Abstract
Background
Corynebacterium striatum is an emerging multidrug-resistant (MDR) pathogen associated with immunocompromised and chronically ill patients, as well as nosocomial outbreaks. In this study, we characterized 23 MDR C. striatum isolated of bloodstream and catheter-related infections from a hospital of Rio de Janeiro.
Methods
C. striatum isolates were identified by 16S rRNA and rpoB genes sequencing. The dissemination of these isolates was accomplished by pulsed-field gel electrophoresis (PFGE). All isolates were submitted to antimicrobial susceptibility testing by disk diffusion and by minimum inhibitory concentration using E-test strips methods. Antimicrobial resistance genes were detected by polymerase chain reaction. Quantitative tests were performed on four different abiotic surfaces and the ability to produce biofilm on the surface of polyurethane and silicone catheter was also demonstrated by scanning electron microscopy.
Results
Eleven PFGE profiles were found. The PFGE profile I was the most frequently observed among isolates. Five different MDR profiles were found and all PFGE profile I isolates presented susceptibility only to tetracycline, vancomycin, linezolid and daptomycin. Only the multidrug-susceptible isolate did not show mutations in the quinolone-resistance determinant region (QRDR) of the gyrA gene and was negative in the search of genes encoding antibiotic resistance. The other 22 isolates were positive to resistance genes to aminoglycoside, macrolides/lincosamides and chloramphenicol and showed mutations in the QRDR of the gyrA gene. Scanning electron microscopy illustrated the ability of MDR blood isolate partaker of the epidemic clone (PFGE profile I) to produce mature biofilm on the surface of polyurethane and silicone catheter.
Conclusions
Genotyping analysis by PFGE revealed the permanence of the MDR PFGE profile I in the nosocomial environment. Other new PFGE profiles emerged as etiologic agents of invasive infections. However, the MDR PFGE profile I was also found predominant among patients with hematogenic infections. The high level of multidrug resistance associated with biofilm formation capacity observed in MDR C. striatum is a case of concern.
Similar content being viewed by others
Background
Corynebacterium genus consists of Gram-positive aerobic or anaerobic facultatively pleomorphic rods with a high G + C content DNA. Some species are part of human skin or mucosa [1, 2]. Corynebacterium striatum has been increasingly associated with severe infections in both immunocompetent and immunocompromised hosts [3, 4]. However, C. striatum isolates have been included among the etiologic agents of bacteremia with or without central venous catheter (CVC) in place [5, 6], endocarditis [7], breast abscesses [8], septic arthritis [2], osteomyelitis [4] and several other invasive diseases. In addition, studies have evidenced C. striatum as an emerging multidrug-resistant (MDR) pathogen related to nosocomial outbreaks in several countries [9,10,11,12,13,14,15,16].
The main risk factors for acquisition MDR C. striatum infections highlighted by Verroken et al. [13] were: prolonged hospital stay, advanced stage of chronic obstructive pulmonary disease, recent administration of antibiotics and exposure to an invasive diagnostic procedure. Besides, empirical antibiotic therapy may select MDR Gram-positive skin flora that can become the etiologic agent of nosocomial invasive diseases [17]. The emergence of MDR C. striatum and its involvement in nosocomial infections require appropriate interpretive criteria to the selection of the adequate antibiotic therapy [16].
Most reports of nosocomial infections and outbreaks caused by C. striatum mainly encompassed the respiratory tract [10, 13, 18]. On the other hand, few studies have investigated bloodstream and catheter-related infections by C. striatum [15, 19,20,21]. In a Brazilian tertiary care hospital located at Rio de Janeiro metropolitan area, a nosocomial outbreak caused by MDR C. striatum mostly isolated from tracheal aspirates samples was initially verified in 2009 [12]. Subsequently, cases of bloodstream and catheter-related infections caused by C. striatum isolates were noticed in the same hospital. In the present study, we aimed to investigate the clonal relationship, antimicrobial susceptibility profiles, ability of biofilm formation, molecular detection of resistance genes to amynoglycosides, quinolones, compounds of the MLSB group (macrolides and lincosamides) and chloramphenicol of these C. striatum invasive isolates.
Methods
Study design and origin of bacterial isolates
Twenty three C. striatum isolates recovered from blood and catheter samples of 21 hospitalised patients with signs and symptoms of bacteremia (n = 13) and catheter-related infections (n = 10) were analysed for microbiological features (Table 1). Two of these isolates (2023 and 2038, PFGE profiles I and II, respectively) were previously studied by Baio and co-workers [12]. The patients were hospitalised during a 42-month period (January 2009 – February 2013) in 13 different wards of Hospital Universitário Pedro Ernesto (HUPE) - a tertiary care hospital belong to Universidade Estado Rio de Janeiro (UERJ) (n = 22) and Hospital Municipal Jesus (HMJ) (n = 1), both located at the metropolitan area of Rio de Janeiro, RJ, Brazil. The 2296 isolate came from a patient with CVC-related infection from HMJ and it was sent to the Laboratório de Difteria e Corinebactérias de Importância Clínica/UERJ for identification of the pathogen. C. striatum was isolated from blood samples and catheters segments of patients with signs and symptoms of bacterial infections as part of the medical care procedures of both hospitals. The consent to participate was not required because all the investigated isolates were taken as a part of standard care (diagnostic purposes). This study focused on bacteria and no identifiable human data were used. All the isolates were deposited in the CBAS/Fiocruz (Coleção de Bactérias do Ambiente e Saúde of Fundação Oswaldo Cruz) culture collection.
Bacterial isolates collection, culture conditions and phenotypic identification procedures
Clinical specimens were inoculated in Bactec Plus anaerobic/aerobic vials and analysed in a Bactec 9240 continuous-monitoring system (Becton-Dickinson Microbiology System, Cockeysville, MD, USA). Corynebacterium-like colonies were selected for identification when they were grown in significant numbers (> 15 colonies) or in pure culture from blood or catheter samples, as recommended by Maki’s semi quantitative method to distinguish infection (> 15 colonies) from contamination of catheter-tips [22]. Bacterial isolates were identified by conventional phenotypic characterization [12, 23] and Vitek 2 (bioMérieux, France) using Anaerobe and Corynebacterium card.
