Abstract
Background
To assess the immune persistence conferred by a Chinese hamster ovary (CHO)-derived hepatitis B vaccine (HepB) 17 to 20 years after primary immunization during early life.
Methods
Participants born between 1997 and 1999 who received a full course of primary vaccination with HepB (CHO) and who had no experience with booster vaccination were enrolled. Blood samples were required from each participant for measurement of hepatitis B surface antibody (anti-HBs), surface antigen and core antibody levels. For those who possessed an anti-HBs antibody < 10 mIU/mL, a single dose of HepB was administered, and 30 days later, serum specimens were collected to assess the booster effects.
Results
A total of 1352 participants were included in this study. Of these, 1007 (74.5%) participants could retain an anti-HBs antibody ≥10 mIU/mL, with a geometric mean concentration (GMC) of 57.4 mIU/mL. HBsAg was detected in six participants, resulting in a HBsAg carrier rate of 0.4% (6/1352). Of those participants with anti-HBs antibodies < 10 mIU/mL, after a challenge dose, 231 (93.1%) presented an anti-HBs antibody ≥10 mIU/mL, with a GMC of 368.7 mIU/mL. A significant increase in the anti-HBs positive rate (≥ 10 mIU/mL) after challenge was observed in participants with anti-HBs antibodies between 2.5 and 10 mIU/mL and participants boosted with HepB (CHO), rather than those with anti-HBs antibodies < 2.5 mIU/mL and those boosted with HepB (SC).
Conclusion
Since satisfactory immune protection against HBV infection conferred by primary vaccination administered 17–20 years ago was demonstrated, there is currently no urgent need for booster immunization.
Similar content being viewed by others
Background
Hepatitis B virus (HBV) is one of the leading causes of infectious diseases and a major public health problem in China. The hepatitis B vaccine (HepB) is the most effective means to control the spread of HBV. In 2002, HepB in China was officially integrated into the China National Immunization Plan, and newborns underwent obligatory vaccination at 0, 1 and 6 months of age. A satisfactory immune response was observed among 90 to 95% of healthy infants, whereas 5 to 10% of the population showed no response or a weak response [1,2,3]. Moreover, for the time being, a decline in hepatitis B surface antibody (anti-HBs) level was observed among those infants with a satisfactory immune response after initial injection. Theoretically, anti-HBs < 10 mIU/mL is considered the threshold for effective protection against HBV infection. To control HBV infection, monitoring the long-term persistence after initial injection in real time to determine the timing for a booster dose is important.
In the 1980s, Zhengding County, Hebei Province, China, was selected as the study site for the clinical evaluation of plasma-derived HepB [4]. Since January 1, 1997, newborns were vaccinated with Chinese hamster ovary (CHO)-derived HepB. To evaluate the immune persistence 17 to 20 years after primary vaccination with CHO-derived HepB and the effects of a booster dose vaccination, we conducted this study between August 2017 and September 2018.
Methods
Study cohort and study design
Universal HepB immunization in newborns began in 1986 in the community. From then on, a database about the assessment of the immunity efficacy of HepB was established in which data about primary immunization were recorded. Between 1997 and 2001, a CHO-derived HepB with a dosage of 10 μg/mL was used for the newborns according to the 0, 1, and 6 month schedule. In total, 92.9% of individuals maintained positive for anti-HBs in the cross-sectional survey in May 1999. By screening the historical database, participants born between 1997 and 1999 living in seven townships in Zhengding County who completed the full course primary vaccination were enrolled in this study. In the present study, a questionnaire, including name, sex, date of birth, and history of HepB booster vaccination, was completed, and those who underwent a booster dose before this study were excluded from the final analysis. A blood sample was collected from each participant who provided written informed consent, and the serum was isolated aseptically and stored at − 20 °C until testing.
Participants who were hepatitis B surface antigen (HBsAg)- and hepatitis B core antibody (anti-HBc)-negative and anti-HBs < 10 mIU/mL were randomly assigned to two groups using the random numbers 1 and 2, receiving a booster dose of HepB (20 μg/ml) derived from either Saccharomyces cerevisiae (SC) (Shenzhen Kangtai Biological Products Co., Ltd., Shenzhen, China) or CHO (Genetech Biotechnology, Huabei Pharmaceutical Co., Ltd., Shijiazhuang, China). Blood samples were collected 30 days after boosting to determine the potential immunological response.
This study was reviewed and approved by the Institutional Review Board (IRB) of the Hebei Center for Disease Control and Prevention (CDC). Written informed consent was obtained from each participant for personal information and blood sample collection. The study was performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its later amendments.
