these proteins involved in the body’s redox systems.
Similarly, the significant drop in estimated glomerular filtration rate for both the CKDEPI and 4v-MDRD equation after the first 60 mg/kg dose shows the body’s initial intolerance to oxidative species from the metabolism of the drug which improved even after monthly repeated doses. It is plausible that sustained higher thresholds of these induced protective antioxidants persist after its initial deficit compared with the highly oxidative drug intermediates associated with organ damage. This suggests possible protective effect of monthly repeated doses of PZQ on the renal filtration with a corresponding decline in schistosome egg count.
It is possible that taking other medications with this treatment regimen may further increase secretion of liver enzymes into blood. However, data backing this assertion is lacking from this study. Moreover, the reported levels of liver enzymes and estimated glomerular filtration rates could have been influenced by viral infections and wasting of muscles respectively. Even though the study finding gives a strong indication that PZQ60 given over 3 months is safe and effective, the willingness of only 32/104 (31%) of egg positive cases to participate in the repeated treatment (Fig. 2) suggests this regimen may have low compliance. It is recommended that further research is done on improving compliance to increase treatment coverage if multiple dosing is to be employed.
Conclusion
This study suggests that treatment of urinary S. hematobium infections with a repeated high monthly dose of 60 mg/kg of praziquantel for 3 months is effective and safe. It provides an option to consider for S. haematobium infection cases with drug resistance.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- CKDEPI:
-
Chronic Kidney Disease Epidemiology Program
- eGFR:
-
Estimated glomerular filtration rate
- GGT:
-
Gamma glutamytransferase
- SN:
-
Schistosomiasis negative
- SP:
-
Schistosomiasis positive
- PZQ:
-
Praziquantel
- 4v-MDRD:
-
4-variable Modification of Diet in Renal Disease
References
Doenhoff M, Hagan P, Cioli D, Southgate V, Pica-Mattoccia L, Botros S, Coles G, Tchuem Tchuenté L, Mbaye A, Engels D. Praziquantel: its use in control of schistosomiasis in sub-Saharan Africa and current research needs. Parasitology. 2009;136(13):1825–35.
Pica-Mattoccia L, Cioli D. Sex-and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int J Parasitol. 2004;34(4):527–33.
Doenhoff MJ, Pica-Mattoccia L. Praziquantel for the treatment of schistosomiasis: its use for control in areas with endemic disease and prospects for drug resistance; 2006.
Jeziorski MC, Greenberg RM. Voltage-gated calcium channel subunits from platyhelminths: potential role in praziquantel action. Int J Parasitol. 2006;36(6):625–32.
Masimirembwa CM, Hasler JA. Characterisation of praziquantel metabolism by rat liver microsomes using cytochrome P450 inhibitors. Biochem Pharmacol. 1994;48(9):1779–83.
Dachman WD, Adubofour KO, Bikin DS, Johnson CH, Mullin PD, Winograd M. Cimetidine-induced rise in praziquantel levels in a patient with neurocysticercosis being treated with anticonvulsants. J Infect Dis. 1994;169(3):689–91.
Ridtitid W, Wongnawa M, Mahatthanatrakul W, Punyo J, Sunbhanich M. Rifampin markedly decreases plasma concentrations of praziquantel in healthy volunteers. Clin Pharmacol Ther. 2002;72(5):505–13.
Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. Philadelphia: Lippincott Williams & Wilkins; 1999.
Chen M, Fu S, Hua X, Wu H. A retrospective survey on side effects of praziquantel among 25,693 cases of schistosomiasis japonica. Southeast Asian J Trop Med Public Health. 1983;14(4):495–500.
Doenhoff MJ, Kusel JR, Coles GC, Cioli D. Resistance of Schistosoma mansoni to praziquantel: is there a problem? Trans R Soc Trop Med Hyg. 2002;96(5):465–9.
GSS. 2010 Population and housing census summary of final results. Ghana: Ghana Statistical Service; 2012.
MSD. Annual Report (Meteorological Statistical Department, Kumasi). Ghana: Ghana Meteorological Agency; 2011.
Cheesbrough M. District laboratory practice in tropical countries. New York: Cambridge University Press; 2006.
Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5(6):1003–9.
Keiser J, N'guessan NA, Adoubryn KD, Silué KD, Vounatsou P, Hatz C, Utzinger J, N'goran EK. Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate, and praziquantel against Schistosoma haematobium: randomized, exploratory open-label trial. Clin Infect Dis. 2010;50(9):1205–13.