C. striatum molecular identification by 16S rRNA and rpoB genes sequencing
Molecular identification was performed according with protocols previously described [12]. The 16S rRNA and rpoB genes sequences were compared to type strains sequences available in the National Center for Biotechnology Information (ncbi.nlm.nih.gov) using the BLAST algorithm and/or the Eztaxon server [24].
Pulsed-field gel electrophoresis (PFGE)
Pulsed-field gel electrophoresis was performed as described by Baio and co-workers [12]. PFGE banding profiles were analyzed using visual comparison among the isolates, according to the criteria established by Tenover and co-workers [25] and by automated analysis using the BioNumerics Fingerprinting software (Version 4.0, Applied Math, Belgium). PFGE profiles were identified by roman numerals and subtypes were identified by roman numerals followed by a letter. The similarity index of the isolates was calculated using the Dice correlation coefficient with a band position tolerance of 1%. The unweighted-pair group method using average linkages (UPGMA) was used to construct a dendrogram. PFGE profiles that showed similarity coefficient ≥ 85% were considered genetically related [26, 27]. The isolates previously studied, 1961 (PFGE profile III) and 1954 (PFGE profile IV) were used as controls [12].
Antimicrobial susceptibility testing
Antimicrobial susceptibility profiles were determined by the disk diffusion and by minimum inhibitory concentration (MIC) using E-test strips methods in cation-adjusted Mueller-Hinton agar supplemented with 5% sheep blood using inoculum equivalent to a 0.5 McFarland standard. Seven antibiotic disks (Oxoid, Hampshire, United Kingdom) were used: clindamycin (2 μg), moxifloxacin (5 μg), gentamicin (10 μg), rifampicin (5 μg) and vancomycin (5 μg), according Brazilian Committee on Antimicrobial Susceptibility Testing – BrCAST [28]. Another three antimicrobials imipenem (10 μg), erythromycin (15 μg) and chloramphenicol (30 μg) were interpreted in accordance to criteria defined by BrCAST for Staphylococcus spp. These antibiotics are not considered in Clinical and Laboratory Standards Institute (CLSI) and BrCAST/EUCAST guidelines for Corynebacterium spp. Linezolid 30 μg was interpreted in accordance to criteria defined by CLSI [29] for Staphylococcus spp. The BrCAST document recommends the use of linezolid 10 μg for antimicrobial susceptibility testing, but this is not found commercially available in Brazil.
Antimicrobial susceptibility to daptomycin, penicillin, ciprofloxacin and tetracycline were determined by MIC using E-test strips (AB Biodisk, Sweden). Interpretation of values to penicillin, ciprofloxacin and tetracycline were performed also according BrCAST [28]. Due to absence of daptomycin breakpoints in this guideline, the interpretation of values was performed according to CLSI M45-A2 document [30]. MDR was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories [31].
Amplification and sequencing of the genes related to resistance
The amplification and sequencing of the QRDR region of the gyrA gene was performed following protocols described by Sierra and co-workers [32]. Mutations in the QRDR region of the gyrA gene were identified by aligning of amino acids sequences and compared to the sequence of C. striatum ATCC 6940 (GenBank accession number AY559038) using Clustal X program [33]. The 23 C. striatum isolates also were screened for the presence of genes codifying antibiotic resistance, ermX (macrolides and lincosamides), aphA (amynoglycoside) and cmx (chloramphenicol), by PCR using primers specific for each gene [11, 34]. For each gene, one amplicon was purified and sequenced according with protocols previously described by Baio and co-workers [12].
Semi-quantitative analyses of biofilm formation on polyurethane and silicone catheter surfaces
Sterile 4 cm segments of 16-gauge percutaneous nephrostomy polyurethane and silicone catheters were immersed in TSB containing 106 CFUml− 1 of C. striatum and incubated at 37 °C for 48 h then Maki’s semi-quantitative roll plate technique were performed. Basically, after washing (three times) with phosphate buffered saline (PBS) 0.1 M pH 7.2, contaminated abiotic substrates were rolled up on Columbia agar plates supplemented with 5% sheep blood (Oxoid, Germany) for 48 h at 37 °C were analyzed presence of bacterial carpet [23, 35].
Quantitative tests of biofilm formation on different on catheter abiotic surfaces
Quantitative analysis of viable sessile cells of representative isolates of PFGE profile I (2023) and II (2038) was evaluated by quantitative tests based on previously described methods [35] and using the following abiotic substrates: surfaces of glass tubes, Thermanox cover slips (Nunc), catheters fragments of polyurethane and metal tips (Intracath; Deseret Pharmaceutical Co., Sandy, Utah). Each experiment was carried out in triplicate and repeated three times. Results of the viable cell counts of experiments performed with the 2023 and 2038 isolates were compared using one-way analysis of variance (ANOVA) and Tukey’s multiple-comparison post-test. The values of p < 0.05 were considered statistically significant. Statistical analyses were performed using GraphPad Prism version 6 (GraphPad Software, San Diego, CA).
Morphological aspects of biofilm formation polyurethane and silicone catheters
The biofilm produced (48 h incubation) on the surface of in vitro prepared on fragments of polyurethane and silicone catheters by C. striatum isolates from blood, 2023 (PFGE profile I) and 2038 (PFGE profile II), were demonstrated by Scanning Electron Microscopy (SEM). Specimen preparation and staining protocol for SEM were performed as described by Souza and co-workers [35]. Catheter segments infected in vitro with C. striatum 1987 (PFGE profile I), 2369 (PFGE profile II), 1961 (PFGE profile III) and 1954 (PFGE profile IV) isolates were used as positive controls [35].