Laboratory assay
A batch test for virological and immunological biomarkers of HBV was performed in the reference laboratory at the Institute for Viral Disease, China Center for Disease Control and Prevention (Beijing) upon the completion of follow-up. Abbott EIA AxSYM (Abbott, Abbott Park, IL, USA) was used for the detection of HBsAg, anti-HBs, and anti-HBc. According to protocols provided by the manufacturer, positive and negative cutoffs were calculated with the positive and negative controls as required by the diagnostic kits. HBsAg < 0.05 IU/mL was considered reactive. The minimum detection limit of anti-HBs was 2.5 mIU/mL.
Statistical analysis
All data were double entered into custom-made data entry programs based on Epidata 3.1. The data management programs included range and consistency checks. An SPSS program (IBM Corporation, Armonk, NY, USA) was used for statistical analysis. Antibody seroprotection rate (SPR), geometric mean concentrations (GMCs) and their 95% confidence intervals (CIs) were calculated. Antibody concentrations were logarithmically converted to allow for assessment of GMCs. For continuous outcome comparisons, Student’s t test or the Mann–Whitney U test was performed, and for dichotomous outcomes, the chi square or Fisher exact test was implemented. The Mantel-Haenszel test was conducted for stratification analysis. Seroprotection was defined as an anti-HBs ≥ 10 mIU/mL. Time after vaccination was defined as the interval between the last visit (time for blood collection during the cross-sectional survey in the present study) and completion of the full primary course. HBV infection was defined as positive for HBsAg or/and anti-HBc. A p-value < 0.05 was considered statistically significant.
Results
Baseline characteristics of enrolled participants
Between 1997 and 1999, 2436 newborns who received a full course of primary vaccination were initially recruited in the study cohort, and 1551 (63.7%) participants were followed in 2017. Of the 1551 individuals, 199 individuals with a history of booster vaccination were excluded, and 1352 (55.5%) participants were included in the final analysis. Of the 1352 participants, 616 (45.6%) were male, and the average age of the enrolled participants was 19.3 years (95% CI: 19.2–19.3). All participants completed primary vaccination at day 187.2 (95% CI: 185.4–189.0) after birth, resulting in an average of 18.8 (95% CI: 18.7–18.8) follow-up years.
Long-term protection against HBV infection
After measurement of the anti-HBs antibody, 1007 (74.5%) participants had an anti-HBs antibody level ≥ 10 mIU/mL, with a GMC of 57.4 mIU/mL (95% CI: 50.4–66.0 mIU/mL) at 17 to 20 years after the initial dose of HBV vaccine. The seroprotection rates were 74.0 and 75.0% in the female and male subgroups, respectively (p > 0.05); moreover, a similar GMC was observed in females (55.1 mIU/mL, 95% CI: 45.6–66.7 mIU/mL) and males (60.9 mIU/mL, 95% CI: 49.9–74.4 mIU/mL) (p > 0.05). A considerable percentage (46.6%; 630/1352) of participants possessed an anti-HBs antibody ≥100 mIU/mL. Of these, 167 participants had an anti-HBs antibody ≥1000 mIU/mL.
During the long-term follow-up, of 1352 participants, 18 (1.3%) experienced HBV infection. HBsAg was detected in 6 participants, all of whom were positive for anti-HBc, and the HBsAg positive rate was 0.4% (6/1352). The other 12 participants did not carry HBV, which led to an anti-HBc positive rate of 0.9% (12/1352).
Recall responses after the booster
Among the 338 participants who were HBsAg- and anti-HBc-negative, with anti-HBs antibody < 10 mIU/mL at 17 to 20 years, 248 (73.4%) participants provided informed consent and received a single booster dose of HBV vaccine. Of these, 128 participants received a single dose of HepB (CHO), while another 120 participants were injected with a single dose of HepB (SC) (Fig. 1).
One month after administration of a single dose of HBV vaccine, 93.1% (231/248) of vaccinees developed detectable anti-HBs antibody with a GMC of 368.7 mIU/mL (95% CI: 278.7–487.8 mIU/mL). The majority (78.2%) of vaccinees displayed a dramatic increase in anti-HBs titers that was ≥10-fold (Table 1). Similar to the abovementioned results, a significant difference was not detected in the anti-HBs antibody level between females and males, neither seroconversion rate (95.0% vs 90.8%), nor antibody concentrations (450.3mIU/mL vs 284.3 mIU/mL) (p > 0.05).