Guidi A, Andolina C, Makame Ame S, Albonico M, Cioli D, Juma Haji H. Praziquantel efficacy and long-term appraisal of schistosomiasis control in Pemba Island. Tropical Med Int Health. 2010;15(5):614–8.
Sissoko MS, Dabo A, Traoré H, Diallo M, Traoré B, Konaté D, Niaré B, Diakité M, Kamaté B, Traoré A. Efficacy of artesunate+ sulfamethoxypyrazine/pyrimethamine versus praziquantel in the treatment of Schistosoma haematobium in children. PLoS One. 2009;4(10):e6732.
Garba A, Lamine MS, Barkiré N, Djibo A, Sofo B, Gouvras AN, Labbo R, Sebangou H, Webster JP, Fenwick A. Efficacy and safety of two closely spaced doses of praziquantel against Schistosoma haematobium and S. mansoni and re-infection patterns in school-aged children in Niger. Acta Trop. 2013;128(2):334–44.
Tchuenté L-AT, Momo SC, Stothard JR, Rollinson D. Efficacy of praziquantel and reinfection patterns in single and mixed infection foci for intestinal and urogenital schistosomiasis in Cameroon. Acta Trop. 2013;128(2):275–83.
Herwaldt BL, Tao L f, van Pelt W, Tsang VC, Bruce JI. Persistence of Schistosoma haematobium infection despite multiple courses of therapy with praziquantel. Clin Infect Dis. 1995;20(2):309–15.
Alonso D, Muñoz J, Gascón J, Valls ME, Corachan M. Failure of standard treatment with praziquantel in two returned travelers with Schistosoma haematobium infection. Am J Trop Med Hygiene. 2006;74(2):342–4.
Dong Y, Chollet J, Vargas M, Mansour NR, Bickle Q, Alnouti Y, Huang J, Keiser J, Vennerstrom JL. Praziquantel analogs with activity against juvenile Schistosoma mansoni. Bioorg Med Chem Lett. 2010;20(8):2481–4.
Gray DJ, Ross AG, Li Y-S, McManus DP. Diagnosis and management of schistosomiasis. Bmj. 2011;342:d2651.
Wang W, Wang L, Liang Y-S. Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitol Res. 2012;111(5):1871–7.
El-Fakahany A, Abdalla K, El-Hady H, Se-A A, Afifi L. The effect of praziquantel treatment on the liver functions, worm burden, and granuloma size using two drug regimen in murine Schistosoma mansoni infection. J Egypt Soc Parasitol. 1993;23(3):877–86.
Rahoud SA, Mergani A, Khamis AH, Saeed OK, Mohamed-Ali Q, Dessein AJ, Elwali NEM. Factors controlling the effect of praziquantel on liver fibrosis in Schistosoma mansoni-infected patients. FEMS Immunol Med Microbiol. 2010;58(1):106–12.
Vale N, Gouveia MJ, Rinaldi G, Brindley PJ, Gärtner F, da Costa JMC. Praziquantel for schistosomiasis: single-drug metabolism revisited, mode of action, and resistance. Antimicrob Agents Chemother. 2017;61(5):e02582–16.
Acknowledgements
Authors are grateful to the study communities and participants for making this research a success. We also thank Dr. Oumou Maiga-Ascofare for her efforts and hard work in coordinating this study. We are further grateful to Tony Sarfo-Acquah and Patrick Obuam for their assistance in data collection.
Funding
This study was funded by the EOD group, Kumasi Centre for Collaborative Research in Tropical Medicine, Kumasi, Ghana.
Author information
Authors and Affiliations
Contributions
SND, EOD and DY conceived the idea. PAK, HH and SBA recruited study participants. SND, PAK and HH conducted sample analysis. SND and STA conducted the statistical analysis. SND drafted the manuscript which was reviewed and approved by all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was obtained from the Committee for Human Research Publication and Ethics, School of Medical Sciences, Kwame Nkrumah University of Science and Technology, Ghana (CHRPE/AP/227/17). All participants were required to sign an informed consent. For minors below 16 years, a signed assent form from the participant and an informed consent from a parent or guardian were requirements for participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Darko, S.N., Hanson, H., Twumasi-Ankrah, S. et al. Three monthly doses of 60 mg/kg praziquantel for Schistosoma haematobium infection is a safe and effective treatment regimen. BMC Infect Dis 20, 323 (2020). https://doi.org/10.1186/s12879-020-05053-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-020-05053-z