Results
C. striatum genotypic identification
The analysis of 16S rRNA and rpoB genes sequences from 23 isolates from blood and catheter samples exhibited the highest similarity values with C. striatum ATCC 6940 type strain, ranging from 99.77–99.12% and 99.28–98.65%, respectively. GenBank accessions for C. striatum 16S rRNA and rpoB genes were deposited: KJ934779 to KJ934789, KM001910 to KM001914, KJ855309 to KJ855313, KR010627 to KR010645, KR020513 and KR020514.
PFGE analysis
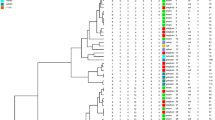
Eleven PFGE profiles among C. striatum isolates were designated I, Ia, Ib, II, V, VI, VIa, VII, VIII, IX and X (Table 1; Fig. 1; Additional files 1 and 2). The PFGE profile I was the most frequently (n = 9) observed among isolates (Table 1). The PFGE profiles I, Ia and Ib showed similarity coefficient ≥ 85% were considered genetically related. The PFGE profiles VI and VIa showed similarity coefficient ≥ 90% were also considered genetically related. PFGE analysis indicated that bloodstream infections in cases 8 and 19 were due to particular clones of C. striatum: PFGE profiles VI (2230 and 2237 isolates) and IX (2425 and 2432 isolates), respectively. PFGE profile X was related to a case of catheter-related infection in a 3 months-old patient from Neonatal ICU. C. striatum PFGE profile VII was isolated only from patient with CVC infection from HMJ-RJ (Table 1). C. striatum PFGE profile I isolates were detected in patients with bloodstream and/or catheter-related infections during the years of 2009 (n = 1), 2010 (n = 2), 2011 (n = 5), 2013 (n = 1) but not 2012.
Dendrogram generated by Dice/UPGMA analysis (Bionumerics, Applied Maths) of SwaI PFGE profiles of C. striatum isolated from bloodstream infection. I- X, PFGE profiles. Previously described non-MDR isolates of PFGE types III and IV were not found in patients with blood and catheter-related infections [12]
Antimicrobial multiresistance profiles
Antimicrobial susceptibility profiles of 23 C. striatum isolates are displayed in Table 2. Except the 2376 isolate from Neonatal ICU, all C. striatum isolates, independent of their PFGE profiles and hospital settings, showed non-susceptibility to at least one agent in three or more antimicrobial categories and were consequently identified as MDR pathogens. Twenty-two C. striatum isolates exhibited five different multiresistance levels corresponding to five different MDR profiles: A, with resistance to 9 antimicrobial agents; B and B1, resistance to 8 antimicrobial agents; C, resistance to 7 antimicrobial agents; and D, with resistance to 6 antimicrobial agents. All C. striatum isolates related to bloodstream and catheter-related infections, representative of different PFGE profiles, showed susceptibility to tetracycline, vancomycin, linezolid and daptomycin. C. striatum isolates, independent of PFGE profiles showed resistance to penicillin (100%), ciprofloxacin and moxifloxacin (95.6%), clindamycin (95.6%), erythromycin (95.6%), gentamicin (91.3%), rifampin (52.2%), imipenem (76.2%) and chloramphenicol (95.6%). MDR profile A was demonstrated for 8 C. striatum PFGE profile I isolates. Moreover, MDR profile A was also shown for C. striatum isolates PFGE profiles Ib, II and VIII.
Molecular detection of resistance genes
The sequences of the QRDR region of the gyrA gene of 22 isolates characterized as resistant to fluoroquinolones were compared to that of the quinolone-susceptible C. striatum ATCC 6940 (MIC = 0.094 mg/L) and with our multidrug-susceptible (MDS) 2376 isolate (MIC = 0.125 mg/L). The relationships between the mutations in the QRDR region gyrA gene and the MICs of ciprofloxacin of the 23 isolates and C. striatum ATCC 6940 are summarized in Table 3. Twenty-one isolates showed MIC > 32 mg/L to ciprofloxacin, fifteen of them carried a mutation only the position 87 generating a change from Ser-87 to Val. The 2324 and 2401 isolates carried a double mutation, generating a change from Ser-87 to Val and Asp-91 to Asn. The 2130 isolate had MIC = 2 mg/L and carried a mutation only in position 87 (Ser-87 to Tyr). All sequences of QRDRs region gyrA gene were deposited in GenBank/NCBI under numbers MG010347-MG010369.
The genes codifying for antibiotic resistance, ermX (macrolides and lincosamides), aphA (amynoglycoside) and cmx (chloramphenicol) were found except in MDS 2376 isolate. Twenty-two isolates resistant to erythromycin, clindamycin and chloramphenicol presented ermX and cmx genes. These isolates were also aphA-positive and resistant to gentamicin, except for one isolate. The 2130 isolate was aphA-positive and susceptible to gentamicin, confirmed by MIC using E-test strip gentamicin. Analysis of genes sequences exhibited high similarity values (above 99%) with those deposited in NCBI. The sequences were deposited in GenBank/NCBI under numbers MF872250 to MF872252.
Quantitative tests of biofilm formation on different catheter abiotic surfaces
Results of quantitative analysis of viable sessile cells of MDR C. striatum isolates 2023 and 2038 (PFGE profiles I and II, respectively) are shown in Fig. 2. Viable sessile bacterial cells were detected 48 h post-infection on surfaces of all types of abiotic substrates tested, but at different levels. C. striatum 2023 isolate (PFGE profile I) showed a higher ability to adhere to glass surface, a hydrophilic and positively charged abiotic surface (p < 0.001). In addition to glass, C. striatum 2023 isolate also exhibited a significantly (p < 0.001) greater level of adherence to an abiotic hydrophilic (polyurethane) positively charged surface compared with C. striatum 2038 isolate (PFGE profile II). Conversely, C. striatum 2038 isolate exhibited a significantly (p < 0.001) greater level of adherence to negatively charged and hydrophobic polystyrene (Thermanox) surface compared with 2023/PFGE profile I isolate. Interestingly, both C. striatum isolates (PFGE profiles I and II) were able to strongly adhere and survive on the metal surface with similar intensities (p = 0.374) (Fig. 2).