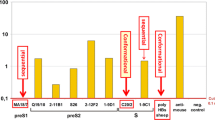
When those participants who received the booster dose of HBV vaccine were further stratified into two subgroups by the minimum detection limit of Abbott reagent of 2.5 mIU/mL, a statistically significant difference was found between participants with pre-booster anti-HBs antibody concentration < 2.5 mIU/mL and participants with pre-booster anti-HBs antibody concentration ≥ 2.5 mIU/mL, either for the seroconversion rate [87.8% (122/139) vs 100% (109/109)] (p < 0.001) or for the GMC [144.0 mIU/mL (95% CI: 97.5–214.9 mIU/mL) vs 1212.0 mIU/mL (95% CI: 934.5–1571.8 mIU/mL)] (p < 0.001) (Fig. 2).
When boostered participants were aggregated into the HepB (CHO) challenge group and the HepB (SC) challenge group, the average age and gender composition were comparable in the two groups (19.2-years vs. 19.3-years, p > 0.05; gender composition ratio: 1.0:1.5 vs. 1.0:1.1; p > 0.05). The difference in the pre-booster anti-HBs titers was not statistically significant in the two groups [HepB (CHO): 3.1 mIU/mL (95% CI: 2.6–3.6 mIU/mL); HepB (SC): 3.2 (95% CI: 2.6–3.7 mIU/mL); p > 0.05]. The seroconversion rates were 97.7% (125/128) and 88.3% (106/120) in the two groups (p < 0.05). A higher anti-HBs concentration was observed in the HepB (CHO) challenge group (578.2 mIU/mL; 95% CI: 419.9–804.3 mIU/mL) rather than in the HepB (SC) challenge group (225.9 mIU/mL; 95% CI: 145.5–354.2 mIU/mL), and a significant difference was reached (p < 0.05) (Fig. 3). After adjusting for the risk factor for pre-booster anti-HBs titers, the difference in the seroconversion rates between the two groups was statistically significant (p = 0.006).
Discussion
In this study, a seroprotection rate of 74.5%, with a GMC of 57.4 mIU/mL, was observed in a longitudinal birth cohort who completed the full primary course of HBV immunization 17 to 20 years ago. Our previous cross-sectional surveys based on the same cohort reported a seroprotection rate of 76.8% (GMC: 276 mIU/mL) and 74.1% (GMC: 209 mIU/mL) at 6 to 8 years and 10 to 12 years after completion of primary vaccination, respectively [5, 6]. Notably, for the time being, the anti-HBs seroprotection rates were quite stable compared to the decline in anti-HBs antibody concentrations. Several studies have reported the persistence of anti-HBs levels after primary vaccination and found that protective anti-HBs antibodies were maintained up to 20 years in approximately 37–77% of vaccinees [7,8,9,10]. The persistence of anti-HBs is related to a variety of factors, including the mother’s carrying status, regimens and type of vaccine used, time interval between the first and last injection of primary vaccination, and anti-HBs antibody peak level after full course of primary immunization [10,11,12].
A series of studies reported a satisfactory anti-HBs seroconversion, which was approximately 84.0 and 98.0%, after a booster dose was achieved [7, 12,13,14,15,16]. In our study, a dramatic increase in the anti-HBs antibody positive rate was measured. More than 90% of participants presented an anti-HBs antibody level ≥ 10 mIU/mL. A robust anamnestic response even in those with pre-challenge anti-HBs antibody < 10 mIU/mL was thus demonstrated. However, an anti-HBs antibody level in 6.9% participants was still less than 10 mIU/mL although it had increased multifold. The likely explanation is the genetic non-response that also occurs during the primary immunization or the decay with time of immunization memory, considering that the anti-HBs levels of the majority of these participants were less than 10 mIU/mL in previous cross-sectional surveys based on the same cohort. It would be interesting to perform genetic analysis on these participants to understand the potential association between gene mutation and response to HBV vaccination.