Adhesive levels, biofilm formation and survival on different types of abiotic surfaces of C. striatum (2023/PFGE profile I MDR and 2038/PFGE profile II MDR isolates) isolated from patients with bloodstream infection evaluated by quantitative tests: glass and polyurethane (hydrophilic and positively charged), polystyrene and thermanox (hydrophobic and negatively charged), and metal (catheter’s tips) surfaces. Mean values and standard deviations of three independent experiments and magnifications are shown in the figure
Semi-quantitative analyses biofilm produced in vitro model of catheter infection
Results of the semi-quantitative roll plate method (> 15 CFU) showed extensively adherent viable sessile forms for both C. striatum isolates (2023 and 2038) on fragments of polyurethane and silicone catheters, as illustrated in Fig. 3a.
Biofilm formation (48 h incubation) on the surface of in vitro infected polyurethane and silicone catheters by the two C. striatum isolates from bloodstream infections: (a-d) 2023/PFGE profile I isolate; (e, f) 2038/PFGE profile II isolate. (c) Microcolony formation (a hallmark of biofilm formation) by autoaggregative C. striatum on catheter surface. SEM assays of biofilm formation on (c, d) polyurethane and (e, f) silicone catheters surfaces. (d, e) Presence of hollow voids indicative of mature biofilm formation
Morphological aspects of biofilm formation on polyurethane and silicone catheters evaluated by SEM
Micrographs illustrating biofilm formation on the surface of polyurethane and silicone catheters by C. striatum 2023/PFGE profile I (Fig. 3b-d) and 2038/PFGE profile II (Fig. 3e, f) isolates demonstrated by SEM are displayed in Fig. 3b-f.; Fig. 3c showed microcolony formation (a hallmark of biofilm formation) by auto aggregative C. striatum on polyurethane surface. SEM assays also evidenced the presence of hollow voids indicative of mature biofilm formation on surfaces of polyurethane (Fig. 3d, e) and silicone (Fig. 3f) catheters.
Discussion
Antimicrobial resistance has a major impact on human health [36]. At present, reports on the emergence and spread of multiresistant bacterial species are important to support the progress of resistance control policies. Our data show bloodstream and catheter-related infections caused by different clones of MDR C. striatum in Brazil. In accordance to Chen and co-workers [37], our findings emphasize that C. striatum from blood and catheter segments should not be considered only as contaminant, since in our study most of the isolates were found in pure cultures (82%) or in significant numbers.
In Brazil the isolation of C. striatum from hospitalized patients with signs and symptoms of infection was observed in some studies [38,39,40]. In a previous study, we documented four PFGE profiles during a nosocomial outbreak caused by C. striatum in Rio de Janeiro, Brazil. MDR clones related to the profiles of PFGE I and II were predominant. In that opportunity only two isolates of PFGE I and II profiles were isolated from blood samples [12]. Due to subsequent increased number of cases of bloodstream and catheter-related infections caused by C. striatum isolates in HUPE, current investigation of the clonal relationship of these C. striatum isolates revealed the permanence of the MDR PFGE profiles I and II in the nosocomial environment as invasive clones. However, the PFGE I profile was found predominant among patients with hematogenic infections. In addition, other new MDR PFGE profiles (V to IX) emerged as etiologic agents of bloodstream and catheter-related infections. Interestingly, one non-multiresistant clone (PFGE profile X) was also related to a case of catheter-related infection in the newborn.
PFGE is a valuable tool to investigate the clonal relatedness of microbial strains during nosocomial outbreaks. Several nosocomial infections and outbreaks studies have employed this methodology for typing of C. striatum isolates [13, 15, 16, 18]. PFGE is a stable and reproducible genotyping method, however, it is time consuming and standardizations for inter-laboratory comparisons do not exist for C. striatum isolates genotyping. Thus, the method is only applicable to compare isolates for regional epidemiology surveillance. Gomila and co-workers described the development of a multilocus sequence typing (MLST) scheme for C. striatum. [9]. However, the proposed MLST scheme has not been adopted by scientific community, perhaps due to the limited number of genes (ITS1 region, gyrA and rpoB) that comprise it. PFGE has limitations but it is the tool available so far for the discrimination of this bacterial species. Future researches are required to evaluate genotyping methods that provide useful data for global surveillance of infections caused by C. striatum.
Antimicrobial susceptibility testing remains rarely performed on Corynebacterium spp. in many laboratories [41]. The method of susceptibility by disk-diffusion is widely used by microbiology laboratories in Brazil and in other countries [12, 42,43,44]. Moreover, CLSI guidelines do not provide breakpoints for disk-diffusion while BrCAST, based in EUCAST document, provides breakpoints for corynebacteria susceptibility testing only for some antibiotics [28, 29], excluding various antimicrobials, such as cephalosporins, carbapenems and lipopeptides, thus many researchers often use staphylococcal breakpoints [12, 16, 45]. Susceptibility testing should be performed on clinically significant C. striatum isolates. However, studies suggest that according to the severity of the infection, empiric treatment should be carried out with vancomycin and linezolid because of low levels of susceptibility to other antimicrobials [13, 46]. In some studies, therapy with an association of at least two of the following antimicrobial agents has been reported: vancomycin, rifampin, linezolid and daptomycin [47, 48].
Resistance to imipenem by C. striatum isolates has been related in some countries, such as Japan [49], Spain [9, 10] and Italy [11]. Most of our C. striatum isolates showed resistance to imipenem (76,2%). Combination therapy that includes imipenem for the treatment of MDR C. striatum infections in Brazil should be more prudent.
Effective treatment of corynebacterial infections with daptomycin has been reported in the literature [48, 50]. In this study, all C. striatum isolates showed susceptibility to daptomycin. However, resistance to daptomycin in C. striatum has been documented and may occur during therapy in patients with invasive infections. Consequently, some authors recommend caution in daptomycin monotherapy for treatment of these infections [41, 51].
Some mechanisms of antimicrobial resistance have been reported in Corynebacterium species. Studies of the sequences encoding the A subunit of the gyrase enzyme in strains of C. striatum, Corynebacterium amycolatum and Corynebacterium macginley have shown that resistance to fluoroquinolones is associated with mutations of a spontaneous nature in this gene and depends on the number of mutations and the type of amino acid that was exchanged [16, 32, 52, 53]. Twenty-two isolates were resistant to the quinolones tested. The combinations of amino acids Val/Asn and Tyr/Asp in positions 87 and 91 of QRDR region gyrA gene, respectively, found in three of our isolates have not been described in the literature for Corynebacterium species until the present moment, conferring resistance to ciprofloxacin and moxifloxacin.
The ermX gene (erythromycin ribosome methylation) encoding the rRNA methylase enzyme leads to simultaneous resistance to macrolides, lincosamides and streptogramins B (MLSB) [45, 54]. This gene was found on chromosomes, plasmids and transposons of corynebacteria. We found this gene in 22 isolates, except in the MDS 2376 isolate, which may indicate that the ermX gene can be involved in the clindamycin and erythromycin resistance phenotype of our isolates.
Antimicrobial susceptibility studies have shown C. striatum isolates resistant to aminoglycosides. Consequently, the use of aminoglycosides as second-line complementary antimicrobial for treatment of C. striatum infections should be cautious [55, 56]. The mechanisms of aminoglycoside resistance most common are the aminoglycoside modifying enzymes (AME’s) classified in 3 classes: aac (acyl-coenzyme A-dependent acetyltransferase), ant (nucleoside triphosphate-dependent nucleotidyl transferases) and aph (nucleoside triphosphate-dependent phosphotransferases). These enzymes are often disseminated by various mobile genetic elements and many aminoglycosides can be inactivated by more than one enzyme [57]. In this study, the aph gene was found in 22 isolates, except in the MDS 2376 isolate, but one aph-positive isolate showed susceptibility to gentamicin, confirmed by MIC E-test strip (MIC = 0.12 mg/L, according to BrCAST). Future analyzes of the region where the gene is located and other mechanisms of resistance to aminoglycosides in all aph-positive isolates should be made.
The cmx gene is responsible for coding the efflux protein to chloramphenicol and has already been found in transposons, plasmids and genomes of Corynebacterium species [58,59,60]. All isolates cmx-positive were resistant to chloramphenicol. The cmx gene sequence showed similarity above 99% with the sequences of cmx gene found in chromosomes, plasmid and genomic island of Pseudomonas aeruginosa and other Corynebacterium species deposited in GenBank/NCBI.
Biofilm is a structure that facilitates several bacterial processes influencing virulence and resistance to antimicrobials such as adhesion capacity, metabolite exchange, cellular communication, protection to antimicrobials, protection against host immune attacks. Consequently, the formation of bacterial biofilms leads to an increase in healthcare costs and extend hospitalization [61, 62]. Previously, Souza and co-workers verified that C. striatum PFGE profiles I to IV formed biofilm on hydrophilic and hydrophobic surfaces. C. striatum PFGE profile I, predominant isolated from nosocomial outbreak, showed the greatest ability to adhere to all surfaces produced much more biofilm than the other profiles [35]. In Japan, Qin and co-workers observed that all 6 C. striatum isolates identified as predominant PFGE profile had high ability to produce biofilm in glass cover-slips after 72 h post-incubation [15]. In the present study, biofilm formation and survival on four abiotic surfaces (glass, metal, polyurethane and silicone) were demonstrated 48 h post-incubation of bacterial cells representative of MDR C. striatum PFGE profiles I and II isolated from patients with bloodstream infections. Similar to C. striatum PFGE profile I isolated from patients undergoing endotracheal intubation procedures, PFGE profile I isolated from bloodstream and catheter-related infections also showed a higher ability to adhere to and to survive on abiotic surfaces of medical devices including those used in invasive procedures. MDR C. striatum viable cells were able to multiply and to produce mature biofilms on both types of catheter surfaces.
Conclusions
The ability of some C. striatum clones to produce biofilm on different types of abiotic surfaces may contribute to the pathogenicity favoring bacterial invasive potential and establishment of bloodstream and catheter-related infections, in addition to permanence in hospital environment and dissemination of antimicrobial resistance. The potential of C. striatum to cause infection should not be underestimated. Therefore, antimicrobial susceptibility testing should be performed on clinically significant C. striatum isolates. Medical surveillance programs should include control strategies in order to decrease potential risk factors of nosocomial infections and outbreaks due to C. striatum.
Availability of data and materials
All data generated of analyses during this study are included in this published article and its additional files. The datasets including sequencing data used and analyzed during the current study are deposited in the Genbank. GenBank accessions for C. striatum 16S rRNA and rpoB genes were deposited: KJ934779 to KJ934789, KM001910 to KM001914, KJ855309 to KJ855313, KR010627 to KR010645, KR020513 and KR020514. C. striatum ATCC 6940 (GenBank accession number AY559038)All sequences of QRDRs region gyrA gene were deposited in GenBank/NCBI under numbers MG010347-MG010369.