Theoretically, primary immunization is the basis of immunity established in human bodies, and a booster effect depends on the pre-challenge antibody level. For people with pre-challenge anti-HBs levels < 10 mIU/mL, after boosting, a higher antibody level could be expected in vaccinees with low anti-HBs levels at pre-challenge compared to that in those vaccinees with undetectable anti-HBs (anti-HBs antibody level < the lower limit of detection) [14, 17]. Our study demonstrated again that a significant increase in seroprotection was found in participants with a pre-challenge anti-HBs antibody between 2.5–10 mIU/mL in comparison with those participants with an anti-HBs antibody < 2.5 mIU/mL. In addition, different booster effects were measured between participants boostered with HepB (CHO) and participants boostered with HepB (SC). The former displayed a better response to boosting. Though controversial conclusions on immune response to a challenge dose were generated from different studies [18,19,20], a higher immune response to primary immunization was always conferred by HepB (CHO) rather than HepB (SC). The possible explanation is the different varieties of vaccines between primary immunization and booster dose. Another study showed that the possibility of the potential mismatch between the antibody elicited by HepB (SC) and the antigen derived from blood donors, which is coated on the reagent plate, cannot be excluded and may affect the sensitivity of the detection [21].
There are several limitations in our study. First, serological surveys were not conducted every year, and the occurrence of a booster due to natural HBV infection could not be detected and eventually could not be excluded from the current analysis. From this point, the persistence of immunity demonstrated in this study was conferred by both vaccine immunization and natural infection. However, because HBV is transmitted through fluids, mainly through sexual transmission, teenagers living in rural areas usually have lower chance of exposure to HBV. Thus, natural infection did not contribute too much to the maintenance of immunity. Second, applying different immunoassay systems with incomparable sensitivity over a long time period is often inevitable and may damage the consistency of serological data. However, in comparison with the seroprotection rate and anti-HBs antibody concentration tested at 6 to 8 years and 10 to 12 years after completion of primary vaccination, a trend of continuous and stable decline, in stead of fluctuation of immunity, was observed. To a certain extent, there was comparability between different generations of Abbott assay systems. Finally, the history of booster immunization was obtained from the questionnaire, in which recall bias might exist.
The demand for booster and the timing of boosters after the completion of primary immunization are always controversial. Some studies concluded that booster vaccination was necessary when antibodies fell below the protective level threshold to reach an effective antibody level against HBV infections [15, 16, 22, 23]. However, other studies indicated that there was no evidence to support booster vaccination thus far [12, 24,25,26]. In particular, the cellular immune memory could be determined from majority of vaccinees whose anti-HBs antibody level was lower than 10 mIU/mL, or even absent [27]. In 2000, the European Consensus Group on Hepatitis B Immunity issued a statement that there were no data supporting a need for booster doses of HepB in immunocompetent individuals who had responded to a primary course [28]. More recently, a WHO position paper also noted that there was no evidence supporting the need for a booster dose of HepB in routine immunization programs [29]. Considering a dramatic and continued reduction in the HBsAg carrier rate in this age group of the entire population in China [30, 31], together with findings from our study, we conclude that there is currently no urgent need for booster immunization.
Conclusion
A protective anti-HBs antibody level conferred by primary vaccination administered 17–20 years ago was maintained in approximately three-quarters of the vaccinees, and a satisfactory anamnestic response was observed in the majority of individuals with a pre-booster anti-HBs antibody level < 10 mIU/mL. Pre-booster anti-HBs antibody concentrations and type of vaccines were factors associated with post-booster anti-HBs antibody levels.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available because the personal information of the participants is confidential but are available from the corresponding author on reasonable request.
Abbreviations
- anti-HBc:
-
Hepatitis B core antibody
- Anti-HBs:
-
Hepatitis B surface antibody
- CHO:
-
Chinese hamster ovary
- GMC:
-
Geometric mean concentration
- HBsAg:
-
Hepatitis B surface antigen
- HBV:
-
Hepatitis B virus
- HepB:
-
Hepatitis B vaccine
- SC:
-
Saccharomyces cerevisiae
References
Alssamei FA, Al-Sonboli NA, Alkumaim FA, Alsayaad NS, Al-Ahdal MS, Higazi TB, Elagib AA: Assessment of immunization to Hepatitis B vaccine among children under five years in rural areas of Taiz, Yemen. Hepat Res Treat 2017, 2017:2131627.
Filippelli M, Lionetti E, Gennaro A, Lanzafame A, Arrigo T, Salpietro C, La Rosa M, Leonardi S. Hepatitis B vaccine by intradermal route in non responder patients: an update. World J Gastroenterol. 2014;20(30):10383–94.
Walayat S, Ahmed Z, Martin D, Puli S, Cashman M, Dhillon S. Recent advances in vaccination of non-responders to standard dose hepatitis B virus vaccine. World J Hepatol. 2015;7(24):2503–9.