Abbreviations
- CFU:
-
Colony-forming unit
- CLSI:
-
Clinical da Laboratory Standards Institute
- CVC:
-
Central venous catheter
- HMJ:
-
Hospital Municipal de Jesus
- HUPE:
-
Hospital Universitário Pedro Ernesto
- ICU:
-
Intensive care unit
- MDR:
-
Multidrug-resistant
- MDS:
-
Multidrug-susceptible
- MIC:
-
Minimum inhibitory concentration
- MLSB:
-
Macrolides, lincosamides and streptogramins B
- PFGE:
-
Pulsed-field gel electrophoresis
- QRDR:
-
Quinolone-resistance determinant region
- UERJ:
-
Universidade do Estado do Rio de Janeiro
- UPGMA:
-
Unweighted-pair group method using average linkages
References
Chandran FL, Puthukkichal DR, Suman E, Mangalore SK. Diphtheroids-important nosocomial pathogens. J Clin Diagn Res. 2016;10:DC28–31.
Collada M, Rico Nieto A. Diaz de Bustamante Ussia M, balsa Criado a.Septic arthritis in a native knee due to Corynebacterium striatum. Reumatol Clin. 2017;17:30033–5.
Severo CB, Guazzelli LS, Barra MB, Hochhegger B, Severo LC. Multiple pulmonary nodules caused by Corynebacterium striatum in an immunocompetent patient. Rev Inst Med Trop São Paulo. 2014;56:89–91.
Verma R, Kravitz G. Corynebacterium striatum empyema and osteomyelitis in a patient with advanced rheumatoid arthritis. BMJ Case Reports. 2016. https://doi.org/10.1136/bcr-2016-214691.
Yoo G, Kim J, Uh Y, Lee HG, Hwang GY, Yoon KJ. Multidrug-resistant Corynebacterium striatum bacteremia: first case in Korea. Ann LabMed. 2015;35:472–3.
Daisuke U, Oishi T, Yamane K, Terada K. Corynebacterium striatum bacteremia associated with a catheter-related blood stream infection. Case Rep Infect Dis. 2017. https://doi.org/10.1155/2017/2682149.
Hascoet S, Mauri L, Claude C, Fournier E, Lourtet J, Riou JY, et al. Infective endocarditis risk after percutaneous pulmonary valve implantation with the melody and sapien valves. JACC Cardiovasc Interv. 2017;10:510–7.
Poojary I, Kurian A, AJ V, Devapriva JD, AT M. Corynebacterium species causing breast abscesses among patients attending a tertiary care hospital in Chennai, South India. Infect Dis (Lond). 2017;6:1–4.
Gomila M, Renom F, Gallegos Mdell C, Garau M, Guerreiro D, Soriano JB, et al. Identification and diversity of multiresistant Corynebacterium striatum clinical isolates by MALDI-TOF mass spectrometry and by a multigene sequencing approach. BMC Microbiol. 2012;4:1–8.
Renom F, Gomila M, Garau M, Gallegos MD, Guerrero D, Lalucat J, et al. Respiratory infection by Corynebacterium striatum: epidemiological and clinical determinants. New Microbes New Infect. 2014;2:106–14.
Campanile F, Carretto E, Barbarini D, Grigis A, Falcone M, Goglio A, et al. Clonal multidrug-resistant Corynebacterium striatum strains. Italy Emerg Infect Dis. 2009;15:75–8.
Baio PVP, Mota HF, Freitas AD, Gomes DL, Ramos JN, Sant’anna LO, et al. Clonal multidrug-resistant Corynebacterium striatum within a nosocomial environment, Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz. 2013;108:23–9.
Verroken A, Bauraingm C, Deplano A, Bogaerts P, Huang D, Wauters G, et al. Epidemiological investigation of a nosocomial outbreak of multidrug-resistant Corynebacterium striatum at one Belgian university hospital. Clin Microbiol Infect. 2014;20:44–50.
Wang J, Du X, Cui J, Guo S, Fu Q, Wang Y, et al. Drug susceptibility and homologous analysis on Corynebacterium striatum strains isolated from inpatients. Zhonghua Yi Xue Za Zhi. 2014;94:2501–5.
Qin L, Sakai Y, Bao R, Xie H, Masunaga K, Miura M, et al. Characteristics of multidrug-resistant Corynebacterium spp. isolated from blood cultures from hospitalized patients in Japan. Jpn J Infect Dis. 2017;70:152–7.
Alibi S, Ferjani A, Boukadida J, Cano ME, Fernández-Martínez M, Martínez-Martínez L, et al. Ocurrence of Corynebacterium striatum as an emerging antibiotic-resistant nosocomial pathogen in a Tunisian hospital. Scienfitic Reports. 2017;7:1.
Fernández-Guerrero ML, Robles I, Nogales MC, Nuevo D. Corynebacterium striatum: an emerging nosocomial drug-resistant endocardial pathogen. J Heart Valve Dis. 2013;22:428–30.
Wang J, Wang Y, Du X, Cui J, Wang K, Zhang L, et al. Rapid transmission of multidrug-resistant Corynebacterium striatum among susceptible patients in a tertiary hospital in China. J Infect Dev Ctries. 2016;10(12):1299–305.
Yanai M, Ogasawasa M, Hayashi Y, Suzuki K, Takahashi H, Satomura A. Retrospective evaluation of the clinical characteristics associated with Corynebacterium species bacteremia. Braz J Infect Dis. 2018;22(1):24–9.
Kimura S, Gomyo A, Hayakawa J, Akahoshi Y, Harada N, Ugai T, et al. Clinical characteristics and predictive factors for mortality in coryneform bacteria bloodstream infection in hematological patients. J Infect Chemoter. 2017;23(3):148–53.
Ishiwada N, Watanabe N, Murata S, Takeuchi N, Taniguchi T, Igari H. Clinical and bacteriological analyses of bacteremia due to Corynebacterium striatum. J Infect Chemother. 2016;22(12):790–3.
Maki DG, Weise CE, Sarafin HW. A semi-quantitative culture method for identifying intravenous-caheter-related infection. N Engl J Med. 1977;296:1305–9.
Funke G, Bernard KA. Coryneform gram-positive rods. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW, editors. Manual of Clinical Microbiology. USA: ASM Press; 2011. p. 413–42.