Shen L, Wang F, Wang F, Cui F, Zhang S, Zheng H, Zhang Y, Liang X, Bi S. Efficacy of yeast-derived recombinant hepatitis B vaccine after being used for 12 years in highly endemic areas in China. Vaccine. 2012;30(47):6623–7.
Wang F, Zhao YL, Ma JC, Bi SL, Zhang Y, Shen LP. Long-term efficacy of 10-12 years after being immunized with Chinese hamster ovary cell derived hepatitis B vaccine in Chinese rural communities. Vaccine. 2012;30(12):2051–3.
Zhang Y, Ma JC, Qi SX, Wang F, Zhao C, Bi SL. Effectiveness of a Chinese hamster ovary cell derived hepatitis B vaccine in Chinese rural communities. Vaccine. 2011;29(22):3905–8.
Coppola N, Corvino AR, De Pascalis S, Signoriello G, Di Fiore E, Nienhaus A, Sagnelli E, Lambert M. The long-term immunogenicity of recombinant hepatitis B virus (HBV) vaccine: contribution of universal HBV vaccination in Italy. BMC Infect Dis. 2015;25(15):149.
Poovorawan Y, Chongsrisawat V, Theamboonlers A, Crasta PD, Messier M, Hardt K. Long-term anti-HBs antibody persistence following infant vaccination against hepatitis B and evaluation of anamnestic response. Hum Vaccin Immunother. 2013;9(8):1679–84.
Poovorawan Y, Chongsrisawat V, Theamboonlers A, Leroux-Roels G, Crasta PD, Hardt K. Persistence and immune memory to hepatitis B vaccine 20 years after primary vaccination of Thai infants, born to HBsAg and HBeAg positive mothers. Human vaccines & immunotherapeutics. 2012;8(7):896–904.
Bagheri-Jamebozorgi M, Keshavarz J, Nemati M, Mohammadi-Hossainabad S, Rezayati MT, Nejad-Ghaderi M, Jamalizadeh A, Shokri F, Jafarzadeh A. The persistence of anti-HBs antibody and anamnestic response 20 years after primary vaccination with recombinant hepatitis B vaccine at infancy. Hum Vaccin Immunother. 2014;10(12):3731–6.
Schonberger K, Riedel C, Ruckinger S, Mansmann U, Jilg W, Kries RV. Determinants of long-term protection after hepatitis B vaccination in infancy: a meta-analysis. Pediatr Infect Dis J. 2013;32(4):307–13.
Romanò L, Galli C, Tagliacarne C, Tosti ME, Velati C, Fomiatti L, Chironna M, Coppola RC, Cuccia M, Mangione R, et al. Persistence of immunity 18–19 years after vaccination against hepatitis B in 2 cohorts of vaccinees primed as infants or as adolescents in Italy. Hum Vaccin Immunother. 2017;13(5):981–5.
Wang ZZ, Gao YH, Lu W, Jin CD, Zeng Y, Yan L, Ding F, Li T, Liu XE, Zhuang H. Long-term persistence in protection and response to a hepatitis B vaccine booster among adolescents immunized in infancy in the western region of China. Hum Vaccin Immunother. 2017;13(4):909–15.
Chen YS, Chu CH, Wang JH, Lin JS, Chang YC. Predictors of booster response to Hepatitis B vaccine at 15 years of age: a cross-sectional school-based study. Pediatr Neonatol. 2016;57(4):302–9.
Lu S, Ren J, Li Q, Jiang Z, Chen Y, Xu K, Ruan B, Yang S, Xie T, Yang L, et al. Effects of hepatitis B vaccine boosters on anti-HBs-negative children after primary immunization. Hum Vaccin Immunother. 2017;13(4):903–8.
Yao J, Shan H, Chen Y, Jiang ZG, Dai XW, Ren JJ, Xu KJ, Ruan B, Yang SG, Li Q. The one year effects of three doses of hepatitis B vaccine as a booster in anti-HBs-negative children 11-15 years after primary immunization; China, 2009-2011. Hum Vaccin Immunother. 2015;11(5):1114–9.
McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, Bulkow L, Fiore AE, Bell BP, Hennessy TW. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis. 2009;200(9):1390–6.
Su YP, Liu XJ, Wang BL, Tang YQ. Evaluation on the immunity effect of different hepatitis B vaccines. Zhongguo Xue Xiao Wei Sheng Za Zhi. 2007;28(9):848–9.
Gong XH, Wang FZ, Wu J, Liu LR, Han LL, Zhang H, Xu ZX, Gan YD, Liu LY, Su YP, et al. Observation on the immunity effects with different Hepatitis B vaccine made by recombinant DNA techniques. Zhongguo Ji Hua Mian Yi Za Zhi. 2007;13(4):316–8.
Zhang AF, Luo GR, Chen XG, Liu CF, Tong GY. The efficacy of reinculation with two recombinant hepatitis B vaccines. Zhongguo Xue Xiao Wei Sheng Za Zhi. 2000;21(2):90–1.
Gong XH, Wang FZ, Li H, Liu LR, Gao J, Han QY, Li YT, Hu Y, Li YH, Tang YW. The follow-up study on immunization effect of Hepatitis B gene recombined vaccine for the new infants during six years. Zhongguo Ji Hua Mian Yi Za Zhi. 2006;12(4):259–61.
Lu IC, Jean MC, Lin CW, Chen WH, Perng DS, Lin CW, Chuang HY. Predictive factors for anti-HBs status after 1 booster dose of hepatitis B vaccine. Medicine (Baltimore). 2016;95(39):e5023.
Pileggi C, Papadopoli R, Bianco A, Pavia M. Hepatitis B vaccine and the need for a booster dose after primary vaccination. Vaccine. 2017;35(46):6302–7.
Gilca V, De Serres G, Boulianne N, Murphy D, Ouakki M, De Wals P, Trudeau G, Massé R, Dionne M. Long-term persistence of immunity after vaccination of pre-adolescents with low doses of a recombinant hepatitis B vaccine. Hum Vaccin Immunother. 2013;9(8):1685–90.
Chang YC, Wang JH, Chen YS, Lin JS, Cheng CF, Chu CH. Hepatitis B virus vaccination booster does not provide additional protection in adolescents: a cross-sectional school-based study. BMC Public Health. 2014;23(14):991.
Poorolajal J, Hooshmand E. Booster dose vaccination for preventing hepatitis B. Cochrane Database Syst Rev. 2016;(7, 6):CD008256.
Makhlouf NA, Farghaly AM, Zaky S, Rashed HA, Abu Faddan NH, Sayed D, El-Badawy O, Afifi N, Hamza WS, El-Sayed Y. The efficacy of hepatitis B vaccination program in upper Egypt: flow cytometry and the evaluation of long term immunogenicity. J Med Virol. 2016;88(9):1567–75.
Are booster immunisations needed for lifelong hepatitis B immunity? European Consensus Group on Hepatitis B Immunity. Lancet (London, England) 2000, 355(9203):561–565.
Hepatitis B. Vaccines: WHO position paper-July 2017. Wkly Epidemiol Rec. 2017;92:369–92.
Cui F, Shen L, Li L, Wang H, Wang F, Bi S, Liu J, Zhang G, Wang F, Zheng H, et al. Prevention of chronic Hepatitis B after 3 decades of escalating vaccination policy, China. Emerg Infect Dis. 2017;23(5):765–72.
Wang FZ, Zhang GM, Shen LP, Zheng H, Wang F, Miao N, Yuan QL, Sun XJ, Bi SL, Liang XF, et al. Comparative analyze on hepatitis B seroepidemiological surveys among population aged 1-29 years in different epidemic regions of China in 1992 and 2014. Zhonghua Yu Fang Yi Xue Za Zhi. 2017;51(6):462–8.
Acknowledgments
We are indebted to Dr. Zhi-Wei Jiang for his technical support on the figures. We thank the people of Zhengding County who participated in the study and the dedicated community health practitioners who made this study possible.
This project titled “Study on protection conferred by hepatitis B vaccine 30 years after initial immunization” (162777290) was supported by the Department of Science & Technology, Hebei Province.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
YLZ, QL and JCM contributed to the study’s conception and design. BHH, XJZ, LLP, HSZ, ZG, ZYH, ZWW and TLM conducted the field work. BHH, LLP, ZYH, ZWW and TLM carried out the laboratory testing. BHH, YLZ, and JCM analyzed the data. BHH and YLZ wrote the paper. FW and SLB provided important contributions in laboratory testing and the writing of the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Institutional Review Board (IRB) of the Hebei CDC. Written informed consent was obtained from each participant for personal information and blood sample collection. The study was performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its later amendments.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhao, YL., Han, BH., Zhang, XJ. et al. Immune persistence 17 to 20 years after primary vaccination with recombination hepatitis B vaccine (CHO) and the effect of booster dose vaccination. BMC Infect Dis 19, 482 (2019). https://doi.org/10.1186/s12879-019-4134-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-019-4134-9