Yoon S, Ha SM, Kwon S, Lim J, Kim Y, Seo H, et al. Introducing EzBioCloud: a taxonomically united database of 16S rRNA and whole genome assemblies. Int J Syst Evol Microbiol. 2016. https://doi.org/10.1099/ijsem.0.001755.
Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–9.
van Belkum A, Tassios PT, Dijkshoorn L, Haeggman S, Cookson B, Fry NK, et al. Guidelines for the validation and application of typing methods for use in bacterial epidemiology. Clin Microbiol Infect. 2007;13(Suppl 3):1–46.
Luo Y, Ma Y, Zhao Q, Wang L, Guo L, Ye L, et al. Similarity and divergence of phylogenies, antimicrobial susceptibilities, and virulence factor profiles of Escherichia coli isolates causing recurrent urinary tract infections that persist or result from reinfection. J Clin Microbiol. 2012;50(12):4002–7.
BrCAST. Tabelas de pontos de corte para interpretação de CIMs e diâmetros de halos. In: Brazil: Brazilian Committee on Antimicrobial Susceptibility Testing. 08/26/2017 version. Brazil: Brazilian Committee on Antimicrobial Susceptibility Testing; 2017.
CLSI. Performance standards for antimicrobial susceptibility testing. In: CLSI supplement M100S. 26th ed. Clinical and Laboratory Standards Institute: Wayne; 2017.
CLSI. Methods for antimicrobial dilution and disk susceptibility testing of Infrenquently isolated or fastidious Bacteria. In: CLSI supplement M45-A2. 2nd ed. Clinical and Laboratory Standards Institute: Wayne; 2010.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81.
Sierra JM, Martinez-Martinez L, Vázques F, Giralt E, Vila J. Relationship between mutations in the gyrA gene and quinolone resistance in clinical isolates of Corynebacterium striatum and Corynebacterium amycolatum. Antimicrobial Agents and Chemother. 2005;49:1714–9.
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–82.
Ortíz-Pérez A, Martín-de-Hijas NS, Esteban J, Fernández-Natal MI, García-Cía JI, Fernández-Roblas R. High frequency of macrolide resistance mechanisms in clinical isolates of Corynebacterium species. Microb Drug Resis. 2010;16:273–7.
Souza C, Faria YV, Sant’Anna LO, Viana VG, Seabra SH, Souza MC, et al. Biofilm production by multiresistant Corynebacterium striatum associated with nosocomial outbreak. Mem Inst Oswaldo Cruz. 2015;110:242–8.
Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645–58.
Chen FL, Hsueh PR, Teng SO, Ou TY, Lee WS. Corynebacterium striatum bacteremia associated with central venous catheter infection. J Microbiol Immunol Infect. 2012;45:255–8.
Camello TCF, Mattos-Guaraldi AL, Formiga LCD, Marques EA. Nondiphtherial Corynebacterium species isolated from clinical specimens of patients in a university hospital, Rio de Janeiro. Brazil Braz J Microbiol. 2003;34:39–44.
Martins CAS, Faria L, Souza M, Camello T, Velasco E, Hirata R Jr, et al. Microbiological and host features associated with corynebacteriosis in cancer patients: a five-year study. Mem Inst Oswaldo Cruz. 2009;104:905–13.
Superti SV, Martins DS, Caierão J, Soares F, Prochnow T, Cantarelli VV, et al. Corynebacterium striatum infecting a malignant cutaneous lesion: the emergence of an opportunistic pathogen. Rev Inst Med Trop São Paulo. 2009;51:115–6.
McElvania TeKippe E, Thomas BS, Ewald GA, Lawrence SJ, Burnham CA. Rapid emergence of daptomycin resistance in clinical isolates of Corynebacterium striatum… a cautionary tale. Eur J Clin Microbiol Infect Dis. 2014;33:2199–205.
Iwalokun BA, Oluwadun A, Akinsinde KA, Niemogha MT, Nwaokorie FO. Bacteriologic and plasmid analysis of etiologic agents of conjunctivitis in Lagos, Nigeria. J Ophthal Inflamm Infect. 2011;1:95–103.
Beltrán-Arroyave C, Díaz-Díaz A, Loaiza-Díaz N. Osteomielitis crónica por Corynebacterium striatum en una adolescente. Rev Chil Infectol. 2016;33:696–9.
Das S, Subba Rao AV, Sahu SK, Sharma S. Corynebacterium spp. as causative agentes of microbial keratitis. Br J Ophthalmol. 2016;100:939–43.
Olender A. Antibiotic resistance and detection of the most common mechanism of resistance (MLSB) of opportunistic Corynebacterium. Chemoterapy. 2013;59:294–306.
Crabtree JH, Garcia NA. Corynebacterium striatum peritoneal dialysis catheter exit site infection. Clin Nephrol. 2003;60:270–4.
Tarr PE, Stock F, Cooke RH, Fedorko DP, Lucey DR. Multidrug-resistant Corynebacterium striatum pneumonia in a heart transplant recipient. Transpl Infect Dis. 2003;5:53–8.
Shah M, Murillo JL. Successful treatment of Corynebacterium striatum endocarditis with daptomycin plus rifampin. Ann Pharmacother. 2005;39:1741–4.
Otsuka Y, Ohkusu K, Kawamura Y, Baba S, Ezaki T, Kimura S. Emergence of multidrug-resistant Corynebacterium striatum as a nosocomial pathogen in long-term hospitalized patients with underlying diseases. Diagn Microbiol Infect Dis. 2006;54:109–14.
Fernández-Guerrero ML, Molins A, Rey M, Romero J, Gadea I. Multidrug-resistant Corynebacterium striatum endocarditis successfully treated with daptomycin. Int J Antimicrob Agents. 2012;40:373–4.
Werth BJ, Hahn WO, Butler-Wu SM, Rakita RM. Emergence of high-level daptomycin resistance in Corynebacterium striatum in two patients with left ventricular assist device infections. Microb Drug Resist. 2016;22:233–7.
Eguchi H, Kuwahara T, Miyamoto T, Nakayama-Imaohji H, Ichimura M, Hayashi T, et al. High-level fluoroquinolone resistance in ophthalmic clinical isolates belonging to the species Corynebacterium macginleyi. J Clin Microbiol. 2008;46:527–32.
Yoon S, Kim H, Lee Y, Kim S. Bacteremia caused by Corynebacterium amycolatum with a novel mutation in gyrA gene that confers high-level quinolone resistance. Korean J Lab Med. 2011;31:47–8.
Fernández-Natal I, Sáez-Nieto JA, Rodríguez-Lázaro D, Valdezate-Ramos S, Parras-Padilla T, Medina MJ, et al. Phenotypic, molecular characterization, antimicrobial susceptibility and draft genome sequence of Corynebacterium argentoratense strains isolated from clinical samples. New Microbes New Infect. 2016;10:116–21.
Navas J, Fernández-Martínez M, Salas C, Cano ME, Martínez-Martínez L. Susceptibility to aminoglycosides and distribution of aph and aac(3)-XI genes among Corynebacterium striatum clinical isolates. PLoS One. 2016;11:12.
Neemuchwala A, Soares D, Ravirajan V, Marchand-Austin A, Kus JV, Patel SN. In Vitro Antibiotic Susceptibility Pattern of Non-diphtheriae Corynebacterium isolates in Ontario, Canada, from 2011 to 2016. Antimicrob Agents Chemother. 2018;62(4):e01776–17.
Shi K, Caldwell SJ, Fong DH, Berghuis AM. Prospects for circumventing aminoglycoside kinase mediated antibiotic resistance. Front Cell Infect Microbiol. 2013;3:1–22.
Tauch A, Trost E, Tilker A, Ludewig U, Schneiker S, Goesmann A, et al. The lifestyle of Corynebacterium urealyticum derived from its complete genome sequence established by pyrosequencing. J Biotechnol. 2008;136:11–21.
Schroder J, Maus I, Meyer K, Wordemann S, Blom J, Jaenicke S, et al. Complete genome sequence, lifestyle, and multidrug resistance of the human pathogen Corynebacterium resistens DSM 45100 isolated from blood samples of a leukemia patient. BMC Genomics. 2012;13:141.
Mollmann S, Albersmeier A, Ruckert C, Tauch A. Complete genome sequence of Corynebacterium imitans DSM 44264, isolated from a five-month-old boy with suspected pharyngeal diphtheria. Genome Announc. 2014. https://doi.org/10.1128/genomeA.01210-14.
Paredes J, Alonso-Arce M, Schmidt C, Valderas D, Sedano B, Legarda J, et al. Smart central venous port for early detection of bacterial biofilm related infections. Biomed Microdevices. 2014;16:365–74.
Donelli G, Vuotto C. Biofilm-based infections in long-term care facilities. Future Microbiol. 2014;9:175–88.
Acknowledgements
Not applicable.
Funding
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001 provided stipend for the doctoral work of Juliana Nunes Ramos and Cassius Souza in Fundação Oswaldo Cruz and Universidade do Estado do Rio de Janeiro, resceptivelly. This work was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Fiocruz covering laboratory reagents to perform all experiments. The Sub-Reitoria de Pós-Graduação e Pesquisa da Universidade do Estado do Rio de Janeiro (SR-2/UERJ) provided stipend to Paulo Victor Pereira Baio and Yuri Vieira Faria. The funders had no role in study design, data collection, analysis and interpretation of this work.
Author information
Authors and Affiliations
Author notes
Raphael Hirata Júnior is deceased. This paper is dedicated to his memory.
- Raphael Hirata Júnior
Contributions
JNR, ALMG. and VVV conceived and designed the study, JNR. collected and identified the clinical isolates, JNR. and JFCV. conducted the experiments of PFGE, JNR and ECS. conducted the antimicrobial assays, CS, YVF, SHS and LOM. conducted the experiments of biofilm, JNR, PVPB, JFCV and VVV. analysed the data, JNR, VVV, ALMG. and RHJ drafted the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was developed in compliance with the Brazilian Government’s Ethical Guidelines for research involving human beings (resolution of the National Health Council/Ministry of Health) and approved by the ethical research committee of Hospital Universitário Pedro Ernesto/Universidade do Estado do Rio de Janeiro (CAAE: 01247512.3.0000.5259). The consent to participate was not required because all the investigated isolates were taken as a part of standard care (diagnostic purposes). The samples were not collected for research purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Figure S1. Pulsed-field gel electrophoresis (PFGE) profiles of Brazilian Corynebacterium striatum isolates from blood and catheter segments. Lane 1: λ DNA ladder PFGE marker; lane 2: profile VIII (isolate 2103); lanes 3, 5 and 6: profile I (isolates 2316, 2439 and 2023, respectively); lane 4: profile Ia (isolate 2324); lane 7: profile X (isolate 2376); lane 8: profile VIa (isolate 2390); lane 9: profile IX (isolate 2425) and lane 10: profile Ib (isolate 2454). (TIF 523 kb)
Additional file 2:
Figure S2. Pulsed-field gel electrophoresis (PFGE) profiles of Brazilian Corynebacterium striatum isolates from blood and catheter segments. Lane 1: λ DNA ladder PFGE marker; lanes 2, 3 and 7: profile I (isolates 2089, 2091 and 2023, respectively); lane 4: profile V (isolate 2130); lanes 5, 6 and 9: profile VI (isolates 2228, 2230 and 2237, respectively); lane 8: profile VII (isolate 2296) and lane 10: profile IV (isolate 1954 – control). (TIF 469 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ramos, J.N., Souza, C., Faria, Y.V. et al. Bloodstream and catheter-related infections due to different clones of multidrug-resistant and biofilm producer Corynebacterium striatum. BMC Infect Dis 19, 672 (2019). https://doi.org/10.1186/s12879-019-4294-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-019-4294-7